Published online by Cambridge University Press: 19 April 2023
Bystrite is redefined as a four-layer cancrinite-group mineral with the four-layer Losod-type framework and the end-member formula Na7Ca(Al6Si6O24)S52–Cl–. The mineral is known only at the Malo–Bystrinskoe gem lazurite deposit, Baikal Lake area, Siberia, Russia. The associated minerals are calcite, lazurite, sodalite, fluorapatite, phlogopite, diopside, dolomite and plagioclase. Bystrite is brittle, with the Mohs hardness of 5 and distinct cleavage on {10$\bar{1}$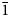 0}. The yellow colour of bystrite is due to the presence of S52– anions occurring in Losod (LOS) cages of the aluminosilicate framework with the ABAC stacking sequence. Measured and calculated density is, respectively, 2.43(1) and 2.412 g cm–3 for the holotype and 2.42(1) and 2.428 g cm–3 for the cotype sample. Bystrite is uniaxial (+), ɛ = 1.660(2) and ω = 1.584(2). The mineral was characterised by infrared and Raman spectra. The empirical formulae of the holotype and cotype samples are Na6.97K0.04Ca0.98(Si6.03Al5.97O24)(S52–)0.93[(SO42–)0.15Cl0.83] and Na6.75K0.04Ca1.11(Si6.09Al5.91O24)(S52–)1.04[(HS–)0.17Cl0.85], respectively. Bystrite is trigonal, space group P31c. The unit-cell parameters are: a = 12.8527(6) Å, c = 10.6907(5) Å, V = 1529.4(1) Å3 and Z = 2. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 4.821 (32) (102), 3.915 (38) (211), 3.712 (100) (300), 3.307 (50) (212), 2.782 (18) (400), 2.692 (22) (401), 2.673 (30) (004) and 2.468 (23) (402). Isomorphism and genesis of bystrite-type minerals is discussed. Bystrite and its K,HS-analogue sulfhydrylbystrite, Na5K2Ca(Al6Si6O24)S52–(HS)–, are indicators of highly reducing conditions.
0}. The yellow colour of bystrite is due to the presence of S52– anions occurring in Losod (LOS) cages of the aluminosilicate framework with the ABAC stacking sequence. Measured and calculated density is, respectively, 2.43(1) and 2.412 g cm–3 for the holotype and 2.42(1) and 2.428 g cm–3 for the cotype sample. Bystrite is uniaxial (+), ɛ = 1.660(2) and ω = 1.584(2). The mineral was characterised by infrared and Raman spectra. The empirical formulae of the holotype and cotype samples are Na6.97K0.04Ca0.98(Si6.03Al5.97O24)(S52–)0.93[(SO42–)0.15Cl0.83] and Na6.75K0.04Ca1.11(Si6.09Al5.91O24)(S52–)1.04[(HS–)0.17Cl0.85], respectively. Bystrite is trigonal, space group P31c. The unit-cell parameters are: a = 12.8527(6) Å, c = 10.6907(5) Å, V = 1529.4(1) Å3 and Z = 2. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 4.821 (32) (102), 3.915 (38) (211), 3.712 (100) (300), 3.307 (50) (212), 2.782 (18) (400), 2.692 (22) (401), 2.673 (30) (004) and 2.468 (23) (402). Isomorphism and genesis of bystrite-type minerals is discussed. Bystrite and its K,HS-analogue sulfhydrylbystrite, Na5K2Ca(Al6Si6O24)S52–(HS)–, are indicators of highly reducing conditions.
Associate Editor: Owen Missen