1. Introduction
The Broken Hill Pb–Zn–Ag deposit (280 Mt of 10.0 % Pb, 8.5 % Zn and 148 g/t Ag; Huston et al. Reference Huston, Stevens, Southgate, Muhling and Wyborn2006) is the world’s largest massive sulfide deposit. It occurs in the southern Curnamona province, New South Wales (Australia), along with hundreds of minor Broken Hill-type (BHT) deposits. They are hosted in the Palaeoproterozoic Willyama Supergroup in an ∼7 km thick package of multi-deformed and metamorphosed clastic sediments, bimodal (felsic and mafic) volcanic and volcaniclastic rocks, chemical sediments and granitoids (Fig. 1) (Willis et al. Reference Willis, Brown, Stroud and Stevens1983; Burton, Reference Burton1994). Metamorphic conditions reached granulite facies. Given the high metamorphic grade and extreme deformation, which have largely removed primary textures in the ore, a variety of origins have been proposed for the formation of the deposit. These ore deposit models are summarized in Greenfield (Reference Greenfield2003) and include: (1) syngenesis in which the deposit was considered to have formed by submarine exhalative/inhalative processes (Plimer, Reference Plimer1979; Wright et al. Reference Wright, Haydon and McConachy1987; Parr & Plimer, Reference Parr, Plimer, Kirkham, Sinclair, Thorpe and Duke1993); (2) syntectonic (Katz, Reference Katz1976; Findlay, Reference Findlay1994; Nutman & Ehlers, Reference Nutman and Ehlers1998); (3) post-tectonic (Andrews, Reference Andrews1922; Williams et al. Reference Williams, Chapman, Richmond, Baker, Heinemann, Pendergrast, Pongratz and Davidson1996); (4) magmatic–hydrothermal (Crawford & Maas, Reference Crawford and Maas2009); and (5) partial melting (Mavrogenes et al. Reference Mavrogenes, MacIntosh and Ellis2001; Frost et al. Reference Frost, Swapp and Mavrogenes2011).

Fig. 1. Geological map of the southern Curnamona Province and study sites: 1 – Broken Hill; 2 – Flying Doctor; 3 – Globe; 4 – Henry George; 5 – Esmeralda; 6 – 11:30; 7 – Pinnacles (modified after Laing et al. Reference Laing, Marjoribanks and Rutland1978; Page et al. Reference Page, Stevens and Gibson2005; O’Brien et al. Reference O’Brien, Spry, Teale, Jackson and Rogers2015).
In attempting to add clarity to how the Broken Hill deposit formed, we evaluate sulfur, zinc and cadmium isotopes of sphalerite along with the Zn/Cd ratios of sphalerite. Although various zinc isotopic studies have been conducted on several types of ore deposits including, Mississippi Valley-type Pb–Zn (MVT) (e.g. Pašava et al. Reference Pašava, Tornos and Chrastný2014; Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016; Zhu et al. Reference Zhu, Liao, Wang, Zhang, Yang, Fan and Wen2018, Reference Zhu, Wen, Zhang, Huang, Cloquet, Luais and Yang2021; Li et al. Reference Li, Liu, Xue and Li2019), Irish-type Pb–Zn (Wilkinson et al. Reference Wilkinson, Weiss, Mason and Coles2005; Gagnevin et al. Reference Gagnevin, Boyce, Barrie, Menuge and Blakeman2012, Reference Gagnevin, Menuge, Kronz, Barrie and Boyce2014); volcanogenic massive sulfide (VMS) (Mason et al. Reference Mason, Weiss, Chapman, Wilkinson, Tessalina, Spiro, Horstwood, Spratt and Coles2005), sedimentary exhalative (Sedex) (e.g. Kelley et al. Reference Kelley, Wilkinson, Chapman, Crowther and Weiss2009; Gao et al. Reference Gao, Zhu, Sun, Luo, Bao, Tang and Ma2018; Baumgartner et al. Reference Baumgartner, Kunzmann, Spinks, Bian, John, Blaikie and Hu2021; Wang et al. Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021) and active hydrothermal vents (e.g. John et al. Reference John, Rouxel, Craddock, Engwall and Boyle2008), Zn isotope studies of sphalerite in regionally metamorphosed ore deposits are restricted to those of Matt et al. (Reference Matt, Powell, Mathur and deLorraine2020, Reference Matt, Peck, Mathur, Nurtgen and Godfrey2022) on sphalerite and zinc oxides (zincite and franklinite) from the carbonate- and evaporate-hosted Balmat Zn deposit (New York) and the marble-hosted Franklin Zn deposit, New Jersey. A preliminary Zn isotope study was also made on sphalerite from the Gamsberg Sedex Zn deposit (South Africa) by S. E. Foulkes (unpub. M.Sc. thesis, Rhodes Univ., 2014), which, along with the Balmat deposit, was metamorphosed to the amphibolite facies. The Franklin district mines, like the Broken Hill district, were metamorphosed to the granulite facies. Although not part of this study, the Zn isotope composition of galena from various unmetamorphosed Chinese Pb–Zn deposits were obtained by Wang et al. (Reference Wang, Zheng, Mathur and Yu2020, Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021).
Cadmium isotopes have been used to evaluate the source of Cd in rocks, ore deposits, unconsolidated sediments, seawater, meteorites and biological samples (e.g. Wohmbacher et al. Reference Wohmbacher, Rehkämper, Mezger and Münker2003, Reference Wohmbacher, Rehkämper and Mezger2004; Lacan et al. Reference Lacan, Francois, Ji and Sherrell2006; Zhu et al. Reference Zhu, Wen, Zhang and Fan2016, Reference Zhu, Wen, Zhang, Huang, Cloquet, Luais and Yang2021; Hohl et al. Reference Hohl, Galer, Gamper and Becker2017), and to understand geochemical processes. Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) suggested that Cd isotope compositions of sphalerite when coupled with Zn/Cd ratios of sphalerite can be used to classify Pb–Zn deposits. They identified three classes of ore systems: high-temperature (i.e. skarn, VMS, porphyry, magmatic–hydrothermal), low-temperature (i.e. MVT) and exhalative (i.e. Sedex, seafloor hydrothermal). To date, no Cd isotope study has been done on an ore deposit subject to regional metamorphism. The criteria for deposit classification as applied by Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) is discussed further in Section 5.b.
Several sulfur isotope studies have been conducted on sulfides from Broken Hill and the smaller BHT deposits (Lawrence & Rafter, Reference Lawrence and Rafter1962; Stanton & Rafter, Reference Stanton and Rafter1966, Reference Stanton and Rafter1967; Both & Smith, Reference Both and Smith1975; Dong et al. Reference Dong, Barnes, Both and Sun1987; Spry, Reference Spry1987; Parr, Reference Parr1992, Reference Parr1994 a; Huston et al. Reference Huston, Power, Gemmell and Large1995), while the major-element composition of sphalerite was determined by, for example, Both (Reference Both1973), Hodgson (Reference Hodgson1975) and Lockington et al. (Reference Lockington, Cook and Ciobanu2014). Trace-element compositions of sphalerite are largely restricted to the studies of Both (Reference Both1973) and Lockington et al. (Reference Lockington, Cook and Ciobanu2014). Both (Reference Both1973) determined the trace-element content (including Cd) of sphalerite separates from each orebody using X-ray fluorescence spectrographic techniques, while Lockington et al. (Reference Lockington, Cook and Ciobanu2014) analysed two samples of sphalerite using a laser ablation inductively coupled plasma mass spectrometer. We have obtained new major- and trace-element compositions of sphalerite because individual sphalerite compositions were not provided by Both (Reference Both1973) and Hodgson (Reference Hodgson1975) and only two samples were obtained by Lockington et al. (Reference Lockington, Cook and Ciobanu2014). Both (Reference Both1973) plotted the compositions to show the ranges of Cd in sphalerite for each orebody, which is unusable for our purposes, while Hodgson (Reference Hodgson1975) analysed Zn, Mn, Fe and S but not Cd. The new sphalerite compositions obtained here, along with Cd isotope analyses from the Broken Hill deposit, are used to evaluate the origin of the Broken Hill deposit given the classification scheme of Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016). These geochemical parameters along with Zn isotope composition of sphalerite have not previously been applied to lead–zinc–silver mineralization in the Broken Hill district. The study of Zn isotopes of sphalerite from Broken Hill is particularly relevant given the partial melt model of Mavrogenes et al. (Reference Mavrogenes, MacIntosh and Ellis2001) and Frost et al. (Reference Frost, Swapp and Mavrogenes2011) for the formation of the deposit, which was recently applied to the metamorphosed Balmat deposit by Matt et al., Reference Matt, Powell, Mathur and deLorraine2020) to explain the fractionation of Zn isotopes in some orebodies. The aim of the study is to utilize Zn, Cd and S isotopes and the Zn/Cd ratios of sphalerite to shed light on the controversy surrounding the origin of the Broken Hill deposit and minor BHT deposits in the Broken Hill district.
2. Geological setting
Depositional ages of the Willyama Supergroup are ∼1720–1640 Ma, with the Broken Hill Group, which hosts the Broken Hill deposit, having formed at ∼1695–1685 Ma (Page & Laing, Reference Page and Laing1992; Page et al. Reference Page, Stevens and Gibson2005) (Fig. 2). Metamorphic conditions in and adjacent to the Broken Hill deposit were ∼700–800 °C and 5–6 kbar (Phillips & Wall, Reference Phillips and Wall1981; Powell & Downes, Reference Powell, Downes, Ashworth and Brown1990; White et al. Reference White, Powell and Halpin2004) but decreased to the amphibolite facies in the northern part of the Willyama Domain. The minor BHT deposits studied here (11:30, Flying Doctor, Esmeralda, Henry George, Globe, Pinnacles) were all subjected to the granulite facies. The deposits were intensely deformed and affected by at least three periods of deformation. Two of these deformational episodes resulted in the Broken Hill deposit being subject to two isoclinal fold events (Laing et al. Reference Laing, Marjoribanks and Rutland1978; Willis et al. Reference Willis, Brown, Stroud and Stevens1983) with the structural data of Laing et al. (Reference Laing, Marjoribanks and Rutland1978) suggesting that the deposit and the contained orebodies were overturned. The Broken Hill deposit is 8 km long and consists of at least six separate orebodies (from stratigraphic bottom to the top, they are C, B and A lodes and 1, 2 and 3 lenses; Figs 3, 4) each of which has a characteristic gangue mineralogy and metal ratio. Details of the vast array of minerals (>350) found in the Broken Hill deposit are given in Plimer (Reference Plimer1984) and Birch (Reference Birch and Birch1999). The main metallic minerals consist of sphalerite and galena, with minor amounts of pyrrhotite, chalcopyrite, arsenopyrite, löllingite, tetrahedrite and various sulfosalts. The most abundant silver-bearing minerals are galena and tetrahedrite with pyrargyrite, polybasite, stephanite, argentite, antimonial silver, allargentum, dyscrasite, argentopyrite and native silver occurring in lesser amounts (Lawrence, Reference Lawrence1968; Both & Stumpfl, Reference Both and Stumpfl1987). The dominant gangue minerals in each orebody are rhodonite, fluorite, quartz (3 lens), calcite, rhodonite, wollastonite (2 lens), quartz, calcite, wollastonite (1 lens), rhodonite, manganoan hedenbergite (A lode), quartz (B lode) and quartz (C lode). Based, in part, on Laing et al’s (Reference Laing, Marjoribanks and Rutland1978) assumption that the deposit was structurally overturned, Groves et al. (Reference Groves, Groves, Bierlein, Broome and Penhall2008) identified a feeder zone system in the C lode, with cross-cutting blue quartz-gahnite ± garnet rocks serving as the metamorphosed alteration zone. However, some workers (e.g. Mavrogenes et al. Reference Mavrogenes, MacIntosh and Ellis2001; Webster, Reference Webster2006; Frost et al. Reference Frost, Swapp and Mavrogenes2011) suggested that the deposit was not overturned so that the orebodies are the correct way up with 3 lens being at the stratigraphic base of the deposit and C lode at the top. The C, B and A lodes and 1 lens are characterized by Zn > Pb, whereas the 2 and 3 lenses have Pb ≥ Zn. By invoking major partial melting of the deposit, Mavrogenes et al. (Reference Mavrogenes, MacIntosh and Ellis2001) and Frost et al. (Reference Frost, Swapp and Mavrogenes2011) argued that the zinc lodes (i.e. A, B and C lodes and 1 lens) are restites of Pb-rich sulfide melts implying that these melts migrated through the stratigraphy to form the Pb-rich orebodies (i.e. 2 and 3 lenses).
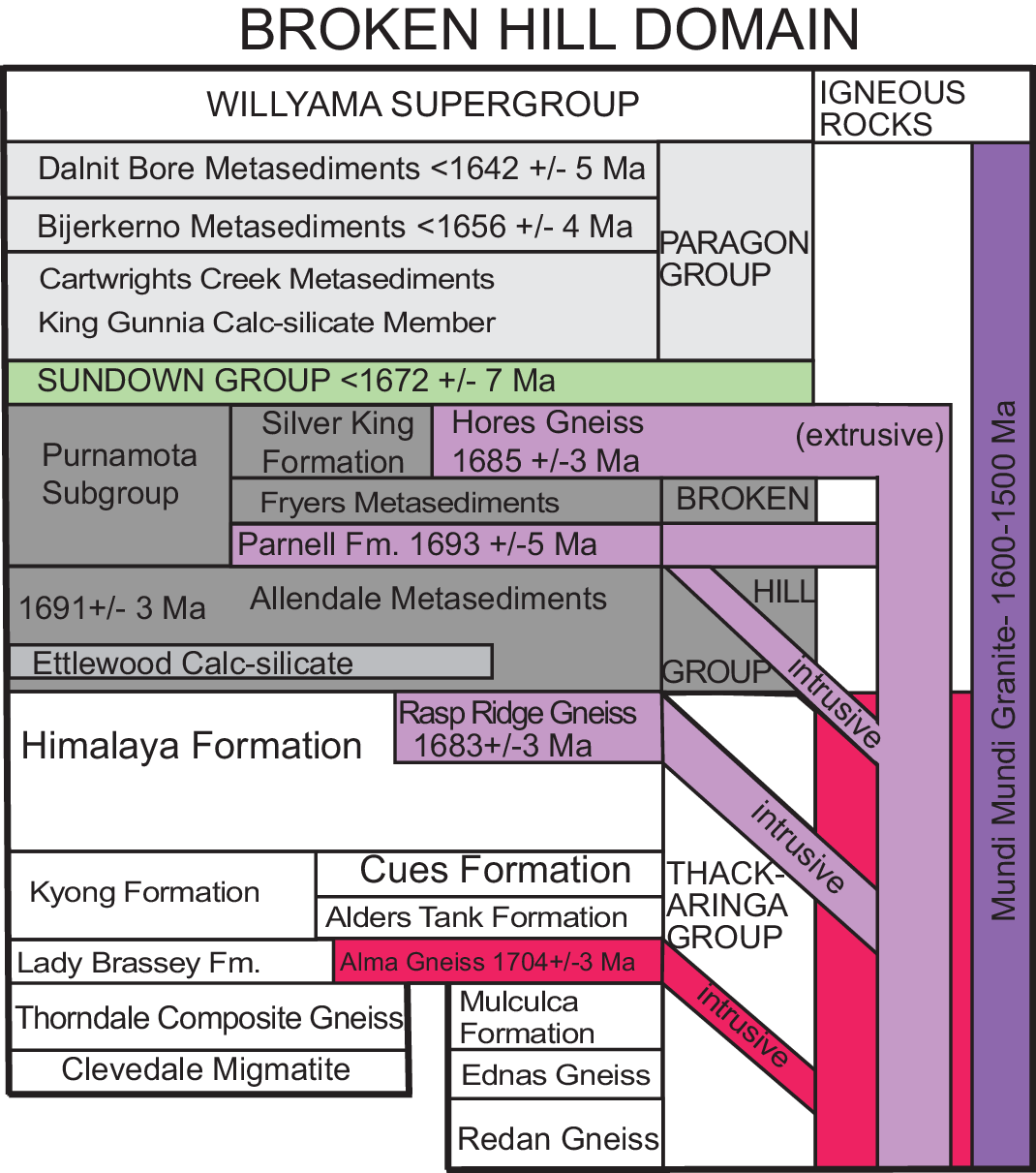
Fig. 2. Stratigraphic column and ages of rocks in the Broken Hill domain (after Conor & Preiss, Reference Conor and Preiss2008). The Broken Hill and BHT deposits occur in the Hores Gneiss of the Purnamota Subgroup, while the Pinnacles deposit likely occurs in the Cues Formation stratigraphically lower in the Broken Hill domain.

Fig. 3. Geological map of the Broken Hill deposit. Abbreviations: N.B.H.C – New Broken Hill Consolidated mine (currently part of Southern Operations operated by Perilya Broken Hill Limited); Z.C. – Zinc Corporation mine (currently part of Southern Operations operated by Perilya Broken Hill Limited); B.H.S. – Broken Hill South mine; N.B.H. – North Broken Hill mine (currently North mine operated by Perilya Broken Hill Limited). Cross-section No. 62 is shown as a bold black line (see Fig. 4 for cross-section).

Fig. 4. Cross-section (No. 62) through the New Broken Hill Consolidated mine haulage shaft, looking south l8 degrees east, Broken Hill deposit. Note that the orebodies are structurally overturned with C lode occurring at the stratigraphic base of the deposit. The 3 lens orebody is not shown here as it only occurs in the central and northern parts of the deposit, where it occurs at the stratigraphic top. The figure has been modified after Pratten (Reference Pratten and McAndrew1965).
The Broken Hill deposit is intimately associated with a package of rocks that Johnson & Klingner (Reference Johnson, Klingner and Knight1975) referred to as the ‘lode horizon’, which consists of quartz garnetite, garnetite, blue quartz and blue quartz-gahnite rocks, and ‘lode’ pegmatite (Spry & Wonder, Reference Spry and Wonder1989; O’Brien et al. Reference O’Brien, Spry, Teale, Jackson and Rogers2015). Apart from metasedimentary rocks, blue quartz-gahnite rocks and quartz garnetite are the two most common rock types spatially associated with minor BHT deposits (Barnes et al. Reference Barnes, Stevens, Stroud, Brown, Willis and Bradley1983). A summary of the geological setting for the deposits from which samples were analysed is given in Table 1.
Table 1. Summary of geological characteristics of Broken Hill and BHT deposits (modified after O’Brien et al. Reference O’Brien, Spry, Teale, Jackson and Rogers2015)

*Estimates of grades and tonnage supplied by Perilya Ltd for North Mine Zinc Lode, Henry George and 11:30.
†Mineral abbreviations after Whitney & Evans (Reference Whitney and Evans2010); Amp – amphibole; Ap – apatite; Asp – arsenopyrite; Az – azurite; Bt – biotite; BHT – Broken Hill-type; BIF – banded iron formation; Cal - calcite; Cer – cerussite; Ccp – chalcopyrite; Chl – chlorite; Di – diopside; Fsp – feldspar; Ghn – gahnite; Gn – galena; Grt – garnet; Lo – löllingite; Mag – magnetite; Mlc – malachite; Ms – muscovite; Pmt - piemontite; Po – pyrrhotite; Py – pyrite; Rhd – rhodonite; Qz – quartz; Sil – sillimanite; Sp – sphalerite; Ttr – tetrahedrite; Tur – tourmaline; Wo – wollastonite.
3. Samples and analytical methods
3.a. Cadmium and zinc isotopes
Thirty-one samples were collected from drill core and from underground locations at the Broken Hill and Pinnacles deposits. Some of the samples were used previously in the studies of Spry & Wonder (Reference Spry and Wonder1989) and O’Brien et al. (Reference O’Brien, Spry, Teale, Jackson and Rogers2015). Approximately 50 mg of sphalerite powder was dissolved in 4 ml of ultrapure heated (80 °C) aqua regia for 8 hours. Complete dissolution was visually confirmed. The solution was cut into two equal aliquots and used for chromatographic separation. The procedure for the preparation of the Cd and Zn isotopes is identical to that given by Wang et al. (Reference Wang, Zheng, Mathur and Yu2020, Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021). All reported results show mass dependence.
The Cd isotopic compositions were measured on a Neptune multicollector inductively coupled plasma mass spectrometer (MS-ICP-MS) at Rutgers University. Cadmium was purified using the anion exchange chromatograph (Cloquet et al. Reference Cloquet, Rouxel, Carignan and Libourel2005) with volumetric yields for the samples greater than 94 % after two rounds of column chromatography. Yield checks were measured on an Agilent 5900 ICP-optical emission spectrometer (ICP-OES) at Juniata College. Zinc and Cd concentrations were determined with standard calibration curves that ranged from 0.5 to 20 ppm, and yttrium was used as an internal standard for analysis.
The chromatography for Cd involved 2 ml of wet BioRad AG MP-1 resin chloride form (100–200 mesh), which was added to a 10 ml BioRad chromatography column. The resin was sequentially cleaned with 10 ml of 2 % HNO3, 10 ml of MQ water (18.2 W) and 5 ml of 1.2 molar HCl. The sample was loaded onto the resin with 1 ml of 1.2 molar HCl and the unwanted ions were sequentially eluted with lower molality HCl and the Cd was collected in 17 ml of 0.0012 molar HCL. This process was repeated with the use of new resin for the second column to eliminate Sn. The chromatography was effective, as no 115Sn voltages were recorded above the 2 mV background. The 115Sn mass was monitored in H4 cup, with 107Ag in L4 cup, 109Ag in L2 cup, 110Cd in L1 cup, 111Cd in Ax cup, 112Cd in H1 cup, 113Cd in H2 cup, 114Cd in H3 cup and 115Sn in H4 cup. Instrumentation setup and introduction was similar to that of Wasylenki et al. (Reference Wasylenki, Swihart and Romaniello2014). All samples were doped with 150 ppb NIST 987 Ag isotope standard, which was used to correct for mass bias using the exponential fractionation correction (Maréchal et al. Reference Maréchal, Telouk and Albarède1999). The 107Ag/109Ag of the NIST 987 Ag isotope standard is reported as 1.07638. Solutions were measured at 200 ppb Cd, with on-peak blank subtraction in one block of 30 ratios. The reported values are an average of two separate measurements, and the data are presented relative to the NIST SRM 3108 Cd standard in per mil notation defined as: δ114/110Cd (‰) = ((114Cd/110Cd)sample/(114Cd/110Cd)NIST SRM 3108 − 1) × 1000 (Abouchami et al. Reference Abouchami, Galer, Horner, Rehkämper, Wombacher, Xue, Lambelet, Gault-Ringold, Stirling, Schönbächler, Shiel, Weis and Holdship2013). All data cited here from the literature are converted relative to the NIST SRM 3108 standard (δ114CdNIST SRM 3108 = δ114CdNancy SPEX – 0.11 ‰; Xu et al. Reference Xu, Zhong, Hu, Wen, Zhu, Bai, Fan, Li and Zhou2020).
Measured errors of ratios were in the fifth or sixth decimal and do not represent a conservative estimation of error. Errors for the measured values are constrained in two ways. The variation of NIST SRM 3108 throughout the measuring session was 0.05 ‰ (2s, n = 27). The second means for error estimation was by measuring a High Purity Standard ICP-MS standard during the two sessions. The value of the standard is δ114Cd = −0.53 ‰ ± 0.06 (2s, n = 10) and is considered the error of measurements. All duplicate measurements fall within reported errors.
The chromatography for Zn also involved the BioRad MP-1 anion exchange resin using the protocol defined by Maréchal et al. (Reference Maréchal, Telouk and Albarède1999). Yields from the columns were tested volumetrically and were all greater than 95 %. The mass bias corrections for Zn using Cu (NIST 976) were employed for these samples and then the corrected values were bracketed by the standards (Archer & Vance, Reference Archer and Vance2004; Chapman et al. Reference Chapman, Mason, Weiss, Coles and Wilkinson2004, Reference Chapman, Mason, Weiss, Coles and Wilkinson2006; Peel et al. Reference Peel, Weiss, Chapman, Arnold and Coles2008). Solutions were measured at 150 ppb Cu and 200 ppb Zn (63Cu = 7V and 66Zn = 4V). One block was 30 ratios in the analytical session, and the Zn isotope values are reported in traditional per mil notation relative to the AA–ETH standard: δ66Zn (‰) = ((66Zn/64Zn)sample/(66Zn/64Zn)AA−ETH − 1) × 1000. All the data cited here from the literature were converted relative to the AA–ETH standard (δ66ZnAA–ETH = δ66ZnJMC3–0749L – 0.28 ‰; Archer et al. Reference Archer, Andersen, Cloquet, Conway, Dong, Ellwood, Moore, Nelson, Rehkämper, Rouxel, Samanta, Shin, Sohrin, Takan and Wasylenki2017).
Errors for Zn isotopes are calculated in a similar manner to that discussed above for Cd isotopes. Throughout the analytical sessions, the reference material AA–ETH standard compared with itself (n = 14) yielded two standard deviations of 0.06 ‰ (2σ) for δ66Zn, which is larger than the error for each sample during the run. The value of USGS BVHO-2 δ66ZnAA–ETH is +0.02 ‰ ± 0.04 (2s, n = 3). The largest error between the two methods is that of the variation of the standard in comparison to itself and is considered the error for reported samples.
3.b. Sulfur isotopes
Sphalerite was separated from ore samples by hand picking under a binocular microscope or was drilled out with a Dremel tool with a 1 mm drill tip. We followed the procedure for sulfur isotope analysis as described by Grassineau (Reference Grassineau2006). Sphalerite was pulverized in an agate mortar to a powder (1.5 mg), which was then loaded into tin capsules and burned using a Thermo Scientific Flash IRMS IsoLInk elemental analyser. The Sn capsules oxidized at ∼1020 °C, and when oxygen was added it flash combusted at 1800 °C (Grassineau, Reference Grassineau2006). The oxygen was added at a rate of 300 ml/minute for three seconds. The SO2 gas produced was purified through a gas chromatography column and then introduced via a Conflo IV Universal Interface system into a continuous flow-type dual-inlet Thermo Scientific Delta V Series Isotope Ratio mass spectrometer under He flow. The analysis time was ∼420 seconds. The δ34SVCDT values were calculated using calibration curves obtained for the following standards, some of which were obtained from the Queen’s Facility for Isotope Research (QFIR), Queen’s University, Canada: NBS 127 barite = +20.3 ‰ (±0.3 ‰), IAEA-SO-6 barite = −34.1 ‰, QFIR pyrite = −0.5 ‰, IAEA-SO-5 barite = +0.5 ‰, MRC pyrite = +0.7 ‰, Q-GEMA pyrite = +3.0 ‰, M6801 barite = +12.5 ‰, and two internal standards provided by Thermo Scientific: peat = −13.15 ‰ (±0.3 ‰) and sulfanilimide = −1.24 ‰ (±0.2 ‰). These values are relative to the internationally recognized sulfur isotope standard Cañon Diablo troilite (FeS). The analytical precision of the data is ±0.1 ‰.
3.c. Major- and trace-element composition of sphalerite
Part of the dissolved separates of sphalerite in solution that were analysed for Zn and Cd isotopes from the Broken Hill deposit as well as smaller BHT deposits (11:30, Esmeralda, Flying Doctor, Henry George) were also analysed for Ag, Cd, Cu, Fe and Zn using an Agilent 5900 ICP-OES at Juniata College. Quantitative analyses of sphalerite were also performed on a JEOL JXA-8530FPlus electron microprobe at the University of Minnesota. Analytical conditions for determining sphalerite compositions used an accelerating voltage of 15 kV, beam current of 50 nA and a beam diameter of 5 microns. Elements were acquired using analysing crystals LIFL for Zn kα, Mn kα, Fe kα and Cu kα, PETL for Cd lα, and PETJ for S kα and Ag lα. The standards were Mn-olivine and synthetic Mn2SiO4 for Mn, Cu metal for Cu, pyrite for Fe, sphalerite for Zn and S, hessite for Ag and cadmium sulfide (CdS) for Cd. The on-peak counting time was 10 seconds for Zn kα, Mn kα, Fe kα, Cu kα and S kα, 40 seconds for Ag lα and 60 seconds for Cd lα. The mean atomic number (MAN) background intensity method was used instead of the traditional off-peak background acquisition (Donovan & Tingle, Reference Donovan and Tingle1996; Donovan et al. Reference Donovan, Singer and Armstrong2016). The MAN background intensity data was calibrated and continuum absorption corrected for Cd lα, Zn kα, Mn kα, Fe kα, Cu kα, S kα and Ag lα. Unknown and standard intensities were corrected for dead-time. The Phi-Rho-Z matrix correction algorithm Armstrong/Love Scott (CitZAF) was used along with the mass absorption coefficients dataset FFAST (Chantler et al. Reference Chantler, Olsen, Dragoset, Chang, Kishore, Kotochigova and Zucker2005).
4. Results
4.a. Sulfur isotopes
Sulfur isotope compositions of 31 samples of sphalerite are in Table 2. The values of δ34S from Broken Hill range from +0.27 to +4.73 ‰ (n = 19), while those for the following smaller BHT deposits are: Pinnacles (−3.08 to −0.94 ‰, n = 3), Esmeralda (+1.24 to +1.28 ‰, n = 2), Henry George (−1.06 to +1.17 ‰, n = 4), 11:30 (−5.11 ‰, n = 2) and Flying Doctor (−0.36 ‰, n = 1) (Fig. 5). Sulfur isotope compositions of sulfides from these minor deposits had not previously been obtained, except for the Pinnacles deposit, the second largest Pb–Zn–Ag deposit in the Willyama Domain, where Parr (Reference Parr1992, Reference Parr1994 a) reported a range of δ34S = −3.5 to +3.7 ‰. Other sulfur isotope studies of sulfides from the Broken Hill area include analyses of sphalerite, galena, pyrrhotite and chalcopyrite. Both & Smith (Reference Both and Smith1975) recorded δ34S values of between −2.1 and +2.4 ‰ per mil and between −3.8 and +5.4 ‰ for sulfides from the main Broken Hill lode and 26 minor BHT deposits (including Pinnacles), respectively, while Spry (Reference Spry1987) showed a broader isotopic range for the Broken Hill deposit of −3.3 to +6.7 ‰. The isotopic compositions obtained by Lawrence & Rafter (Reference Lawrence and Rafter1962; δ34S = +0.4 to +1.7 ‰) and Stanton & Rafter (Reference Stanton and Rafter1966; δ34S = −2.2 to +4.7 ‰, 1967; δ34S = −1.5 to +2.8 ‰) for Broken Hill fall within the range given by Spry (Reference Spry1987).
Table 2. Zn, Cd and S isotope data and major–trace-element contents of sphalerite (ppm) from Broken Hill and minor BHT deposits

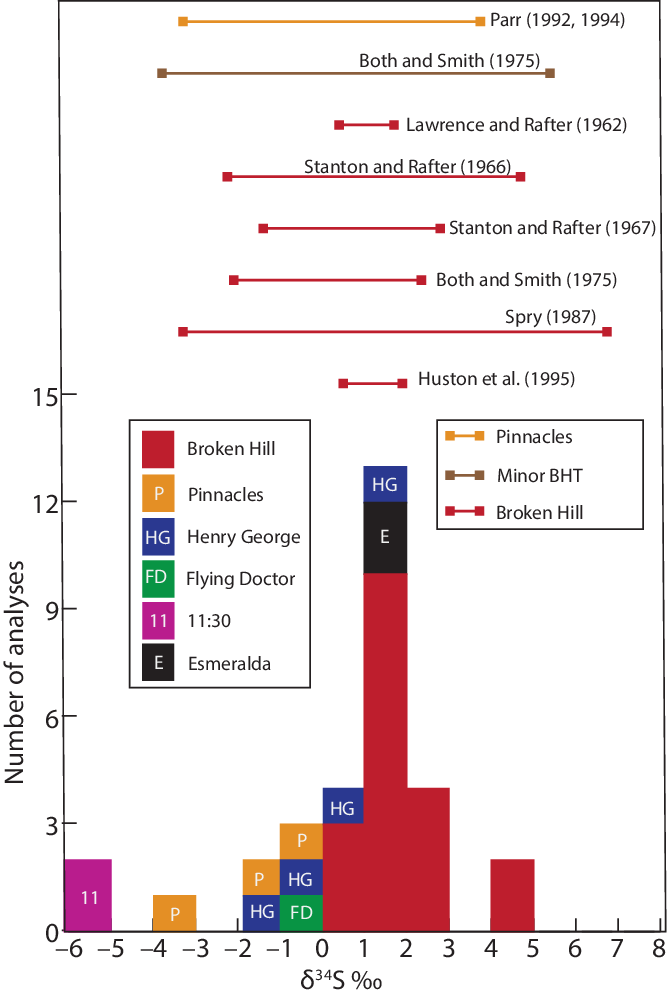
Fig. 5. Histogram of sulfur isotope compositions of sphalerite (this study) from the Broken Hill deposit and minor Broken Hill-type deposits (P – Pinnacles; HG – Henry George; FD – Flying Doctor; 11 – 11:30). Also shown as bar lines are the ranges of previously published sulfur isotope studies by Lawrence & Rafter (Reference Lawrence and Rafter1962), Stanton & Rafter (Reference Stanton and Rafter1966, Reference Stanton and Rafter1967), Both & Smith (Reference Both and Smith1975), Spry (Reference Spry1987), Parr (Reference Parr1992, Reference Parr1994 a) and Huston et al. (Reference Huston, Power, Gemmell and Large1995). Sulfur isotope compositions of sulfides from the Pinnacles deposit, minor BHT deposits and Broken Hill from these studies are shown as orange, brown and red bar lines, respectively.
By combining the S isotope data for sphalerite, galena, pyrrhotite and chalcopyrite of Both & Smith (Reference Both and Smith1975) and Spry (Reference Spry1987), the latter proposed that there may be a weak increase in isotopic values from the stratigraphic footwall (C lode) through to the hanging wall (3 lens). However, by adding the S isotope data for sphalerite obtained here with these two studies it is apparent that there is no systematic increase from the footwall to the hanging wall. Instead, there is an increase in the average isotopic compositions for sphalerite in C lode (δ34S = 0 ‰) to the top of the Zn lodes (i.e. 1 lens; δ34S = +2.2 ‰) with a slight decrease to 1 ‰ in 3 lens (Fig. 6a). Although galena was not analysed here, the combined data of Both & Smith (Reference Both and Smith1975) and Spry (Reference Spry1987) show a steady increase in the average δ34S galena composition from C lode (−1.4 ‰) to 2 lens (+1.3 ‰) and 3 lens (+1.2 ‰). Data for the Zn mineralization from the North mine is shown in Figure 6a but was not included owing to uncertainty in its stratigraphic position (possibly 1 lens or A lode; Plimer, Reference Plimer1979). It yields the highest average sulfur isotopic composition for sphalerite and galena for any of the orebodies.

Fig. 6. (a) Sulfur isotope compositions of sphalerite and galena as a function of stratigraphic position (with C lode in the stratigraphic footwall) in the Broken Hill deposit. Note that isotopic compositions of sphalerite in the so-called Zinc lode from the North mine is also shown but its stratigraphic position is uncertain. Plimer (Reference Plimer1979) proposed that it equated to either 1 lens or A lode. (b) Zinc isotope compositions as a function of stratigraphic position in the Broken Hill deposit. (c) Cadmium isotope compositions as a function of stratigraphic position in the Broken Hill deposit.
4.b. Zinc isotopes
Values of δ66Zn range from −1.15 to +0.46 ‰ (n = 19) for the Broken Hill deposit and from −0.97 to +0.10 ‰ (n = 9) for the smaller BHT deposits (Table 2). The isotopically lightest value for the smaller BHT deposits is sample JB-10-53 (δ66Zn = −0.97 ‰) from the Esmeralda deposit, while the isotopically heaviest sample is δ66Zn = +0.10 ‰ for sample JB-10-100 from the Flying Doctor deposit. Sample JB-10-53, along with samples BH-1 (3 lens), JB-10-87 (Zinc lode, North mine) and JB-10-52 (Esmeralda) have values of δ66Zn of −1.15, −0.77 and −0.76 ‰, respectively, which are among the most negative values for sphalerite ever reported for an ore deposit, with that from sample BH-1 being the lowest value yet recorded. There appears to be no systematic variation of Zn isotopes from the stratigraphic footwall to the hanging wall of the Broken Hill deposit (Fig. 6b).
4.c. Cadmium isotopes
Values of δ114Cd for sphalerite from Broken Hill and smaller BHT deposits range from −0.48 to +0.01 ‰ for the Broken Hill deposit and from −1.02 to +2.59 ‰ for the smaller BHT deposits (Table 2). The isotopically lightest value is from the Henry George deposit, while the heaviest is from the 11:30 deposit. The range observed here for the BHT deposits are the most negative and positive yet reported for sphalerite from an ore deposit. Although it should be noted that Cd isotope compositions of galena from the unmetamorphosed Zhaxikang and Keyue Sedex deposits, Tibet, show the isotopically lightest (δ114Cd = −2.19 ‰, Zhaxikang; Wang et al. Reference Wang, Zheng, Mathur and Yu2020) and the heaviest (δ114Cd = +3.17 ‰, Keyue; Wang et al. Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021) for a sulfide deposit reported to date. At first glance, the extraordinarily heavy sample from the 11:30 deposit of +2.59 ‰ may appear to be due to analytical error. However, it should be noted that the sample was analysed twice and yielded the same value (Table 2). Moreover, this same sample yielded the most isotopically light sulfur isotope value (−5.11 ‰) of any sample yet reported in the Broken Hill district. Like the Zn isotope compositions of sphalerite, there appears to be no systematic variation of Cd isotopes from the stratigraphic footwall to the hanging wall of the Broken Hill deposit for the limited number of data obtained here (Fig. 6c).
4.d. Composition of sphalerite and Zn/Cd ratios
Samples were analysed for Ag, Cd, Cu, Fe and Zn using the Agilent 5900 ICP-OES but only the Ag, Cd and Cu concentrations are accurate since the Zn and Fe are major elements (per cent levels) and thus not suitable for analysis by ICP-OES analysis, given the ppm level concentrations of Zn and Fe in the standard. The Ag, Cd and Cu concentrations of sphalerite from Broken Hill (17–2842 ppm Ag, 181–10 882 ppm Cd, 0–151 369 ppm Cu), Esmeralda (18–81 ppm Ag, 2123–2960 ppm Cd, 1664–2928 ppm Cu), Flying Doctor (196 ppm Ag, 4100 ppm Cd, 2684 ppm Cu) and Henry George (8–532 ppm Ag, 1950–2210 Cd, 576–3563 ppm Cu). To further explore the concentrations of both major- (Zn, Fe and S) and trace-element (Ag, Cd, Cu and Mn) compositions of sphalerite, a suite of ore samples containing sphalerite from the main Broken Hill deposit, as well as minor BHT deposits (Flying Doctor, Globe, Henry George) were analysed by electron microprobe (Fig. 7; Table 3). Note that samples analysed here are not the same as those analysed for Zn, Cd and S isotopes owing to the limited sample size. The Zn and Cd concentrations in sphalerite from 14 samples from the Broken Hill deposit range from 52.93 to 58.73 wt % Zn and 1740 to 2810 ppm Cd, respectively, for Zn/Cd ratios of 203 to 303 with an average of 220 (Fig. 7). The narrow range of ratios obtained by electron microprobe analysis is remarkable given the enormous size of the deposit and that the samples were collected from widely spaced localities. Samples of sphalerite were analysed from Globe (n = 1), Flying Doctor (n = 2) and Henry George (n = 1) and yield compositions considerably more variable than those from Broken Hill (i.e. Globe, 56.99–58.63 wt % Zn, 42–70 ppm (average Zn/Cd ratio of 1111); Flying Doctor, 51.05–56.66 wt % Zn, 2400–3420 ppm (average Zn/Cd ratios of 153 to 226); Henry George, 54.94–55.44 wt % Zn, 2410–2550 ppm Cd (average Zn/Cd ratio of 221)). The Zn/Cd ratio of 1111 for the Globe sample is anomalous relative to all other samples of sphalerite from the Broken Hill district. This is likely due to the low Fe content of the sphalerite, which is consistent with sphalerite not being buffered by a member of the system Fe–S. The Fe, Mn and Cu concentrations of sphalerite from Broken Hill range from 7.12 to 11.89 wt % Fe (average = 10.35 wt %), 0.08 to 1.02 wt % Mn (average = 0.34 wt %) and 0 to 0.09 wt % Cu (average = 0.03 wt %). Up to 0.08 wt % Ag was also obtained. The range of concentrations of Fe, Cu and Ag in sphalerite is similar to the ranges of these elements in the minor BHT deposits (6.85 to 12.93 wt % Fe, 0 to 0.04 wt % Cu, up to 0.06 wt % Ag). However, the Mn concentration is considerable lower (0.01 to 0.08 wt % Mn) in the minor BHT occurrences. The concentrations of Cd and the Zn/Cd ratios derived from electron microprobe analysis are evaluated further in this contribution rather than those obtained by ICP-OES.

Fig. 7. Box and whisker plot of Zn/Cd ratios for sphalerite from the Broken Hill deposit and minor BHT deposits (Henry George and Flying Doctor). Data from the single sample from the Globe BHT deposit gave an anomalous average value of 1100 due to the low Fe content of the sphalerite, which is commensurate with sphalerite not being buffered by a member of the system Fe–S; see Table 3) Data are shown for MVT deposits: Fule, Dadongla, Jinding (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) and Jinding, Nanchang, Beichang (Li et al. Reference Li, Liu, Xue and Li2019); VMS deposits: Cayeli (Revan et al. Reference Revan, Genç, Maslennikov, Maslennikova, Large and Danyushevsky2014), Bankshapa, Bhuyari, Biskhan, Jangaldheri (Mishra et al. Reference Mishra, Patia, Dora, Baswani, Meshram, Shareef, Pattanayak, Suryavanshi, Mishra and Raza2021), Bukit Ketaya, Bukit Botol (Basori et al. Reference Basori, Gilbert, Zaw and Large2021), Gacun (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016); and Sedex deposits: Langshan (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016), Mt Isa (Cave et al. Reference Cave, Lilly and Barovich2020), Sullivan (Lydon & Reardon, Reference Lydon, Reardon, Lydon, Höy, Slack and Knapp2000).
Table 3. Zn and Cd concentrations and Zn/Cd ratios of sphalerite from Broken Hill and BHT deposits

NBHC – New Broken Hill Consolidated mine; NM – North mine; ZC – Zinc Corporation mine.
5. Discussion
5.a. Previous genetic models
The origin of the Broken Hill deposit and the minor BHT deposits is controversial with a variety of origins having been proposed in the vast literature on the deposit (see Greenfield, Reference Greenfield2003). Essentially these disparate views can be distilled down to syngenetic, epigenetic and magmatic–hydrothermal models. For the syngenetic models, sulfide formed by subaqueous hydrothermal processes and subsequently underwent high-grade metamorphism, deformation and possibly partial melting (e.g. Johnson & Klingner, Reference Johnson, Klingner and Knight1975; Laing et al. Reference Laing, Marjoribanks and Rutland1978; Mavrogenes et al. Reference Mavrogenes, MacIntosh and Ellis2001). For the syngenetic models, there has been debate regarding whether or not the BHT deposits are Sedex deposits (e.g. Goodfellow et al. Reference Goodfellow, Lydon, Turner, Kirkham, Sinclair, Thorpe and Duke1993; Sangster, Reference Sangster2020), a separate class of deposit (e.g. Walters, Reference Walters, Pongratz and Davidson1996; Walters et al. Reference Walters, Skrzeczynski, Whiting, Bunting, Arnold, Goldfarb and Nielsen2002; Spry & Teale, Reference Spry and Teale2021) or deposits that are possibly transitional between Sedex and VMS deposits (e.g. Walters, Reference Walters1998; Leach et al. Reference Leach, Sangster, Kelley, Large, Garven, Allen, Gutzmer, Walters, Hedenquist, Thompson, Goldfarb and Richards2005; Spry et al. Reference Spry, McFadden, Teale and Steadman2010).
Epigenetic models revolve around the introduction of metals during peak metamorphism or by post-tectonic replacement (e.g. Nutman & Ehlers, Reference Nutman and Ehlers1998; Gibson & Nutman, Reference Gibson and Nutman2004). Crawford & Maas (Reference Crawford and Maas2009) proposed a magmatic–hydrothermal model where they argued that the ore-forming components were derived from fractionated rift-related ferrotholeiite magmas in which fractional crystallization of Fe-rich oxide gabbros separated Cu from Pb and Zn. They suggested that magmatic fluid evolved from these magmas transported Pb and Zn in a saline-rich hydrothermal fluid and deposited metals below the seafloor.
Of these models, the epigenetic (i.e. syntectonic and post-tectonic models) of formation can be rejected since the orebodies were deformed and metamorphosed by the Olarian Orogeny. However, the syngenetic and magmatic–hydrothermal models will be considered further based on the geochemical data obtained here.
5.b. Zn/Cd ratios and Cd isotopes of sphalerite as an indicator of ore genesis
Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) pointed out that the Cd content of sphalerite is dependent on a variety of physicochemical parameters including temperature (T), the nature and concentration of ligands in the ore fluid that bond to Zn and Cd, pH and the total sulfur in solution. Wen et al. classified Pb–Zn deposits into three groups: low-temperature (i.e. MVT deposits), high-temperature (i.e. porphyry, magmatic–hydrothermal, skarn and VMS deposits) and exhalative systems (i.e. Sedex, seafloor hydrothermal sulfides). Distinctions between the three classes of deposits were based on Cd concentrations and Zn/Cd ratios in sphalerite as well as a plot of Cd isotopes versus Zn/Cd ratios. However, an issue with Wen et al.’s study is that it was based on only ten occurrences, four of which were MVT deposits with single examples of a Sedex (i.e. Langshan) and a VMS deposit (i.e. Gacun).
There are several difficulties with the categorization technique proposed by Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016). Their study evaluated low- and high-temperature classes of deposits, which include different types of deposits that form under very different ore-forming conditions. For example, Wen et al.’s (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) high-temperature deposits include both VMS and porphyry-style deposits, yet VMS deposits form at much lower temperatures (up to ∼400 °C) than those of porphyry-style deposits that form above magmatic solidus temperatures (∼600–750 °C) (Franklin et al. Reference Franklin, Gibson, Jonasson, Galley, Hedenquist, Thompson, Goldfarb and Richards2005; Seedorff et al. Reference Seedorff, Dilles, Proffett, Einaudi, Zurcher, Stavast, Johnson, Barton, Hedenquist, Thompson, Goldfarb and Richards2005). Volcanogenic massive sulfide deposits also generally form from low salinity fluids (i.e. equivalent to seawater compositions), although higher salinity fluids are reported in some deposits that have a magmatic component (Franklin et al. Reference Franklin, Gibson, Jonasson, Galley, Hedenquist, Thompson, Goldfarb and Richards2005). Metals from porphyry-style deposits are either carried in the vapour phase or from highly saline fluids (commonly >50 wt % NaCl). Finally, Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) also provided a simplistic set of physicochemical conditions of formation for Sedex deposits proposing that they formed under reducing conditions, which is characteristic of Selwyn-type Pb–Zn Sedex deposits. Cooke et al. (Reference Cooke, Bull, Large and McGoldrick2000) recognized the McArthur River-type Sedex deposits form from more oxidized fluids at T generally <200 °C, while Selwyn-type deposits form from reduced fluids at T >200 °C. These concerns notwithstanding, Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) demonstrated that competing physicochemical conditions produce different Cd concentrations and Zn/Cd ratios.
Figure 8 shows a plot of the Cd isotope composition versus Zn/Cd ratios of the four MVT deposits, along with the Langshan Sedex deposit and the Gacun VMS deposit of Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016), and recent data from the Jinding MVT deposit (Li et al. Reference Li, Liu, Xue and Li2019), the Keyue and Zhaxikang Sedex deposits (Wang et al. Reference Wang, Zheng, Mathur and Yu2020, Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021) and the Xiaobaliang VMS deposit (Yang et al. Reference Yang, Song, Wen, Zhang, Fan, Wang, Li, Yang, Zhou, Liao and Zhu2022). Also shown are data from the Broken Hill and minor BHT deposits. Although the data of Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) for the MVT, Sedex (Langshan) and VMS (Gacun) deposits suggest that these three deposit types can be discriminated, with MVT deposits showing the lowest Zn/Cd ratios and the Sedex deposit showing the highest Zn/Cd ratio, with the VMS deposit somewhere in between, it is clear when data for the Keyue and Zhaxikang Sedex deposits and the Xiaobaliang VMS deposits are added that these two classes of deposits overlap and cannot be distinguished. Although Cd isotope data were not obtained, Zn/Cd ratios of published sphalerite from several Sedex (Kanmantoo, Mt Isa, Gamsberg, Bleikvassli, Aclare, Sullivan) and VMS (Bankshapa, Jangaldheri, Biskan, Bhuyari, Bukit Botol, Arminius, Attu, Pontide) further show that the Zn/Cd ratios of sphalerite overlap (see Table 4) are not a useful discriminator of Wen et al.’s (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) ‘high-temperature’ and ‘exhalative’ deposits.
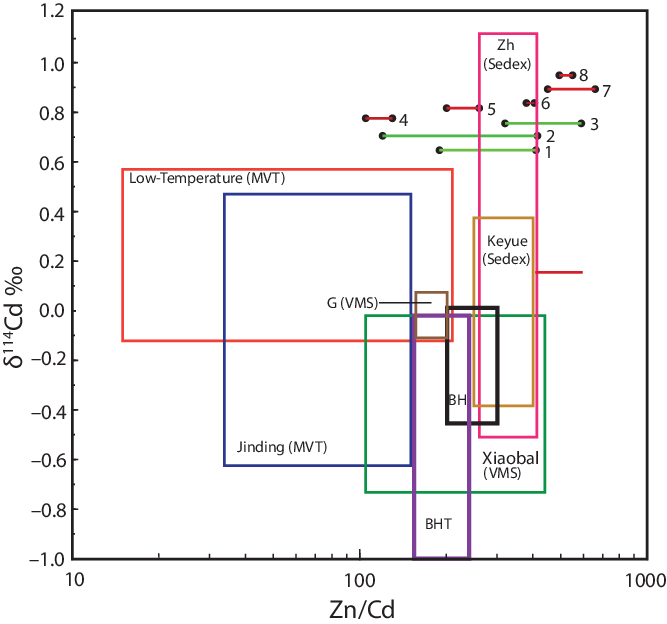
Fig. 8. Plot of δ114Cd versus Zn/Cd ratios for sphalerite from MVT, Sedex and VMS deposits along with ranges shown for the Broken Hill and BHT deposits. Note that the boxes shown for Broken Hill and BHT deposits are based on samples of sphalerite analysed by MC-ICP-MS whereas the Zn/Cd ratios are derived from different samples (see Table 3) that were analysed by electron microprobe. Data from the literature are for the Fule, Tianbaoshan, Dadongla and Jinding MVT deposits (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016; Zhu et al. Reference Zhu, Liao, Wang, Zhang, Yang, Fan and Wen2018), Jinding MVT deposit (Li et al. Reference Li, Liu, Xue and Li2019), Gacun and Xiaobaliang VMS deposits (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016; Yang et al. Reference Yang, Song, Wen, Zhang, Fan, Wang, Li, Yang, Zhou, Liao and Zhu2022) and Keyue and Zhaxikang Sedex deposits (Wang et al. Reference Wang, Zheng, Mathur and Yu2020). Abbreviations: BH – Broken Hill; BHT – Broken Hill-type; G – Gacun; Xiaobal – Xiaobaliang; Zh – Zhaxikang. Ranges of Zn/Cd ratios for sphalerite for the various deposits are shown as bar lines, which were not analysed for their Cd isotope compositions: 1 – Kanmantoo (Sedex) deposit (H. Arbon, unpub. B.Sc. Hons thesis, Univ. Adelaide, 2011); 2 – Mt Isa Cu–Pb–Zn (Sedex) deposit (Cave et al. Reference Cave, Lilly and Barovich2020); 3 – Gamsberg Zn (Sedex) deposit (Höhn et al. Reference Höhn, Frimmel and Westley2021); 4 – Bukit Botol (VMS) deposit, massive sulfides (Basori et al. Reference Basori, Gilbert, Zaw and Large2021); 5 – Bukit Botol (VMS) deposit, vein sulfides (Basori et al. Reference Basori, Gilbert, Zaw and Large2021); 6 – Bankshapa VMS deposit (Mishra et al. Reference Mishra, Patia, Dora, Baswani, Meshram, Shareef, Pattanayak, Suryavanshi, Mishra and Raza2021); 7 – Jangaldheri VMS deposit (Mishra et al. Reference Mishra, Patia, Dora, Baswani, Meshram, Shareef, Pattanayak, Suryavanshi, Mishra and Raza2021); 8 – Biskan VMS deposit (Mishra et al. Reference Mishra, Patia, Dora, Baswani, Meshram, Shareef, Pattanayak, Suryavanshi, Mishra and Raza2021).
Table 4. Cd concentrations and Zn/Cd ratios of sphalerite in MVT, VMS and Sedex deposits

* below detection limits (0.11 wt % Cd) were not included.
† below detection limits (0.05 wt % Cd) were not included.
Since the Broken Hill deposit has been regarded as a Sedex deposit by, for example, Sangster (Reference Sangster2020), the focus here is to see if there are differences in these concentrations and ratios between sphalerite in Sedex and VMS deposits. Table 3 lists the Cd concentrations and Zn/Cd ratios of sphalerite in the Broken Hill area that were analysed by electron microprobe. The highest concentrations of Cd are generally associated with the 3 lens and Lead lode (i.e. undifferentiated 2 and 3 lenses), which is consistent with the findings of Both (Reference Both1973) who determined the trace-element compositions of sphalerite concentrate in the Broken Hill orebodies. Although the Cd concentrations overlap in the current study for the various orebodies, Both (Reference Both1973) found a decline in Cd content of sphalerite from 3 lens to A lode. This was not observed in the current study but is likely a result of the fewer number of samples analysed here. Given that Broken Hill and minor BHT deposits occur in metasedimentary rocks spatially associated with meta-igneous rocks, it is not surprising that both sets of data for these deposits have Cd isotope compositions and Zn/Cd ratios that overlap the compositions of both Sedex and VMS deposits (Figs 7, 8). The only magmatic–hydrothermal deposit for which there are Cd isotope compositions and Zn/Cd ratio data is the Shagou deposit, China, which has Zn/Cd ratios of 154–191 and values of δ114Cd = −0.05 to 0 ‰. These values overlap those for MVT deposits with the Zn/Cd ratios being lower than the range observed for the Broken Hill and the minor BHT deposits. Although the number of data are limited, the range of values obtained by Wen et al. (Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) cannot be used to support a magmatic–hydrothermal model for the Broken Hill and minor BHT deposits.
5.c. Cd, Zn and S isotopes and the origin of Broken Hill and minor BHT deposits
By incorporating data from the present study with those of Lawrence & Rafter (Reference Lawrence and Rafter1962), Stanton & Rafter (Reference Stanton and Rafter1966, Reference Stanton and Rafter1967), Both & Smith (Reference Both and Smith1975), Spry (Reference Spry1987), Parr (Reference Parr1992, Reference Parr1994 a) and Huston et al. (Reference Huston, Power, Gemmell and Large1995), sulfur isotope compositions of sulfides in the Broken Hill and minor BHT deposits show ranges of δ34S = −3.3 to +6.7 ‰ and −5.1 to +5.4 ‰, respectively. Plimer (Reference Plimer1985), in recognizing that the sulfur isotope compositions were centred around 0 ‰, proposed a single primordial source of sulfur, while Parr (Reference Parr1992) suggested that the values near 0 ‰ were the result of sulfide formation from a modified magmatic–hydrothermal source of sulfur in which hydrothermal fluids mixed with reduced sulfur source or that magmatic sulfur was oxidized. The scenarios proposed by Plimer (Reference Plimer1985) and Parr (Reference Parr1992) are supportive of a magmatic source associated with the magmatic–hydrothermal model of Crawford & Maas (Reference Crawford and Maas2009).
Alternatively, Spry (Reference Spry1987) suggested an inorganic source of sulfur in which thermochemical considerations at a T of ∼350 °C show that the range of isotopic compositions observed for Broken Hill and the minor BHT deposits occur along the pyrrhotite–magnetite join, which is the dominant assemblage in the system Fe–S–O in the Broken Hill district, although rare primary pyrite is also present (e.g. Parr, Reference Parr1994 b). A log fO2–pH diagram incorporates the current S isotope data along with those of previously published S isotope data (Fig. 9). The temperature used here is higher than that proposed by Large et al. (Reference Large, Bodon, Davidson, Cooke, Pongratz and Davidson1996) who suggested that BHT deposits were derived from slightly acid or near neutral, high salinity fluids between 100 and 250 °C. The upper temperature limit was largely based on solubility constraints of chalcopyrite. However, it should be emphasized that minor amounts of chalcopyrite are present throughout the deposit but its paucity may simply be due to the limited amount of Cu in the source rocks. Regardless, if thermochemical sulfate reduction (TSR) is assumed, it is not possible to obtain the observed range of isotopic compositions along the pyrrhotite–magnetite join for geologically reasonable values of ionic concentrations, pH and δ34SΣS for the ore fluid at the temperatures proposed by Large et al. (Reference Large, Bodon, Davidson, Cooke, Pongratz and Davidson1996). While the range of sulfur isotope values can be explained by TSR, the range in isotope data can also be accounted for by reduced sulfur produced by TSR that is mixed with magmatic sulfur or that sulfate from seawater was reduced by biogenic processes at low temperatures (Spry, Reference Spry1987). It should be noted that Both & Smith (Reference Both and Smith1975) suggested that sulfur isotopic differences among BHT deposits are due to differences in the relative proportion of biogenic sulfur contributed to each deposit.

Fig. 9. A logfO2–pH diagram for BHT mineralization. Sulfur isotope contours for sphalerite are drawn for δ34S = +4 ‰ and T = 350 °C. Minerals in the system Fe–S–O are shown for ΣS = 0.1 moles/kg H2O as red dashed lines. The shaded region shows the approximate range of conditions for δ34S of sphalerite over fO2–pH range indicated (primarily along the pyrrhotite–magnetite join). Note that the shaded area starts at the pyrite–magnetite–pyrrhotite triple point to accommodate the rare presence of primary pyrite. Modified after Ohmoto (Reference Ohmoto1972).
In an attempt to further evaluate ore-forming processes that may be gleaned from the sulfur isotopes obtained here, we plotted δ34S versus δ114Cd (Fig. 10) and δ66Zn versus δ34S (Fig. 11). However, these isotope pairs involving S show no systematic variation suggesting that Zn, Cd and S were decoupled from each other. This is further demonstrated by the lack of correlation between δ66Zn and δ114Cd (Fig. 12). Two exceptions exist for one sample (Z3590 15.6 m) from A lode at Broken Hill and one from 11:30. The former shows the highest Zn (δ66Zn = +0.46 ‰) and S (δ34S = +4.47 ‰) isotope compositions in the deposit, while sample JB-10-46 from 11:30 shows the highest Cd (δ114Cd = 2.59 ‰) and lowest S (δ34S = −5.11 ‰) isotope values for the samples analysed here.

Fig. 10. A plot of δ34S versus δ114Cd for sphalerite from the Broken Hill deposit and minor BHT deposits.

Fig. 11. A plot of δ66Zn versus δ34S for sphalerite from the Broken Hill deposit and minor BHT deposits.

Fig. 12. A plot of δ66Zn versus δ114Cd for sphalerite from the Broken Hill deposit and minor BHT deposits.
The range of δ66Zn for sphalerite from the Broken Hill deposit is among the largest (1.61 ‰) yet reported, being exceeded only by sphalerite from the Yuhuang-1 hydrothermal field (1.67 ‰; Liao et al. Reference Liao, Tai, Zhu, Li, Li, Liang, Yang and Wang2019). Fourteen of the 18 samples of sphalerite samples from Broken Hill have values of δ66Zn that range from −0.39 to +0.46 ‰, which overlap most Zn isotope compositions reported from previous studies of MVT, Sedex and VMS deposits (Fig. 13), as well as the compositions of most igneous and sedimentary rocks (e.g. Maréchal et al. Reference Maréchal, Emmanuel, Chantal and Francis2000; Toutain et al. Reference Toutain, Sonke, Munoz, Nonell, Polvé, Viers, Freydier, Sortino, Joron and Sumarti2008; Telus et al. Reference Telus, Dauphas, Moynier, Tissot, Teng, Nabelek, Craddock and Groat2012). A question remains as to why the remaining four Zn isotope isotopic compositions, two from Broken Hill and two from the Esmeralda deposit, yield the very negative values of δ66Zn between −1.15 and −0.76 ‰.

Fig. 13. Histogram of δ66ZnAA-ETH, compositions of sphalerite from the Broken Hill deposit and minor Broken Hill-type deposits. Also shown as bar lines are the ranges of δ66Zn for sphalerite from the Dongshengmiao (Gao et al. Reference Gao, Zhu, Sun, Luo, Bao, Tang and Ma2018), Gamsberg (S. E. Foulkes, unpub. M.Sc. thesis, Rhodes Univ., 2014), Keyue (Wang et al. Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021), Red Dog (Kelley et al. Reference Kelley, Wilkinson, Chapman, Crowther and Weiss2009) and Zhaxikang (Wang et al. Reference Wang, Zheng, Mathur and Wu2018, Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021) Sedex deposits; the Alexandrinka (Gao et al Reference Gao, Zhu, Sun, Luo, Bao, Tang and Ma2018) and Xiaobaliang (Yang et al. Reference Yang, Song, Wen, Zhang, Fan, Wang, Li, Yang, Zhou, Liao and Zhu2022) VMS deposits; the Cantabria (Pašava et al. Reference Pašava, Tornos and Chrastný2014), Cévennes (Albarède, Reference Albarède2004), Jinding (Deng et al. Reference Deng, Wang, Bagas, Selvaraja, Jeon, Wu and Yang2017; Li et al. Reference Li, Liu, Xue and Li2019), Maoping (Wu et al. Reference Wu, Huang, He, Yang, Fan, Wei, Ye, Hu, Xiang and Lai2021) and Wuishe (Zhu et al. Reference Zhu, Liao, Wang, Zhang, Yang, Fan and Wen2018) MVT deposits; Irish-type deposits including Navan (Wilkinson et al. Reference Wilkinson, Weiss, Mason and Coles2005; Gangevin et al. Reference Gagnevin, Boyce, Barrie, Menuge and Blakeman2012) and the Balmat (Matt et al., Reference Matt, Powell, Mathur and deLorraine2020), Banbanqiao, Tianqiao (Zhou et al. Reference Zhou, Huang, Zhou, Zhu and Muchez2014 b), Daliangzi, Fusheng, Jinshachang, Mazou, Tianbaoshan (Xu et al. Reference Xu, Zhong, Hu, Wen, Zhu, Bai, Fan, Li and Zhou2020) and Shanshulin (Zhou et al. Reference Zhou, Huang, Lv, Zhu, Gao and Mirnejad2014 a) carbonate-hosted Pb–Zn or Zn deposits. Note that the anomalous value of sample of δ66Zn = +1.05 ‰ for late-stage sphalerite that was obtained by Wilkinson et al. (Reference Wilkinson, Weiss, Mason and Coles2005) from the Galmoy Irish-type Zn–Pb deposit is not shown on this figure. The reader can view the individual data points for most of the deposits shown here in Wang et al. (Reference Wang, Zheng, Mathur and Wu2018, Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021).
Variations in Zn isotopes in a given hydrothermal orebody can result from a variety of processes including (Li et al. Reference Li, Liu, Xue and Li2019): Rayleigh fractionation (e.g. Wilkinson et al. Reference Wilkinson, Weiss, Mason and Coles2005; Kelley et al. Reference Kelley, Wilkinson, Chapman, Crowther and Weiss2009; Wang et al. Reference Wang, Zheng, Mathur and Yu2020); biological processes (Li et al. Reference Li, Liu, Xue and Li2019); equilibrium fractionation related to T (Mason et al. Reference Mason, Weiss, Chapman, Wilkinson, Tessalina, Spiro, Horstwood, Spratt and Coles2005); different Zn species in the hydrothermal fluid (Fuji et al. Reference Fuji, Moynier, Pons and Albarède2011); volatilization/evaporation/boiling (e.g. Paniello et al. Reference Paniello, Day and Moynier2012; Wang et al. Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021); and mixing of different sources of Zn (Wilkinson et al. Reference Wilkinson, Weiss, Mason and Coles2005). Although the effects of metamorphism on the fractionation of Zn isotope compositions in natural systems have received limited attention, Xu et al. (Reference Xu, Liu and Li2021) showed that basalts metamorphosed to the greenschist, amphibolite and eclogite facies showed no detectable fractionation. This contrasts with the observations of Pons et al. (Reference Pons, Debret, Bouihol, Delacour and Williams2016) who showed that small isotopic variations (up to 0.16 ‰) occur in subducted Alpine serpentinites that were metamorphosed from greenschist to blueschist through to the eclogite facies. They ascribed the decrease in δ66Zn to the release of oxidized Zn sulfate-rich fluids to the mantle wedge. Regardless, metamorphism to high grades would appear to only produce a very small amount (i.e. <0.2 ‰) of fractionation. Relatively small isotopic ranges were reported by S. E. Foulkes (unpub. M.Sc. thesis, Rhodes Univ., 2014) for sphalerite from the Gamsberg (δ66Zn = −0.22 to −0.08 ‰, n = 7) and by Matt et al., (Reference Matt, Powell, Mathur and deLorraine2020) for sphalerite from the Balmat deposits (δ66Zn = −0.30 to 0.28, n = 47) that were both metamorphosed to the amphibolite facies. This further supports the idea that the metamorphism does not modify the original Zn isotopes in the Broken Hill district and is not the cause for the wide isotopic range in the Broken Hill district. Owing to the intense deformation at Balmat, Matt et al., (Reference Matt, Powell, Mathur and deLorraine2020) showed there was a δ66Zn fraction of up to 0.4 ‰ down the length of some ore bodies. They ascribed this to syntectonic isotopic fractionation that resulted from the interaction between the ore and sulfide melts that were fluxed by H2S. Peak metamorphic conditions at Balmat reached ∼640 °C and 6.5 kbar. However, given that sulfide in the Balmat deposit primarily consists of sphalerite with only minor to trace amounts of other sulfides/sulfosalts (e.g. arsenopyrite, bournonite, tetrahedrite, galena, pyrite, tennantite, chalcopyrite, jordanite, realgar; P. Matt, unpub. MS thesis, City Univ. New York, 2019) to potentially lower the melting point of a sulfide mix, there is some question regarding whether or not there was sufficient quantity of these minerals to lower the melting point to the metamorphic conditions reached at Balmat given the high melting point of sphalerite (i.e. 1827 °C). Regardless of whether or not there was a sulfide melt, or whether the light Zn isotopes fractionate due to deformation in a fluid-bearing or fluid-free environment remains uncertain. However, a similar question was raised by Spry et al. (Reference Spry, Plimer and Teale2008) regarding whether a partial sulfide melt was produced at Broken Hill and whether or not it was possible to produce the Pb lode ore bodies as a result of sulfide migration from the restite Zn lodes as proposed by Mavrogenes et al. (Reference Mavrogenes, MacIntosh and Ellis2001). Despite these uncertainties, fractionation of S isotopes in veins due to deformation was reported by Spry (Reference Spry1987) at Broken Hill and it may be the same mechanism that is responsible for some of the light Zn isotopes in the Broken Hill district where isoclinal folding exists for the first two phases of deformation that affected the Broken Hill deposit and which caused the migration of sulfides into fold hinges (e.g. Laing et al. Reference Laing, Marjoribanks and Rutland1978; Parr & Plimer, Reference Parr, Plimer, Kirkham, Sinclair, Thorpe and Duke1993).
Mechanisms involving Rayleigh fractionation may not produce the large fractionation in Zn isotopes observed in the Broken Hill and the minor BHT deposits, although it may account for the Zn isotopic compositions >0 ‰ at Broken Hill. Nonetheless, ab initio calculations by Fuji et al. (Reference Fuji, Moynier, Pons and Albarède2011) show that negative values of δ66Zn of up to 0.6 ‰ can occur in sulfides in high pH fluids (likely associated with carbonates) at low temperatures but a considerably smaller fractionation occurs under neutral to acidic fluids at higher temperatures. Regardless, Rayleigh fractionation is inconsistent with the isotopic compositions in the Esmeralda deposit and two samples from Broken Hill that have values of δ66Zn < −0.7 ‰ since they are hosted in clastic metasedimentary rocks rather than marbles (although carbonates are relatively common in 2 lens at Broken Hill). It is, therefore, unlikely that high pH ore fluids were associated with the formation of deposits in the Broken Hill district (including Broken Hill) and cannot account for the observed wide isotopic range.
The largest Zn isotopic variations in the solar system are associated with devolatilization processes related to the formation of terrestrial bodies where variations of several per mil have been reported (e.g. Luck et al. Reference Luck, Othman and Albarède2005; Creech & Moynier Reference Creech and Moynier2019). Wang et al. (Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021), in evaluating the Zn isotopic compositions of sphalerite in the Keyue and Zhaxikang Sedex deposits, showed that vapour–liquid–solid partitioning from hydrothermal fluids would result in lighter Zn and Cd isotopes in the vapour and heavier Zn and Cd isotopes in the solid phase (i.e. sphalerite). Given the possibility that the Broken Hill and minor BHT deposits may have formed from magmatic–hydrothermal fluids (i.e. possibly in the range of 400–700 °C, see Williams-Jones & Heinrich, Reference Williams-Jones and Heinrich2005) rather than the lower temperature fluids (i.e. <350 °C) associated with the previously discussed syngenetic model, then a vapour phase may have been generated. However, Wang et al. (Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021) also showed that when the fraction of the initial Zn and Cd partitioned into the volatile phase is extremely high (i.e. >0.8), the resultant sphalerite precipitated from the vapour can produce very light isotopic values. This scenario could conceivably account for the very negative Zn isotopes observed in sphalerite from the Esmeralda deposit and two samples from the Broken Hill deposit even though such high partitioning of metals such as Zn and Pb into the vapour seems far-fetched. Such an extraordinarily high value is unlikely to produce the enormous amount of sphalerite in the supergiant deposit. Therefore, vaporization of the ore-forming fluid potentially associated with the magmatic–hydrothermal model does not appear to be a likely scenario to account for compositions of the Zn or Cd isotopes observed in the Broken Hill district.
Fractionation of Cd isotopes can be large with extreme values of between ∼ −8 and +16 ‰ being reported for meteorites as a result of condensation and evaporation processes (e.g. Wohmbacher et al. Reference Wohmbacher, Rehkämper, Mezger and Münker2003, Reference Wohmbacher, Rehkämper and Mezger2004, Reference Wohmbacher, Rehkämper, Mezger, Bischoff and Münker2008). Previous studies of hydrothermal ore deposits show ranges of −0.74 to +1.01 ‰ in sphalerite based on studies of the Zhaxikang VMS and Fule MVT deposits (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016; Wang et al. Reference Wang, Zheng, Mathur and Yu2020). Cadmium isotope compositions of igneous and sedimentary rocks are essentially indistinguishable with δ114Cd values generally around 0 ± 0.2 ‰ (e.g. Wohmbacher et al. Reference Wohmbacher, Rehkämper, Mezger and Münker2003; Schmitt et al. Reference Schmitt, Galer and Abouchami2009; Liu et al. Reference Liu, Zhang, Zhang, Zhang, Huang and Yu2019). Values of δ114Cd for sphalerite from Broken Hill and smaller BHT deposits range from −0.48 to +0.01 ‰ for the Broken Hill deposit and from δ114Cd = −1.02 to 2.59 ‰ for the smaller BHT deposits (Fig. 14). Although values of δ114Cd for sphalerite from Broken Hill overlap those of igneous and sedimentary rocks, 10 of the 17 samples analysed range from δ114Cd = −0.48 to −0.23 ‰ suggesting that some mechanism other than hydrothermal processes, where Cd was extracted from the meta-igneous and metasedimentary package associated with the Zn–Pb deposits in the Broken Hill district, was responsible. In a Cd, S and Zn isotope study of the Xiaobaliang VMS deposit, Yang et al. (Reference Yang, Song, Wen, Zhang, Fan, Wang, Li, Yang, Zhou, Liao and Zhu2022) proposed that the values of δ114CdNIST-3108 values for sphalerite, which range from −0.74 to −0.08 ‰, are the result of biological processes that enrich the fluid in the light Cd isotope, resulting in the precipitation of sulfides with light values of δ114Cd. Such processes are considered here.

Fig. 14. Histogram of δ114CdNIST SRM 3108 compositions of sphalerite from the Broken Hill deposit and minor Broken Hill-type deposits. Also shown as bar lines are the ranges of δ114Cd for sphalerite from the Keyue (Wang et al. Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021), Langshan (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) and Zhaxikang (Wang et al. Reference Wang, Zheng, Mathur and Yu2020, Reference Wang, Zheng, Mathur, Qiu, Wu, Ren, Wang, Li and Yi2021) Sedex deposits; Gacun (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016) and Xiaobaliang (Yang et al. Reference Yang, Song, Wen, Zhang, Fan, Wang, Li, Yang, Zhou, Liao and Zhu2022) VMS deposits; Dadongla (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016), Fule1,2 (Zhu et al. Reference Zhu, Wen, Zhang, Fan, Fe, Xu and Qin2013 1; Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016 2), Jinding1,2 (Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016 1; Li et al. Reference Li, Liu, Xue and Li2019 2), Maoping (Wu et al. Reference Wu, Huang, He, Yang, Fan, Wei, Ye, Hu, Xiang and Lai2021) and Niujiatong (Zhu et al. Reference Zhu, Wen, Zhang, Fan, Fe, Xu and Qin2013) MVT deposits; and Daliangzi, Fusheng, Jinshachang, Mazou, Tianbaoshan1 (Xu et al. Reference Xu, Zhong, Hu, Wen, Zhu, Bai, Fan, Li and Zhou2020), Tianbaoshan2 (Zhu et al. Reference Zhu, Wen, Zhang and Fan2016) Huize1,2 (Zhu et al. Reference Zhu, Wen, Zhang, Fan, Fe, Xu and Qin2013 2, 20211) and Shanshulin carbonate-hosted Pb–Zn or Zn deposits (Zhu et al. Reference Zhu, Wen, Zhang, Fan, Fe, Xu and Qin2013). The reader can view the individual data points for some of the deposits shown here in Li et al. (Reference Li, Liu, Xue and Li2019), although their data are reported as δ114CdSPEX.
5.d. A biological syndepositional model for the formation of the Broken Hill deposit and minor BHT deposits
Although the S isotopic compositions of sphalerite can be interpreted in the light of a magmatic–hydrothermal or a syngenetic model, where TSR occurs at a temperature of around 350 °C, neither model can account for the wide range of Zn and Cd isotope compositions of sphalerite. Microbially mediated dissimilatory sulfate reduction to H2S produces isotopically light H2S with Δ34SSO4-H2S up to 72 ‰ (e.g. Canfield & Teske, Reference Canfield and Teske1996; Balci et al. Reference Balci, Shanks, Mayer and Mandernack2007). However, biological processes are also likely to be important for Zn and Cd (e.g. Li et al. Reference Li, Liu, Xue and Li2019). Although fractionation factors associated with biological processes are not large (1.0002 to 1.0008; Abouchami et al. Reference Abouchami, Galer, Horner, Rehkämper, Wombacher, Xue, Lambelet, Gault-Ringold, Stirling, Schönbächler, Shiel, Weis and Holdship2013), biological partial assimilation of Cd from seawater can generate a range of δ114Cd of 7 ‰ in surface waters as a result of the uptake of dissolved Cd by photosynthesis (e.g. Lacan et al. Reference Lacan, Francois, Ji and Sherrell2006; Ripperger et al. Reference Ripperger, Rehkämper, Porcelli and Halliday2007; Schmitt et al. Reference Schmitt, Galer and Abouchami2009; Wen et al. Reference Wen, Zhu, Zhang, Cloquet, Fan and Fu2016), while a range of δ66Zn of up to ∼0.7 ‰ occurs as a result of biological processes (e.g. Conway & John, Reference Conway and John2014; John & Conway Reference John and Conway2014; Zhao et al. Reference Zhao, Vance, Abouchami and de Baar2014) (Fig. 15). Theoretical calculations and experimental studies of Fuji et al. (Reference Fuji, Moynier, Pons and Albarède2011) and Marković et al. (Reference Marković, Manzoor, Humphreys-Williams, Kirk, Vilar and Weiss2017), respectively, show that organic compounds (e.g. Zn-carboxylate) generally have heavier isotopic compositions than Zn2+, which can result in sphalerite having very low δ66Zn values in a low T solution (i.e. <100°C). Li et al. (Reference Li, Liu, Xue and Li2019) suggested that Cd, like Zn, may have bonded to carboxylate molecules in hydrothermal solutions resulting in light isotopic values for sphalerite.

Fig. 15. Schematic plot showing the fractionation of Zn, Cd and S isotopes as a result of biogenic sulfate reduction (BSR) and its relationship to isotopic values reported for these elements for sulfides from the Broken Hill deposit and minor BHT deposits. For the sulfur isotope fractionation, we include the amount of fractionation caused by bacterial sulfate reduction for δ34S of up to ∼70 ‰ reported by Canfield & Teske (Reference Canfield and Teske1996) and Lefticariu et al. (Reference Lefticariu, Behum, Bender and Lefticariu2017) and a sulfur isotope value of +20 ‰ of Strauss (Reference Strauss, Amend, Edwards and Lyons2004) for Proterozoic seawater. A value of δ66Zn = +0.5 ‰ for deep seawater (John & Conway Reference John and Conway2014; Zhao et al. Reference Zhao, Vance, Abouchami and de Baar2014) with preferential uptake of light isotopes during biological assimilation by as much as −1.3 ‰ (i.e. the sum of values for biological assimilation (up to 0.7 ‰) and scavenging/adsorption (−0.3 to −0.6 ‰) (Conway & John Reference Conway and John2014; John & Conway Reference John and Conway2014; Li et al. Reference Li, Liu, Xue and Li2019) and scavenging/adsorption of Zn on phytoplankton membranes causing an additional isotopic fraction of δ66Zn of −0.3 to −0.6 ‰. A value of δ114Cd of +0.3 ‰ is given for deep seawater (e.g. Chen et al. Reference Chen, Little, Kreissig, Severmann and McManus2021). Biological fractionation causes phytoplankton to have values of δ114Cd lower by 0.2 to 0.8 ‰.
However, two features of the Zn and Cd isotope ranges for sphalerite from Broken Hill need to be addressed: why there is no apparent linear relationship between Zn and Cd isotopic compositions (see Fig. 12) and why the range of isotopic values for Cd is smaller than that for Zn isotopes. Cadmium and Zn behave differently with regards to biological productivity. While Zn can be adsorbed and assimilated by phytoplankton, the scavenging of isotopically heavy Zn onto biological particles, which sink through the water column, leave the remaining fluid characterized by lighter Zn isotope compositions (John et al. Reference John, Kunzmann, Townsend and Rosenberg2017). Scavenging/adsorption can lower the Zn isotope compositions by −0.3 to −0.6 ‰ (Li et al. Reference Li, Liu, Xue and Li2019). Therefore, the sum of values related to preferential uptake of light isotopes during biological processes is as much as −1.3 ‰ (i.e. the sum of values for biological assimilation (up to 0.7 ‰; Conway & John, Reference Conway and John2014; John & Conway, Reference John and Conway2014) and scavenging/adsorption (−0.3 to −0.6 ‰; Li et al. Reference Li, Liu, Xue and Li2019)). On the other hand, Cd is not scavenged by biological particles but is controlled by biological assimilation as it substitutes for P. If Zn in the Broken Hill area was released from organic material during decay to form sphalerite this process could result in the light isotopic composition of both Zn and Cd. Alternately, sphalerite formation from the residual seawater would not account for the Cd isotope composition because surface seawater trends to high values of δ114Cd.
A similar explanation for the small range in Cd isotopes compared to Zn isotopes, a feature observed in sphalerite from the Broken Hill area, was proposed by John et al. (Reference John, Kunzmann, Townsend and Rosenberg2017) for the Neoproterozoic dolostones from the Nuccaleena Formation, South Australia. In the Nuccaleena dolostone, Cd was buried in biological material (i.e. phytoplankton) to produce light Cd isotope compositions, while scavenging of heavy Zn left surface seawater with light Zn isotope compositions. Organic matter formed in surface seawater when buried will produce a larger isotopic range for Zn than Cd.
A mechanism involving Zn and Cd being bonded to organic molecules best accounts for the light Zn and Cd isotopic compositions in the Broken Hill district, including the light isotopic values from the Flying Doctor (δ114Cd = −0.77 ‰) and Henry George (δ114Cd = −1.02 ‰). This scenario is analogous to biogenic processes proposed by Li et al. (Reference Li, Liu, Xue and Li2019) to explain similar Zn, Cd and S isotope compositions for sulfides from the giant Jinding MVT deposit, China, and by Yang et al. (Reference Yang, Song, Wen, Zhang, Fan, Wang, Li, Yang, Zhou, Liao and Zhu2022) to account for the isotopically light Cd and S isotope values of sphalerite in the Xiaobaliang Cu–Au VMS deposit, China. Fractionation by biogenic sulfate reduction (BSR) is consistent with the process proposed by Both & Smith (Reference Both and Smith1975) to explain the differences in S isotopes in the district. To this end, Heimann et al. (Reference Heimann, Spry, Teale, Leyh, Conor, Mora and O’Brien2013) also reported C and O isotopes values in calcite from the Esmeralda and Broken Hill deposits (the two deposits that show the most negative values of δ66Zn; Table 2), which range from −25 to −21 ‰ for δ13CVPDB and +10 to +11.0 ‰ for δ18OSMOW, respectively. The low carbon isotope values also overlap (δ13CVPDB = −26 to −14 ‰) for graphite in graphitic schists in the southern Curnamona province (including the Broken Hill deposit) and calcite at the RW Iron Clad and Little Broken Hill BHT prospects (M. Schuler et al., The Broken Hill Line of Lode Study, unpub. report to Pasminco Mining Company, 1993; Bierlein et al. Reference Bierlein, Ashley and Seccombe1996). Biogenic processes occur at low temperatures (i.e. <100 °C). Notwithstanding the proposal here that biological processes are important in producing the Zn, Cd and S isotopic compositions reported for sphalerite in the Broken Hill district, the cause of the outlier value of δ114Cd = +2.59 ‰, coupled with the isotopically lightest value of δ34S of −5.11 ‰, for the samples studied here from the small 11:30 deposit remains unknown. However, these anomalous isotopic values may be due to kinetic and/or equilibrium effects similar to those responsible for the isotopically anomalous value of δ66Zn > +1 ‰ reported by John et al. (Reference John, Rouxel, Craddock, Engwall and Boyle2008) for sulfides in active hydrothermal vents on the seafloor.
6. Conclusions
Geological and geochemical considerations suggest that the Broken Hill deposit as well as minor BHT deposits likely formed by either syngenetic processes at T <350 °C or from magmatic–hydrothermal fluids at a T of between 400 and 700 °C. Although S isotope studies are compatible with either process, Cd and Zn isotope studies are incompatible with high T processes because both the lighter isotopes for both isotopic systems will fractionate into the vapour phase leaving sphalerite exhibiting heavy isotopic compositions. Even though the S, Cd and Zn isotope values show no correlation with each other, suggesting they were decoupled, the isotopic ranges are commensurate with fractionation being caused by low-temperature biogenic processes.
The Zn and Cd isotope variations for sphalerite from the Broken Hill deposit and minor BHT deposits are among the largest yet reported. Although biogenic processes appear to be the most likely explanation for the isotopically light values reported for both isotopes, fractionation caused by mechanical processes whereby the lighter isotope Zn and Cd isotopes migrate more easily in a fluid assisted or depleted system is uncertain, but this remains a possibility given the extreme deformation that resulted in two isoclinal fold episodes at Broken Hill and the migration of sulfides into fold hinges.
Syngenetic scenarios for the Broken Hill deposit have previously considered it being a VMS or Sedex deposit. Although Cd isotopes have been combined with the Zn/Cd isotope ratio of sphalerite to classify Pb–Zn deposits in the past and may have helped in distinguishing these two deposit models, such an exercise is fraught with problems. Although MVT deposits tend to have lower Zn/Cd ratios in sphalerite than those formed by high-temperature (e.g. VMS) and exhalative (Sedex) systems, the Zn/Cd ratios for VMS and Sedex deposits overlap and cannot be used to distinguish between these deposit types. Sphalerite from the Broken Hill and BHT deposits have average Zn/Cd ratios for sphalerite that range from 203 to 303 and fit within the overlap region for Sedex and VMS deposits. This is hardly surprising given the spatial association of sulfide mineralization with bimodal mafic and felsic igneous rocks within a thick package of metasedimentary rocks.
Acknowledgements
Funding for the electron microprobe facility used in this research was provided by the National Science Foundation under award number EAR-1625422. Dan-Layton Matthews and Evelyne Leduc Queen’s Facility for Isotope Research (Queen’s University, Canada) are kindly thanked for providing sulfur isotope standards, while Ben Johnson (ISU) helped with S isotope analyses. Assistance with drafting by Justin Glenn is gratefully appreciated. The reviews of Peter Matt and Da Wang are greatly appreciated and improved the quality of the manuscript.





















