Plant materials are widely used in human foods and animal feedstuffs( Reference Shewry, Beaudoin and Jenkins1 ). However, the large use of plant materials aggravated persistent diarrhoea in children( Reference Mattos, Ribeiro and Mendes2 ), resulted in the poor growth performance in early-weaned pigs( Reference Yun, Kwon and Lohakare3 ) and induced enteritis in common carp (Cyprinus carpio L.)( Reference Urán, Gonçalves and Taverne-Thiele4 ). These adverse influences may be largely attributed to various antinutritional factors (ANF) in plant materials, such as tannins, gossypol, saponins, protease inhibitors, glycinin and plant lectins( Reference Siddhuraju, Makkar and Becker5 ). By reports, ANF mostly interfered with digestive and absorptive abilities in animals( Reference Lajolo and Maria Inés6 , Reference Zhang, Guo and Feng7 ). The digestive and absorptive abilities of animals rely on the intestinal health, which is correlated with intestinal immune function( Reference Li, Tang and Hu8 ). Surprisingly, except for the study of gossypol from our laboratory( Reference Wang, Feng and Jiang9 ), the limited literature concerning the influence of ANF on the intestinal immune function of animals merely determined partial cytokines (such as TNF-α, interferon-γ (IFN-γ), IL-1, IL-4, IL-6, IL-8 and IL-17a) and not involved in signalling pathway, lacking of systematicness and depth so far(Reference Sun, Li and Qiao10–Reference Krogdahl, Gajardo and Kortner12). Additionally, the impacts of different ANF on intestinal immunity seem to be not yet identical in animals. For instance, our previous studies in the distal intestine (DI) of juvenile Jian carp (C. carpio var. Jian) observed that glycinin down-regulated the mRNA level of IL-1 ( Reference Zhang, Guo and Feng7 ), whereas β-conglycinin had no impact on this gene expression( Reference Jiang, Hu and Zhang13 ). Accordingly, it can be seen that diverse ANF have distinct effects on the intestinal immunity of animals, so it is more meaningful to conduct a systematic and in-depth exploration on a kind of ANF.
Tannins, in which condensed tannins (CT) are the most typical types, are common kinds of ANF and are widely distributed in plant foods and feeds( Reference Chung, Wong and Wei14 ). The contents of tannins in soyabean, rapeseed, sorghum, barley, faba bean and cottonseed are up to 1·0, 5·9, 72·0, 12·3, 24·0 and 16·0 g/kg, respectively( Reference Terrill, Rowan and Douglas15 , Reference Sarwar, Wu and Cockell16 ). Studies have shown that tannins reduced the weight gain in weanling pigs( Reference Lee, Shinde and Choi17 ) and Nile tilapia (Oreochromis niloticus)( Reference Buyukcapar, Atalay and Kamalak18 ). It is generally well known that animal growth is always dependent on intestinal health, which is closely related to intestinal immune function( Reference Betancor, Li and Bucerzan19 ). Zhu et al. have reported that intestinal immune function is closely related to the innate immune components such as lysozyme (LZ), acid phosphatase (ACP), complements (e.g. C3 and C4) and antimicrobial peptides (e.g. β-defensin) and adaptive immune components such as IgM in fish( Reference Zhu, Nie and Zhu20 ). However, only fragmentary research has considered the effects of tannins on those innate and adaptive immune components in animals. One study showed that tannins stimulated arachidonic acid release in bovine tracheal epithelial cells( Reference Cloutier and Guernsey21 ). Ding et al. reported that excess arachidonic acid could weaken serum LZ activity in Macrobrachium nipponense (De Haan)( Reference Ding, Zhou and Kong22 ). Tannins inhibited histamine release in rat peritoneal mast cells( Reference Kanoh, Hatano and Ito23 ). In humans, it was confirmed that low concentrations of histamine could depress the secretion of C3 and C4 in monocytes( Reference Lappin and Whaley24 ) and reduce β-defensin-3 mRNA levels in keratinocytes( Reference Ishikawa, Kanda and Hau25 ). A study reported that tannins could result in a reduction of vitamin A content in rat liver( Reference Suschetet26 ), while depressed vitamin A level down-regulated the serum IgM concentration in broilers( Reference Niu, Wei and Liu27 ). The above observations indicate that there might be a possible relationship between tannins and the innate and adaptive immune function in animals, which deserves research.
Besides the innate and adaptive immune components, cytokines also play vital roles in the intestinal immune function of fish( Reference Zhang, Zeng and Yang28 ). Cytokines contain pro-inflammatory and anti-inflammatory cytokines which could be modulated by NF-κB( Reference Gomes, Capela and Ribeiro29 ) and mammalian target of rapamycin (TOR)( Reference Weichhart, Costantino and Poglitsch30 ) in humans, respectively. Up to now, excepting inflammatory infiltration was observed in the ileum of sheep( Reference Hervás, Pérez and Giráldez31 ) induced by tannins, the influences on intestinal inflammatory cytokines and potential signalling molecules in animals have never reported. A study displayed that tannins lessened the butyric acid concentrations in the caecum of rat( Reference Levrat, Texier and Régerat32 ). It was confirmed that butyrate inhibited the mRNA levels of pro-inflammatory cytokines IL-1β and IL-6 by depressing NF-kB in human peripheral blood mononuclear cells( Reference Segain, Raingeard de la Blétière and Bourreille33 ). Moreover, Lee et al. showed that tannin supplementation decreased plasma Fe concentration in weanling pigs( Reference Lee, Shinde and Choi17 ). Our previous study demonstrated that dietary Fe deficiency can down-regulate TOR signalling to reduce anti-inflammatory cytokines transforming growth factor-β (TGF-β) and IL-10 mRNA levels in the spleen of grass carp( Reference Guo, Jiang and Wu34 ). Based on these observations, we hypothesised that tannins might influence intestinal immune function of fish associated with the inflammatory cytokines as well as potential NF-κB and TOR signalling.
Taken together, the present study for the first time systematically investigated the influences of tannins on the innate and adaptive immune components, inflammatory cytokines as well as the related signalling pathways (such as NF-κB and TOR signalling), which may partially reveal the impacts of tannins on intestinal immune function and the underlying regulatory mechanisms in animals. Meanwhile, we also determined the maximum allowable level of tannins for on-growing grass carp, which might provide a partial basis for the feed efficiently formulating on-growing grass carp.
Materials and methods
Experimental diets preparation
The formulation of basal diet is shown in Table 1. Fishmeal, casein and gelatin were used as main dietary protein sources. Fish oil and soyabean oil were used as main dietary lipid sources. CT (purity, 78 %; purchased from Guangzhou Senlai Trading Co. Ltd), one of the most widely distributed and abundant tannins in plants( Reference Chung, Wong and Wei14 ), were added to the basal diet to provide graded concentrations of 0 (as the control group), 10·00, 20·00, 30·00, 40·00 and 50·00 g/kg diet according to Buyukcapar et al. ( Reference Buyukcapar, Atalay and Kamalak18 ), and the amounts of maize starch were reduced to compensate as described by Rasid et al. ( Reference Rasid, Brown and Pratoomyot35 ). The prepared diets were air-dried using an electrical fan at room temperature and then stored at –20°C until being used.
Table 1. Composition and nutrient content of basal diet

CT, condensed tannins.
* Crude protein and crude lipid contents were measured values (mean values).
† n-3 and n-6 were according to Zeng et al. (Reference Zeng, Jiang and Liu103) and calculated according to the National Research Council (2011).
‡ Available P was according to Wen et al. (Reference Wen, Jiang and Feng50) and calculated according to the National Research Council (2011).
§ Per kg of vitamin premix (g/kg): retinyl acetate (500 000 IU/g), 0·39; cholecalciferol (500 000 IU/g), 0·20; dl-α-tocopheryl acetate (50 %), 23·23; menadione (22·9 %), 0·83; thiamine nitrate (98 %), 0·10; calcium-d-pantothenate (98 %), 3·85; pyridoxine hydrochloride (98 %), 0·45; cyanocobalamin (1 %), 0·94; niacin (99 %), 3·44; d-biotin (2 %), 0·75; meso-inositol (98 %), 28·23; folic acid (95 %), 0·17; riboflavin (80 %), 0·73; ascorhyl acetate (95 %), 9·77. All ingredients were diluted with maize starch to 1 kg.
‖ Mineral premix (g/kg): MnSO4·H2O (31·8 % Mn), 2·6590; MgSO4·H2O (15·0 % Mg), 200·0000; FeSO4·H2O (30·0 % Fe), 12·2500; ZnSO4·H2O (34·5 % Zn), 8·2460; CuSO4·5H2O (25·0 % Cu), 0·9560; KI (74·9 % iodine), 0·0668; Na2SeO3 (44·7 % Se), 0·0168. All ingredients were diluted with maize starch to 1 kg.
¶ Per kg of CT premix: the CT was diluted with maize starch to obtain graded levels of CT according to Rasid et al. (Reference Rasid, Brown and Pratoomyot35).
Feeding trial and sample collection
The procedures used in the present study were approved by the University of Sichuan Agricultural Animal Care Advisory Committee. The on-growing grass carp was obtained from fishery (Sichuan, China). According to the normal feeding and swimming of fish and microscopic examination to observe no diseases and parasites, fish were acclimatised to the experimental environment for 2 weeks before experiment, as our laboratory study of Feng et al. ( Reference Feng, Li and Liu36 ). Subsequently, a total of 540 healthy fish (initial mean weight 232·67 (sd 0·60) g) were randomly assigned to eighteen experimental cages (1·4 length × 1·4 width × 1·4 height, m), resulting in three cages per treatment and thirty fish per cage, as described by Tian et al. (Reference Tian, Zhou and Jiang37 ). Each cage was equipped with a disc of 1 m diameter in the bottom to collect the uneaten feed. In the feeding trial, fish were fed with their respective diets four times per d for 70 d, mainly according to the great differences of feed intake (FI) among the different treatments and referring to the literatures of tannin on fish( Reference Buyukcapar, Atalay and Kamalak18 ,Reference Prusty, Sahu and Pal38) . According to Cai et al. (Reference Cai, Luo and Xue39 ), 30 min after feeding, uneaten feed was collected, dried using an electrical fan at room temperature and weighed to calculate the FI. During the experimental period, the dissolved O2 was higher than 6·0 mg/l. The temperature was measured to be 28·1 (sd 2·0)°C using the thermometer with a precision of 0·1°C. The pH value was estimated to be 7·4 (sd 0·3) using a commercial test kit by the colorimetric method and pH indicator paper with a precision of 0·1. The experiment was conducted under a natural photoperiod (approximately 14 h light–10 h dark).
Fish in each cage were weighed at the origination and termination of the feeding trial to determine the percentage weight gain (PWG), specific growth rate and feed efficiency (FE). After that, seven fish were randomly selected from each treatment and anaesthetised in a benzocaine (50 mg/l) bath as described by Geraylou et al. (Reference Geraylou, Souffreau and Rurangwa40 ). After weighing and measuring the fish body, they were killed and their intestines were quickly separated, measured and weighed for calculating the intestinal length index (ILI) and intestinal somatic index (ISI) (The precisions of electronic scale and ruler are 0·01 g and 0·1 cm, respectively.). According to the description of Stroband(Reference Stroband41 ) and Stroband et al. (Reference Stroband, van deer Meer and Timmermans42 ), the whole intestine was divided into the proximal intestine (PI), mid intestine (MI) and DI, of which the PI is started from the ending of intestinal bulb to the last second bend, the MI is from the ending of PI to the last bend and the DI is from the last bend to the anus. And then, the intestine samples were frozen in liquid N2 and stored at –80°C for later analysis as described by Ji et al. (Reference Ji, Zhang and Huang43 ). Intestinal histological samples from three fish in each group were douched with physiological saline and preserved in 4 % paraformaldehyde for histological examination according to Wang et al. (Reference Wang, Meng and Li44 ). At the same time, the intestinal content of six fish was collected from each group for measuring the counts of Aeromonas spp., Escherichia coli, Lactobacillus spp. and Bifidobacterium spp., according to Spanggaard et al. (Reference Spanggaard, Huber and Nielsen45 ). These intestinal micro-organisms were enumerated depending on their respective colony characteristics in the selective medium(Reference Allen, Austin and Colwell46–Reference Simpson, Fitzgerald and Stanton49) (Aeromonas spp., shiny, smooth and round on tryptone soya agar; E. coli, black centre and metallic sheen on eosin-methylene blue agar; Lactobacillus spp., white protrusion on Lactobacillus selective agar; Bifidobacterium spp., cream and soft texture on Bifidobacterium selective medium).
Digestibility trial and sample collection
The digestibility trial was conducted on the basis of our laboratory’s previous study(Reference Wen, Jiang and Feng50 ). During the digestibility trial, according to the growth performance after the 70 d’ feeding trial, only the 0, 20·00 and 50·00 g/kg CT diet treatments were used for the apparent digestibility coefficient (ADC) measurements of nutrients. According to Che et al. (Reference Che, Su and Tang51 ), the three group diets were all added to the inert marker chromic oxide (Cr2O3) with 5 g/kg dry diet, measured with an electronic scale with 0·01 g precision. A total of 270 healthy fish (initial mean weight 202·67 (sd 0·02) g) were randomly assigned to nine above-mentioned experimental cages (three cages per treatment and thirty fish per cage, one ADC per cage). According to Burel et al., the adaptation lasted for a week(Reference Burel, Boujard and Escaffre52 ) and then fish fed with their respective treatment diets four times per d under the same feeding trial conditions for 10 d as described by Peres et al. (Reference Peres, Lim and Klesius53 ). At the end of the experiment, the faeces of each cage (all of the thirty fish) were respectively collected by applying slight pressure between the ventral fin and anus, following the method of Austreng(Reference Austreng54 ). Finally, the faecal samples were stored at –20°C for further analyses according to Omnes et al. (Reference Omnes, Goasduff and Delliou55 ). The crude protein content was assayed using the Kjeldahl method (N-Kjeldahl × 6·25). The crude fat content was determined by using the Soxhlet exhaustive extraction technique. The Cr content was determined using atomic absorption spectrometry.
Immunisation test and sample collection
After the 70 d’ feeding trial, an immunisation test was conducted via infecting fish with a widespread pathogenic bacterium to investigate the influence of dietary CT on the intestinal immune function of on-growing grass carp(Reference Deng, Kang and Tao56 ). Aeromonas hydrophila, one of the most common pathogenic bacteria causing bacterial enteritis in freshwater fish(Reference Song, Jie and Bo57 ), was friendly provided by College of Veterinary Medicine, Sichuan Agricultural University. From each treatment group, twenty-four fish with a similar body weight were injected intraperitoneally with 1·0 ml A. hydrophila at a concentration of 2·5 × 108 colony-forming units/ml under ventral fin by an 1·0 ml syringe and 26-gauge needle. The injection angle was about 50°, with the injection depth being 1 cm approximately. At the same time, the other twenty-four fish from the control group were intraperitoneally injected with 1·0 ml physiological saline as a positive control (saline) (online Supplementary Figs. S1 and S2). The injected concentration of bacteria was a nonlethal dosage which was sufficient to activate the immune system and consequently enable the investigation of effluent on reactivity against a threatening disease according to our preliminary study (unpublished results). The immunisation test lasted for 14 d, according to our previous study(Reference Zhang, Feng and Jiang58 ). During the test, the experimental conditions and feeding management were same as the feeding trial. At the end of the immunisation test, a scoring system was conducted to evaluate the severity of intestine haemorrhage and red swelling morbidity with a semiquantitative method according to Song et al. (Reference Song, Jie and Bo57 ). According to the sample collection method of feeding trial, all fish were anaesthetised in a benzocaine (50 mg/l) bath, then their intestine was quickly removed, segmented (PI, MI and DI), frozen in liquid N2 and stored at –80°C.
Immunological analysis
The intestinal samples were homogenised in 10 volumes (w/v) of ice-cold physiological saline and centrifuged at 6000 g at 4°C for 20 min; then, the collected supernatant was stored for the analysis of related parameters as described by Chen et al. (Reference Chen, Feng and Kuang59 ). The LZ and ACP activities were assayed by using the commercial kit (Nanjing Jiancheng Bioengineering Institute) according to the method of Jiang et al. (Reference Jiang, Li and Gao60 ). The contents of C3, C4 and IgM were measured using the immunoturbidimetric kit (Zhejiang Elikan Biological Technology Co. Ltd), according to the method of Wan et al. (Reference Wan, Ge and Bo61 ).
Histopathological analysis
According to the changes of growth performance results, only the 0, 30, 50 g/kg diet CT groups were used to observe histopathological damage. The intestines were fixed in 4 % paraformaldehyde, dehydrated in ethanol/methanol and embedded in paraffin. Then tissue was sectioned to 4 μm. The sections were stained using standard haematoxylin–eosin and examined by a light microscope (Nikon Eclipse TS100, Nikon Corporation) according to the method of Wu et al. (Reference Wu, Jiang and Jiang62 ). Then a scoring system was used to evaluate the proportion of histology changes in different treatments according to Liu et al. (Reference Liu, Ran and Liu63 ). The morphological changes are based on the evaluation of ten images randomly selected from each fish in each group (three fish in each group). The symptoms were scored as follows: 0 = not observed; 1 = low frequency (1–3 out of 10 images); 2 = moderate frequency (4–6 out of 10 images) and 3 = high frequency (≥7 out of 10 images).
Real-time PCR analysis
The procedures of RNA analysis were conducted according to the method of a study from our laboratory(Reference Liu, Zhou and Jiang64 ). Total RNA samples were extracted from the PI, MI and DI using an RNAiso Plus Kit (Takara), and the RNA was dissolved in DEPC treated-water. The RNA quality and quantity were assessed by agarose gel (1 %) electrophoresis and spectrophotometric analysis (A260:280 nm ratio). The total RNA was used to synthesise cDNA by a PrimeScript™ RT Reagent Kit (TaKaRa), according to the manufacturer’s instructions. The specific primers for the genes were designed according to the grass carp sequences (online Supplementary Table S1). And then, analysing melt curve was used to confirm the specificity and purity of all PCR products after amplification. According to the results of our preliminary experiment about the evaluation of internal control genes (data not shown), β-actin was used as a reference gene to normalise cDNA loading. Based on the specific gene, standard curves were generated from 10-fold serial dilutions to calculate the target and housekeeping gene amplification efficiency. The 2–ΔΔCT method was used to calculate the expression results after verifying that the primers amplified with an efficiency of approximately 100 %, as described by Aditya et al. (Reference Aditya, Shim and Yang65 ).
Western blot analysis
The method of Western blotting was based on our previous study(Reference Huang, Feng and Jiang66 ). Briefly, protein homogenates of PI, MI and DI were prepared and determined by a BCA assay kit (Beyotime Biotechnology Inc.). The protein samples were separated by SDS-PAGE and then transferred to a polyvinylidene difluoride membrane for the Western analysis. The membrane was blocked for 1·5 h with 0·5 % BSA at room temperature and then incubated with a primary antibody overnight at 4°C. The total TOR (T-TOR, AF6308, 1:1000 dilution), phosphorylation of TOR on residue Ser2448 (p-TOR Ser 2448) (AF3308, 1:1000 dilution), NF-κBp65 (DF7003, 1:1000 dilution), β-actin (AF7018, 1:3000 dilution) and Lamin B1 (AF5161, 1:1000 dilution) antibodies were same with our previous studies(Reference Liu, Zhou and Jiang64 ,Reference Zheng, Feng and Jiang67 ). The β-actin and Lamin B1 were used as control proteins for total and nuclear protein, respectively. After being washed, the polyvinylidene difluoride membrane was incubated for 1·5 h with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) in TRIS-buffered saline with Tween. The immune complexes were visualised using electrochemiluminescence reagents (Affinity Biosciences Inc.). The Western bands were quantified using NIH Image 1.63 software (National Institutes of Mental Health). The density of the protein was quantified and then normalised to the control CT group as relative density referring to Jiang et al. (Reference Jiang, Wen and Liu68 ). This experiment was repeated at least three times, and similar results were obtained each time.
Calculations and statistical analysis
The PWG, specific growth rate and FE were calculated by initial body weight (IBW), final body weight and FI, respectively. The values of growth performances are means of three replicate groups, with thirty fish in each group. The intestinal length and intestinal weight were used to calculate ILI and ISI, respectively. All above were according to Wu et al. (Reference Wu, Feng and Kuang69 ). The ADC of nutrient (one ADC per cage) were calculated, based on the ratio of the marker in the diets and faeces, according to the formula (NRC, 2011).
PWG = 100 × (final body weight (g/fish) – IBW (g/fish))/IBW (g/fish);
Specific growth rate = 100 × ln (final body weight (g/fish)/IBW (g/fish))/d;
FI = total feed consumption(dry) (g/fish) – total uneaten feed(dry) (g/fish);
FE = (final body weight (g/fish) – IBW (g/fish))/FI (g/fish);
ILI = 100 × (intestinal length (cm)/total body length (cm));
ISI = 100 × (intestinal weight (g)/wet body weight (g));
ADCDM = 100 × (1 − (Crdiet/Crfaeces));
ADCnutrient = 100 × (1 − (Crdiet/Crfaeces) × (Nutrientfaeces/Nutrientdiet)).
where Crdiet/faeces is the Cr content in diet or faeces, respectively; nutrientdiet/faeces is the crude protein and crude fat in diet or faeces.
The results are represented with mean values and standard deviation. Data were subjected to one-way ANOVA followed by Duncan’s multiple range tests to determine significant differences among six treatment groups at P < 0·05 with SPSS 18.0 (SPSS Inc.). Pearson correlation coefficient analysis was conducted using the Bivariate Correlation program in SPSS 18.0. On the basis of the means and standard deviations of growth and intestinal immune-related parameters, the minimum effect size was calculated to be 0·68 according to the method of Searcy-Bernal et al. (Reference Searcy-Bernal70 ). With the effect size of 0·68, a significance level of 0·05 and the six replicates in each treatment, the statistical power was calculated to be 0·84 using the R pwr package according to Grey et al. (Reference Grey, Chiasson and Williams71 ). Broken-line analysis was used to evaluate the maximum allowable level of CT in the diets of on-growing grass carp, according to Wang et al. ( Reference Wang, Feng and Jiang9 ).
Results
Growth performance and intestinal function related indices of on-growing grass carp
Effects of CT on growth performance, intestinal growth and intestinal bacterial counts of on-growing grass carp and feed ADC are presented in Table 2 and Fig. 1. Compared with the control group, the growth performance (FI, FE and PWG) of on-growing grass carp had no significant differences with increasing CT to 10 and 20 g/kg, but were significantly decreased when CT levels were up to 30 g/kg and above (P < 0·05). Compared with the control group, the intestinal growth parameters (intestinal length, ILI, intestinal weight, ISI and protein content) of on-growing grass carp had no significant differences with increasing CT to 10 and 20 g/kg, but were strikingly decreased when CT levels were up to 30 g/kg and above (P < 0·05). Meanwhile, in comparison with control treatment, the ADC of DM and crude lipid were significantly decreased when adding 20 g/kg CT and 50 g/kg (P < 0·05). The ADC of crude protein had no significant difference when adding 20 g/kg CT but significantly decreased with CT levels up to 50 g/kg (P < 0·05). Additionally, compared with the control diet, adding 10 g/kg CT to the diet has significantly decreased the Aeromonas spp. and E. coli amounts (P < 0·05). The Lactobacillus spp. and Bifidobacterium spp. amounts had no significant differences when the CT levels were up to 10 g/kg but strikingly lowered when the CT levels were up to 20 g/kg and above (P < 0·05).
Table 2. Growth performance and intestinal growth and bacterial counts of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of condensed tannins (CT) for 70 d
(Mean values and standard deviations)

IBW, initial body weight (g/fish); FBW, final body weight (g/fish); FI, feed intake (g/fish); FE, feed efficiency; PWG, percentage weight gain (%); SGR, specific growth rate (%/d); IL, intestine length (cm); ILI, intestine length index; IW, intestine weight (g/fish); ISI, intestinal somatic index; PI, proximal intestine; MI, mid intestine; DI, distal intestine.
a,b,c,d,eMean values within a row with unlike superscript letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests).
* Mean values and standard deviations for three replicate groups, with thirty fish in each group.
† Mean values and standard deviations (n 7).
‡ Mean values and standard deviations (n 6).
§ Protein content (%) = protein content (g)/intestine weight (g) × 100.

Fig. 1. Apparent digestibility coefficients of DM, crude protein and crude lipid of experimental diets. Values are means and standard deviations for three replicate groups, with thirty fish in each group. a,b Mean values with unlike letters in the same row are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests). ![]() , 0 g/kg diet condensed tannins (CT);
, 0 g/kg diet condensed tannins (CT); ![]() , 20 g/kg CT;
, 20 g/kg CT; ![]() , 50 g/kg CT.
, 50 g/kg CT.
The histopathological results are shown in Fig. 2. Compared with the control group, goblet cell hyperplasia and blood capillary hyperaemia were observed in the PI, MI and DI of on-growing grass carp with the CT levels up to 30 g/kg. Increasing the dietary levels of CT to 50 g/kg resulted in goblet cell heavily hyperplasia, thickening of lamina propria and leucocytes infiltration in the lamina propria in three intestinal segments of fish. The summary scoring results of morphological changes are presented in Table 3. Compared with the control group, the morphological change was not significant with CT level up to 30 g/kg but significantly aggravated with CT level up to 50 g/kg (P < 0·05) in the PI of on-growing grass carp. In the MI and DI, the intestinal morphological changes were significantly aggravated with the CT levels up to 30 and 50 g/kg CT (P < 0·05) in fish. The survival rate of all of the groups after infection with A. hydrophila was 100 % (unpublished results). Based on the noticeable hypertrophy and hyperaemia of intestine (shown in Fig. 3), the enteritis severity of twenty-four fish from each treatment was evaluated as described by Huang et al. (Reference Huang, Feng and Jiang66 ) and Song et al. (Reference Song, Jie and Bo57 ). As shown in Fig. 4, the enteritis morbidity of on-growing grass carp had no significant differences with increasing CT to 10 and 20 g/kg, but were significantly enhanced with CT levels up to 30 g/kg and above (P < 0·05).

Fig. 2. Histology of the proximal intestine (PI), mid intestine (MI) and distal intestine (DI) in on-growing grass carp (Ctenopharyngodon idella) fed diets containing different levels of condensed tannins (CT) (g/kg diet) for 70 d. (A) Control (0), (B) 30 g/kg diet and (C) 50 g/kg diet group in PI; (D) control (0), (E) 30 g/kg diet and (F) 50 g/kg diet in MI; (G) control (0), (H) 30 g/kg diet and (I) 50 μg/kg diet in DI. The sections were stained with haematoxylin–eosin and observed at 200 × original magnification. In each panel, BH, blood capillary hyperaemia; GH, goblet cell hyperplasia; TL, thickening of lamina propria; LI, leucocyte infiltration.
Table 3. Intestinal morphological changes in on-growing grass carp (Ctenopharyngodon idella) fed diets containing different levels of condensed tannins (CT) for 70 d*
(Mean values)
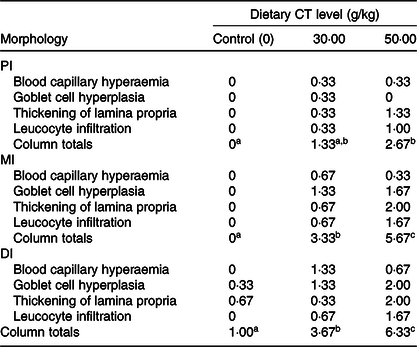
PI, proximal intestine; MI, mid intestine; DI, distal intestine.
a,b,c Mean values with unlike superscript letters among groups are significantly different (P < 0·05).
* The morphological changes are based on the microscopy evaluation of ten micrographs from each fish in each treatment group. Values are means, n 3 fish in each treatment. Tissue changes were assessed as follows: 0 = not observed; 1 = low frequency (1–3 out of 10 images); 2 = moderate frequency (4–6 out of 10 images) and 3 = high frequency (≥7 out of 10 images).

Fig. 3. Enteritis symptoms of on-growing grass carp (Ctenopharyngodon idella) fed diets containing different levels of condensed tannins (CT) after challenge with Aeromonas hydrophila for 14 d.

Fig. 4. Effects of graded levels of condensed tannins (CT) on enteritis morbidity of on-growing grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Values are means (n 24 individuals in each group), with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests).
Immune parameters in the intestine of on-growing grass carp
As shown in Table 4, the activities of LZ and ACP as well as C3, C4 and IgM contents in the intestine of on-growing grass carp were significantly down-regulated when the CT was added to appropriate levels, and then sustainably decreased or gradually plateaued. In the PI, compared with the control group, the activities of LZ and ACP as well as C3 and IgM contents were all obviously lower with increasing CT levels to 30 g/kg diet (P < 0·05), while the C4 contents were obviously decreased as the CT levels increased to 20 g/kg diet (P < 0·05). In the MI, compared with the control group, the LZ and ACP activities as well as C4 and IgM contents were significantly decreased as the CT levels increased to 20 g/kg diet (P < 0·05), while the C3 contents were obviously decreased as the CT levels increased to 30 g/kg diet (P < 0·05). In comparison with the control group, with CT levels up to 20, 20, 30, 20 and 30 g/kg diet (P < 0·05), the LZ and ACP activities as well as C3, C4 and IgM contents in the DI were remarkably decreased, respectively.
Table 4. Effect of condensed tannin (CT) levels on immune parameters in the proximal intestine (PI), mid intestine (MI) and distal intestine (DI) of on-growing grass carp (Ctenopharyngodon idella) with fed diets containing graded levels of CT for 70 d after injection infected with Aeromonas hydrophila for 14 d (n 6) (Mean values and standard deviations)
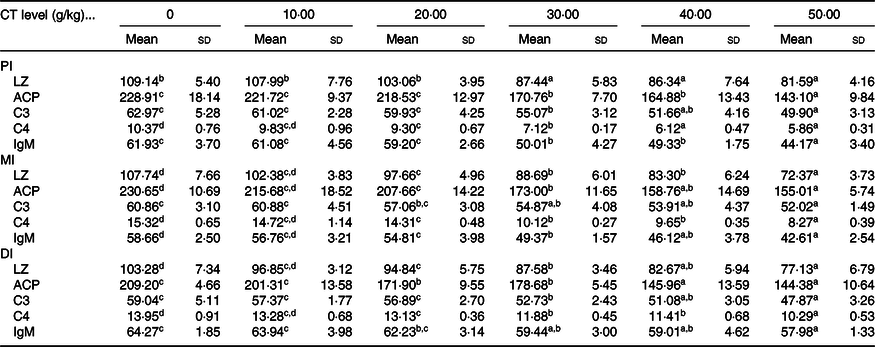
LZ, lysozyme activity (U/mg protein); ACP, acid phosphatase (U/mg protein); C3, complement 3 (mg/g protein); C4, complement 4 (mg/g protein).
a,b,c,d Mean values within a row with unlike superscript letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests).
Gene expression in the intestine of on-growing grass carp
Relative mRNA levels of antimicrobial peptides and anti-inflammatory cytokines in the intestine of on-growing grass carp
As shown in Fig. 5, the relative mRNA levels of Hepcidin, liver-expressed antimicrobial peptide (LEAP)-2A, -2B, β-defensin-1, Mucin-2, TGF-β1, IL-4/13A, IL-10 and IL-11 in the intestine of on-growing grass carp were significantly down-regulated when the CT was added to appropriate levels, and then sustainably decreased or gradually plateaued. In the PI, compared with the control group, the relative mRNA levels of Hepcidin, LEAP-2A, -2B, β-defensin-1, Mucin-2, TGF-β1, TGF-β2, IL-4/13A, IL-10 and IL-11 were significantly down-regulated when the CT levels were up to 40, 30, 30, 20, 30, 30, 30, 40, 40 and 30 g/kg diet (P < 0·05), respectively. Compared with the control group, the mRNA levels of Hepcidin, LEAP-2A, -2B, β-defensin-1, Mucin-2, TGF-β1, IL-4/13A, IL-10 as well as IL-11 in the MI of on-growing grass crap were significantly down-regulated when the CT levels reached 30, 30, 30, 30, 30, 40, 30, 30 and 30 g/kg diet (P < 0·05), respectively. In the DI, compared with the control group, the mRNA levels of Hepcidin, LEAP-2A, -2B, β-defensin-1, Mucin-2, TGF-β1, IL-4/13A, IL-10 as well as IL-11obviously descended when the CT increased to 20, 10, 30, 20, 30, 30, 50, 40 and 20 g/kg diet (P < 0·05), respectively. Interestingly, CT had no effect on the mRNA expression of TGF-β2 in the MI and DI and IL-4/13B in the three intestinal segments of on-growing grass crap (P > 0·05).

Fig. 5. Effects of condensed tannins (CT) on relative mRNA levels of antibacterial and anti-inflammatory cytokines in the proximal intestine (PI) (A), mid intestine (MI) (B) and distal intestine (DI) (C) of on-growing grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Values are means of six fish in each group, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests). LEAP, liver-expressed antimicrobial peptide; TGF, transforming growth factor. ![]() , 0 g/kg diet CT;
, 0 g/kg diet CT; ![]() , 10 g/kg CT;
, 10 g/kg CT; ![]() , 20 g/kg CT;
, 20 g/kg CT; ![]() , 30 g/kg CT;
, 30 g/kg CT; ![]() , 40 g/kg CT;
, 40 g/kg CT; ![]() , 50 g/kg CT.
, 50 g/kg CT.
Relative mRNA levels of pro-inflammatory cytokines in the intestine of on-growing grass carp
As shown in Fig. 6, the relative mRNA levels of IFN-γ2, IL-1β, -6, -12p35, -12p40, -15 and -17D in the intestine of on-growing grass carp were significantly up-regulated when the CT was added to appropriate levels, and then sustainably increased or gradually plateaued. In the PI of on-growing grass crap, compared with the control group, the relative mRNA levels of IFN-γ2, IL-1β, -6, -12p35, -12p40, -15 and -17D were significantly up-regulated with the CT levels up to 30, 30, 40, 40, 30, 30 and 30 g/kg diet (P < 0·05), respectively. In the MI, compared with the control group, the relative mRNA levels of IFN-γ2, IL-1β, -6, -8, -12p35, -12p40, -15 and -17D significantly increased when the CT levels reached 40, 30, 30, 30, 30, 30, 40 and 20 g/kg diet (P < 0·05), respectively. Similarly, when the CT levels were up to 30, 30, 20, 30, 30, 30, 30 and 30 g/kg diet (P < 0·05) in the DI, the relative mRNA levels of IFN-γ2, IL-1β, -6, -8, -12p35, -12p40, -15 and -17D were significantly increased, respectively. Specifically, the CT level had no significant impact on the relative mRNA level of IL-8 in the PI and TNF-α in the three intestinal segments of on-growing grass carp (P > 0·05).

Fig. 6. Effects of condensed tannins (CT) on relative mRNA levels of pro-inflammatory cytokines in the proximal intestine (PI) (A), mid intestine (MI) (B) and distal intestine (DI) (C) of on-growing grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Values are means of six fish in each group, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests). IFN-γ2, interferon-γ2. ![]() , 0 g/kg diet CT;
, 0 g/kg diet CT; ![]() , 10 g/kg CT;
, 10 g/kg CT; ![]() , 20 g/kg CT;
, 20 g/kg CT; ![]() , 30 g/kg CT;
, 30 g/kg CT; ![]() , 40 g/kg CT;
, 40 g/kg CT; ![]() , 50 g/kg CT.
, 50 g/kg CT.
Relative mRNA levels of inflammation-related signalling molecules in the intestine of on-growing grass carp
As shown in Fig. 7, the relative mRNA levels of TOR, S6K1 and IκBα in the intestine of on-growing grass carp were significantly down-regulated as the CT added to appropriate levels, and then sustainably decreased or gradually plateaued, while the relative mRNA levels of 4E-BP2, NF-κBp65, c-Rel, IKKα, β and γ were significantly advanced as the CT added to appropriate levels, and then sustainably increased or gradually plateaued. In the PI, compared with the control group, the relative mRNA levels of TOR, S6K1 and IκBα were all significantly decreased with the CT levels reached to 20 g/kg diet (P < 0·05), while the relative mRNA levels of 4 E-BP2, NF-κBp65, c-Rel, IKKα, β and γ were significantly advanced with the CT levels reached to 20, 20, 30, 20, 30 and 20 g/kg diet (P < 0·05), respectively. In the MI, compared with the control group, the relative mRNA levels of TOR, S6K1 and IκBα were all significantly decreased with the CT levels reached to 30 g/kg diet (P < 0·05), while the relative mRNA levels of 4E-BP2, NF-κBp65, c-Rel, IKKα, β and γ were significantly advanced with the CT levels reached to 30, 30, 20, 30, 30 and 20 g/kg diet (P < 0·05), respectively. In the DI, compared with the control group, the relative mRNA levels of TOR, S6K1 and IκBα were significantly decreased with the CT levels reached to 40, 30 and 30 g/kg diet (P < 0·05), while the relative mRNA levels of 4E-BP2, NF-κBp65, c-Rel, IKKα, β and γ were significantly advanced with the CT levels reached to 30, 20, 40, 30, 30 and 30 g/kg diet (P < 0·05), respectively. Interestingly, CT had no effect on the mRNA expression of 4 E-BP1 and NF-κBp52 in the three intestinal segments of on-growing grass crap (P > 0·05).

Fig. 7. Effects of condensed tannins (CT) on relative mRNA levels of inflammatory related signalling molecules in the proximal intestine (PI) (A), mid intestine (MI) (B) and distal intestine (DI) (C) of on-growing grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Values are means of six fish in each group, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests). TOR, target of rapamycin. ![]() , 0 g/kg diet CT;
, 0 g/kg diet CT; ![]() , 10 g/kg CT;
, 10 g/kg CT; ![]() , 20 g/kg CT;
, 20 g/kg CT; ![]() , 30 g/kg CT;
, 30 g/kg CT; ![]() , 40 g/kg CT;
, 40 g/kg CT; ![]() , 50 g/kg CT.
, 50 g/kg CT.
Protein levels of NF-κB p65, phosphorylated target of rapamycin Ser2448 and total target of rapamycin in the intestine of on-growing grass carp
As shown in Figs. 8 and 9, in comparison with the control group, the protein levels of T-TOR and p-TOR Ser2448 protein levels in the PI, MI and DI were both significantly decreased when CT levels were up to 30, 20 and 30 g/kg (P < 0·05), respectively. Compared with the control group, the protein levels of NF-κBp65 in the PI, MI and DI were significantly increased when CT levels were up to 20, 20 and 10 g/kg (P < 0·05), respectively.

Fig. 8. Western blot analysis of target of rapamycin (TOR) phosphorylation levels at Ser2448 and total TOR (T-TOR) protein levels in the proximal intestine (PI) (A), mid intestine (MI) (B) and distal intestine (DI) (C) of on-growing grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila (β-actin is added as a loading control). Values are means of three fish in each group, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests). ![]() , 0 g/kg diet condensed tannins (CT);
, 0 g/kg diet condensed tannins (CT); ![]() , 10 g/kg CT;
, 10 g/kg CT; ![]() , 20 g/kg CT;
, 20 g/kg CT; ![]() , 30 g/kg CT;
, 30 g/kg CT; ![]() , 40 g/kg CT;
, 40 g/kg CT; ![]() , 50 g/kg CT.
, 50 g/kg CT.

Fig. 9. Western blot analysis of NF-κBp65 protein levels in the proximal intestine (PI) (A), mid intestine (MI) (B) and distal intestine (DI) (C) of on-growing grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila (Lamin B1 is added as a loading control). Values are means of three fish in each group, with standard deviations represented by vertical bars. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple range tests). ![]() , 0 g/kg diet condensed tannins (CT);
, 0 g/kg diet condensed tannins (CT); ![]() , 10 g/kg CT;
, 10 g/kg CT; ![]() , 20 g/kg CT;
, 20 g/kg CT; ![]() , 30 g/kg CT;
, 30 g/kg CT; ![]() , 40 g/kg CT;
, 40 g/kg CT; ![]() , 50 g/kg CT.
, 50 g/kg CT.
Discussion
Condensed tannins reduced the growth performance and enteritis resistance ability of fish
The results in the present study illustrated that CT reduced the growth performance (FI, FE and PWG) and decreased the feedstuff utilisation (DM, crude protein and crude lipid) of on-growing grass carp. As we know, the fish growth is closely associated with intestinal function(Reference Gisbert, Andree and Quintela72 ). The present study observed that CT also decreased the intestinal growth (intestinal length, ILI, intestinal weight, ISI and protein content), lessened all of the studied intestinal bacteria amounts (Aeromonas spp., E. coli, Lactobacillus spp. and Bifidobacterium spp.), induced the fish intestinal histopathological lesions (goblet cell hyperplasia, thickening of lamina propria, blood capillary hyperaemia and leucocytes infiltration) and diminished enteritis resistance ability of on-growing grass carp (the enteritis morbidity of fish in 50 g/kg CT treatment was 18·6 %, which increases by 77·1 % when compared with that of control group (10·5 %) after infection with A. hydrophila). These observations indicated that CT weakens the intestinal function of on-growing grass carp. Interestingly, not only beneficial bacteria amounts but also harmful bacteria amounts have been reduced by CT. Among these, the decrease of Lactobacillus amount caused by CT is similar to the report in weaned piglets(Reference Biagi, Cipollini and Paulicks73 ). And, bacteria amount of E. coli depressed by CT might be partly associated with the cytoplasmic membrane integrity. Hoshino et al. (Reference Hoshino, Kimura and Yamaji74 ) have discovered that CT exposure killed E. coli cells by damaging the cytoplasmic membrane.
As shown in Fig. 10, according to the broken-line analysis of the PWG, the maximum allowable level of CT for on-growing grass carp was estimated to be 18·6 g/kg diet, which was a little higher than that based on the enteritis resistance ability (17·4 g/kg). This phenomenon was in agreement with the study of gossypol in on-growing grass carp( Reference Wang, Feng and Jiang9 ). The result indicated that infection with intestinal pathogenic bacteria could decrease the tolerance of on-growing grass carp on CT. In addition, research has confirmed that the enteritis resistance ability is closely associated with the intestinal immune function in fish(Reference Rauta, Nayak and Das75 ). Therefore, we next investigated the influence of CT on the intestinal function of on-growing grass carp.

Fig. 10. Broken-line analysis of percentage weight gain (PWG) and enteritis morbidity of on-growing grass carp (Ctenopharyngodon idella) containing graded levels of condensed tannins (CT).
Condensed tannins decreased innate and adaptive immunity in the intestine of fish
The immune function of fish relies on the immune response, which is closely related to innate and adaptive immune components such as LZ, ACP, complements, antibacterial peptides and Ig(Reference Uribe, Folch and Enriquez76 ). From our research, CT (≥30 g/kg) decreased the LZ and ACP activities, C3, C4 and IgM contents and down-regulated the Hepcidin, LEAP-2A, -2B, β-defensin-1 and Mucin2 mRNA levels in the three intestinal segments of on-growing grass carp. These results demonstrated that CT decreased intestinal immune function of fish.
In addition, another dietary ANF, gossypol, also determined above antibacterial peptides in the intestine of on-growing grass carp( Reference Wang, Feng and Jiang9 ), but interestingly, partial results were unalike the present study. Firstly, CT decreased the Hepcidin mRNA levels in the whole of three intestinal segments. However, gossypol caused down-regulation of the Hepcidin mRNA levels only in the PI and MI (not in DI) of fish. The reason for causing such results might be in part associated with the TGF-β1. It was reported that the inhibition of TGF-β1 could down-regulate the Hepcidin mRNA level in mice hepatic cells(Reference Wang, Li and Xu77 ). In the present study, CT down-regulated TGF-β1 mRNA levels in all the three intestinal segments, while gossypol down-regulated it only in the PI and MI (not in DI) of fish, which, to some extent, might explain the differences of Hepcidin mRNA level induced by CT and gossypol. Secondly, in the three intestinal segments of fish, CT down-regulated the mRNA levels of LEAP-2A, whereas this change was unobserved in the study of gossypol, which might be partially associated with IKKα. A study from mice found that IKKα could increase the mRNA level of IL-22 (Reference Giacomin, Moy and Mario78 ), which could up-regulate the LEAP-2A gene expression in splenocytes of rainbow trout (Oncorhynchus mykiss)(Reference Monte, Zou and Wang79 ). However, the comparison found that CT increased the IKKα gene expression but gossypol had no distinct effect on it in the three intestinal segments of fish. Therefore, we speculated that the different influences of CT and gossypol on the LEAP-2A mRNA level might be partially correlated with their different impacts in IKKα. Certainly, these diversities induced by CT and gossypol occurred in intestinal immune function of on-growing grass carp need more researches to explicate. In addition, the fish intestinal immune function is also correlated with its inflammatory responses, which are mainly mediated by cytokines(Reference Krogdahl, Gajardo and Kortner80 ) and related signalling molecules such as TOR and NF-κB(Reference Dong, Jiang and Liu81 ). Thus, we next examined the effects of CT on inflammatory cytokines and researched the potential regulation mechanism in the intestine of fish.
Condensed tannins aggravated inflammatory responses by regulating inflammatory cytokines partly related to the target of rapamycin and NF-κB signalling pathways in the intestine of fish
Condensed tannins aggravated intestinal inflammation by regulating inflammatory cytokines in fish
Research indicated that down-regulating anti-inflammatory cytokines (like IL-10) and up-regulating pro-inflammatory cytokines (like IL-1β) mRNA levels could aggravate inflammatory responses in fish(Reference Wang and Secombes82 ). The present results showed that compared with the control group, CT (≥30 g/kg) down-regulated most of the studied anti-inflammatory cytokines (such as TGF-β1, IL-4/13A, IL-10 and IL-11) and up-regulated the majority of studied pro-inflammatory cytokines (such as IFN-γ2, IL-1β, -6, -12p35, -12p40, -15 and -17D) mRNA levels in the three intestinal segments of on-growing grass carp. These results illustrated that CT could aggravate the intestinal inflammatory responses of fish.
Surprisingly, we discovered four interesting phenomena on the changes of intestinal inflammatory cytokines mRNA levels in fish caused by CT. These might be analysed as follows. Firstly, CT had no effect on the TNF-α mRNA levels in the three intestinal segments of on-growing grass carp, which might be partially relevant to the growth hormone. Research has found that growth hormone deficiency caused plasma TNF-α level to increase in children(Reference Andiran and Yordam83 ). In ewes, CT had no impact upon plasma concentrations of growth hormone(Reference Wang, Douglas and Waghorn84 ). Hence, we speculated that CT had no influence on the level of growth hormone, leading to unaffected TNF-α mRNA levels in the three intestinal segments of fish, but the concrete mechanism needs further study. Secondly, CT down-regulated IL-4/13A mRNA levels (rather than IL-4/13B) in the three intestinal segments of on-growing grass carp, which might be partially associated with TOR and GATA-3. A previous study reported that the absence of mammalian TOR decreased the protein expression of GATA-3 in mice Th2 cells(Reference Cook and Miller85 ). Ohtani et al. indicated that GATA-3 could directly enhance the gene transcription of IL-4/13A rather than IL-4/13B in most teleost(Reference Ohtani, Hayashi and Hashimoto86 ). Our data found that CT down-regulated the p-TOR Ser2448 protein levels in three intestinal segments of fish, supporting our hypothesis. Thirdly, CT up-regulated IL-8 mRNA levels in the MI and DI (rather than PI) of on-growing grass carp, which might be partially associated with cholesterol and glucocorticoid. It was reported that CT decreased the cholesterol level in an in vitro experiment(Reference Zhu, Zou and Zhenzhen87 ). Cholesterol serves as a precursor of glucocorticoid(Reference Sheen, Liu and Chen88 ), and lessened glucocorticoid could raise the IL-8 gene expression in human fibroblasts(Reference Tobler, Meier and Seitz89 ). Moreover, the mRNA abundance of glucocorticoid receptor in the MI and DI was higher than that in the PI of tilapia (Oreochromis mossambicus)(Reference Takahashi, Sakamoto and Hyodo90 ). Therefore, we supposed that CT up-regulated IL-8 mRNA levels in the MI and DI (rather than PI) which is partially associated with the higher mRNA levels of glucocorticoid receptor in the MI and DI than PI of fish. However, this supposition needs deeper investigation. Fourthly, CT down-regulated TGF-β2 mRNA level in the PI (rather than MI and DI) of fish, which might be partially associated with butyric acid, cholecystokinin (CCK) and protein kinase C. It was reported that CT lessened the butyric acid concentrations in the caecum of rat( Reference Levrat, Texier and Régerat32 ). In neonatal piglets, supplementation with Na-butyrate increased the CCK concentrations in plasma(Reference Kotunia, Woliński and Laubitz91 ). A literature discovered that CCK could activate protein kinase C in pancreatic acinar cells(Reference Tapia, Bragado and García-Marín92 ), whereas inhibited protein kinase C down-regulated proteins of TGFβ-2 in the guinea pig myopic eye(Reference Mao, Liu and Qin93 ). However, Yuan et al. found that the mRNA expression of CCK in the PI was much higher than that in the MI and DI of Schizothorax prenanti (Reference Yuan, Wang and Zhou94 ). Consequently, we hypothesised that CT down-regulated butyric acid level resulting in decreasing the expression of CCK only in the PI (rather than MI and DI) of fish, which depressed the activity of protein kinase C and then leaded to down-regulate the TGF-β2 mRNA level only in the PI (rather than MI and DI) of fish. However, further investigation should be conducted to verify this assumption.
The identical inflammatory cytokines were also investigated in the study of gossypol( Reference Wang, Feng and Jiang9 ); however, these detrimental effects showed that CT and gossypol differed in ways whereby regulating cytokines to aggravate intestinal inflammatory responses in on-growing grass carp. Examples of CT down-regulated anti-inflammatory cytokines TGF-β1 and IL-10 mRNA levels and enhanced pro-inflammatory cytokine IL-6 mRNA levels in all the three intestinal segments, whereas gossypol decreased TGF-β1 only in the PI and MI and IL-10 only in the PI and increased IL-6 just in the PI of fish. These observations indicated that CT-induced inflammatory responses might be relative equilibrium in the three intestinal segments, whereas gossypol impaired the PI more severely than MI and DI of fish. However, the reasons for these different results are largely unknown, but the difference of IL-6 could be speculated, which might be partly associated with c-Rel. A study found that c-Rel could increase the IL-6 gene expression in the primary B cells(Reference Tumang, Hsia and Tian95 ). In the present study, in the whole of three intestinal segments of fish, the mRNA level of c-Rel was up-regulated by CT, while that was up-regulated by gossypol just in the PI (rather than MI and DI). Hence, this indicated that CT increased IL-6 mRNA levels in the three intestinal segments, while gossypol up-regulated it only in PI (rather than MI and DI) of fish, which might be related to their diverse influences on c-Rel. For other differences on inflammatory cytokines between CT and gossypol in the intestine of on-growing grass carp, further studies are needed.
Condensed tannin-induced intestinal inflammation partially associated with target of rapamycin and NF-κB signalling in fish
The study proved that mammalian TOR signalling partly regulated anti-inflammatory cytokines by activating S6K1 and inhibiting 4E-BP associated with eIF4E in humans(Reference Zhao, Benakanakere and Hosur96 ). The phosphorylation of TOR on residue Ser2448 can be used to monitor the activation of TOR signalling in rainbow trout(Reference Seiliez, Gabillard and Skiba-Cassy97 ). In our study, CT (≥30 g/kg) down-regulated TOR and S6K1 mRNA levels, up-regulated the mRNA levels of 4E-BP2 and depressed p-TOR Ser2448 protein levels in the three intestinal segments of on-growing grass carp. Furthermore, correlation analysis indicated that anti-inflammatory cytokines (except IL-4/13B and TGF-β2 in MI and DI) were positively correlated with the p-TOR Ser2448 protein levels (online Supplementary Table S2). Hence, these results implied that CT down-regulated most of the studied anti-inflammatory cytokines gene expressions partly associated with (TOR/(S6K1, 4 E-BP2)) signalling pathways in the intestine of fish. Interestingly, CT up-regulated 4E-BP2 mRNA levels rather than 4E-BP1 in the three intestinal segments of fish, which might be partly related to the low protein level. In the present study, our data found that CT reduced the protein contents in the three intestinal segments of fish. A previous study from our laboratory observed that a low level of protein led to the increase of 4E-BP2 mRNA levels, but had no influence on the 4E-BP1 mRNA levels in the three intestinal segments of on-growing grass carp(Reference Xu, Wu and Jiang98 ). Hence, we supposed that CT enhanced 4E-BP2 mRNA levels rather than 4E-BP1 in the three intestinal segments of fish partly due to the decrease of protein level in the intestine. However, further investigation should be conducted to verify this assumption.
Additionally, one study reported that the IKK complex (including IKKα, β and γ) promoted the phosphorylation and proteasome degradation of IκBα and then induced the activation of NF-κB (including p65, p52 and c-Rel), which regulated the expression of pro-inflammatory cytokines in mammalian cells(Reference Vallabhapurapu and Karin99 ). The NF-κB protein levels could be considered hallmarks for the activation of the NF-κB signalling pathway in humans(Reference Yi, Hu and Zhang100 ). In the present study, our results showed that CT (≥30 g/kg) induced the up-regulation of NF-κBp65, c-Rel, IKKα, β, γ and the NF-κBp65 protein levels and the down-regulation of IκBα mRNA levels in the three intestinal segments of on-growing grass carp. Furthermore, the correlation analysis showed that pro-inflammatory cytokines (except TNF-α and IL-8 in PI) mRNA levels were positively correlated with the NF-κBp65 protein levels and c-Rel mRNA levels (online Supplementary Table S2). The above data indicated that CT up-regulated the mRNA levels of most of the studied pro-inflammatory cytokines which might be partly associated with ((IKKα, β, γ)/IκBα/(NF-κBp65, c-Rel)) signalling pathways in the intestine of fish. However, it is interesting that CT have up-regulated the mRNA levels of NF-κBp65, but unaffected NF-κBp52 mRNA levels in the three intestinal segments of on-growing grass carp, which might be relevant to the different activated NF-κB signalling pathways. In mammals, NF-kBp65 and NF-kBp52 could be activated by classical NF-kB signalling pathways and noncanonical NF-kB signalling pathways, respectively(Reference Bonizzi and Karin101 ). Classical NF-kB activity could suppress noncanonical NF-κB signalling in peripheral blood mononuclear cells(Reference Gray, Remouchamps and McCorkell102 ). In the present study, we found that CT increased NF-κB p65 rather than NF-κB p52 mRNA levels. Hence, we speculated that CT might regulate inflammatory responses in the intestine of fish through classical NF-κB signalling pathways rather than alternative NF-κB signalling pathways. Nevertheless, the supposition still remains to be elucidated.
Interestingly, about TOR and NF-κB signalling pathways, discrepancies still presented between CT and gossypol in the intestine of on-growing grass carp. CT might regulate inflammatory cytokines which are partially associated with (IKKα, β, γ/IκBα/NF-κBp65, c-Rel) and (TOR/S6K1,4E-BP2 (not 4E-BP1)) signalling pathways, but gossypol activated (IKKβ, γ (not IKKα)/IκBα/NF-κBp65, c-Rel (not in MI and DI)) and (TOR/S6K1, 4E-BP1(not 4E-BP2)) signalling pathways in the intestine of fish( Reference Wang, Feng and Jiang9 ). The reasons of these discrepant results have been still unclear. Besides, whether the different signalling pathways activated by CT and gossypol were the accounts for the discrepancies on inflammatory cytokines in the intestine of on-growing grass carp needs to be found.
Conclusion
In summary (Fig. 11), we found that dietary CT decreased the growth performance, feed utilisation, intestinal growth and bacterial counts of on-growing grass carp. Meanwhile, we for the first time found that dietary CT impaired intestinal immune function of fish as displayed in the following aspects. Compared with the control group, dietary CT (1) induced intestinal histopathological lesions and aggravated enteritis of on-growing grass carp; (2) reduced the innate and adaptive immune immunity partly related to decrease the immune components including LZ, ACP, C3, C4, IgM and antimicrobial peptides; (3) aggravated intestinal inflammatory responses partially by down-regulating most of the studied anti-inflammatory cytokines (except IL-4/13B and TGF-β2 in the MI and DI) mRNA levels and up-regulating the majority of studied pro-inflammatory cytokines (except TNF-α and IL-8 in the PI) in the intestine of fish, which were partially associated with (TOR/(S6K1 and 4E-BP2)) and (IKKα, β, γ/IκBα/NF-κB (p65 and c-Rel)) signalling pathways, respectively, but not TOR/4E-BP1 and NF-κBp52 signalling pathways; (4) induced inflammatory responses might be relative equilibrium in the three intestinal segments, whereas gossypol, our previous study by Wang et al. (Reference Wang, Feng and Jiang9), impaired the PI more severely than MI and DI of fish. In addition, based on the PWG, enteritis morbidity, the maximum allowable levels of CT for on-growing grass carp (232·22–890·11 g), were estimated to be 18·6 and 17·4 g/kg diet, respectively. Furthermore, our experiment has discussed the on-growing grass carp, so what the effect of CT on adult grass carp will be much interesting. Moreover, on the basis of our result that infection with pathogenic bacteria decreased the tolerance of on-growing grass carp on CT, it is betokening that the maximum allowable level of fish like carps even other animals on CT might be relatively lower in the wild or commercial farms, which needs more exploration and research.

Fig. 11. Potential action pathways of condensed tannins (CT)-disrupted intestinal immune function in fish. TOR, target of rapamycin; LZ, lysozyme; ACP, acid phosphatase; IFN-γ2, interferon-γ2; PI, proximal intestine; TGF, transforming growth factor; MI, mid intestine; DI, distal intestine.
Acknowledgements
The authors would like to thank the personnel of these teams for their kind assistance.
This research was financially supported by National Key R&D Program of China (2018YFD0900400), the Earmarked Fund for China Agriculture Research System (CARS-45), Outstanding Talents and Innovative Team of Agricultural Scientific Research (Ministry of Agriculture), Foundation of Sichuan Youth Science and Technology Innovation Research Team (2017TD0002) and Supported by Sichuan Science and Technology Program (2019YFN0036).
The authors’ contributions are as follows: X.-Q. Z. and L. F. designed the study; M. L. and L. F. conducted the study; M. L. and P. W. analysed the data; Y. L., J. J., S.-Y. K. and L. T. participated in the interpretation of the results; M. L. and W.-D. J. wrote the manuscript; X.-Q. Z. had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519003295


















