INTRODUCTION
The incidence of fungal infection has increased in recent years, particularly in immunocompromised patients and those in intensive care units (ICUs) [Reference Banerjee1–Reference Silva, Diaz and Febre3]. Fungi are frequently detected on culture of airway secretions, urine, wound and blood in long-stay ICU patients with Candida spp. being the most prevalent [Reference Petri4]; In the hospital setting Aspergillus and Cryptococcus spp. are often associated with bloodstream infections following organ transplantation [Reference Silva, Diaz and Febre3, Reference Singh5, Reference Patterson6]. The presence of Aspergillus in ICU patients is indicative of a poor clinical outcome [Reference Khasawneh7, Reference Vandewoude8] while Candida in the blood stream has been associated with significantly higher hospital mortality regardless of immunocompetence of the patient [Reference Dimopoulos9, Reference Singh10]. Thus the diagnosis of fungal infection may be an important prognostic factor in ICU patients.
Cryptococcus neoformans is an encapsulated yeast that rarely infects individuals with normal immune function, but is often found in HIV infection. Besides HIV, cryptococcal infection has been asssociated with other underlying disorders including haematological malignancy, collagen vascular disease, cancer, solid organ transplantation, immunosuppressive drug use, and diabetes mellitus [Reference Pappas11–Reference Vilchez13]. Nevertheless, 22% of non-HIV patients with cryptococcosis had no apparent immune suppression [Reference Pappas11]. The most common infections are meningitis and pneumonia but any organ system can be affected [Reference Jean14, Reference Perfect15] and sepsis and septic shock are not uncommon in cryptococcaemia [Reference Jean14]. Cryptococcal infection is associated with a high mortality rate regardless of the presence of HIV infection [Reference Jongwutiwes, Sungkanuparph and Kiertiburanakul16], but older age, previous ICU admission and mechanical ventilation have been cited as prognostic factors in HIV-infected patients with cryptococcosis [Reference Darras-Joly17]. There are few reports in the literature on clinical characteristics, outcome and prognostic factors of patients with cryptococcosis in ICUs and hence these factors were addressed in this study.
METHODS
Setting
The study was performed at National Taiwan University Hospital, a 2200-bed teaching hospital with active programmes for a wide range of surgical transplantation. The medical and microbiological records of all patients with a diagnosis of cryptococcal infection from January 2000 to December 2005 were reviewed.
Microbiology
Isolates were identified as C. neoformans by conventional methods, including characteristic morphotypes on India ink staining, positive reaction on caffeic acid and compatible biochemical reactions generated by API 20C and Vitek YBC systems (bioMérieux-Vitek, USA) [Reference Hazen, Howell, Murray, Baron, Jorgensen, Pfaller and Yolken18]. l-canavanine-glycine-Bromothymol Blue (CGB) agar and genotyping by PCR fingerprinting and URA5 gene RFLP analysis were used to differentiate among varieties of C. neoformans [Reference Hazen, Howell, Murray, Baron, Jorgensen, Pfaller and Yolken18, Reference Meyer19].
Patient selection and data collection
The inclusion criterion for the study was that patients had cryptococcal infection established by microbiological study from any specimen during an ICU stay. The data collected were: age, gender, predisposing factors, current immunosuppressant treatment, clinical symptoms, laboratory data at ICU admission, duration of hospitalization and ICU stay, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores and Multiple Organ Dysfunction (MOD) scores during ICU admission; treatment regimen, complications and outcome. The date of diagnosis of cryptococcal infection was defined as the date of collection of the specimen that yielded a positive culture. If the diagnosis date was later than the first day of ICU stay, cryptococcal infection was classified as being diagnosed after ICU admission.
Statistical analysis
Data were recorded as median or mean (range) for continuous variables, or as a percentage of the group from which they were derived for categorical variables. Differences in variables between 14-day ICU survivors and non-survivors were analysed by χ2 test or Fisher's exact test as necessary. Further multivariate analysis was performed by logistic regression. A univariate model was used to evaluate the hazard ratio of patient characteristics and hospital prognosis individually. A Cox regression model was used to determine the independent prognostic factors for hospital survival. All statistical analyses were performed using SPSS software, version 13 (SPSS Inc., USA). A value of P⩽0·05 was considered statistically significant.
RESULTS
Epidemiological data and underlying diseases/conditions
During the study period, there were 31601 ICU admissions and cryptococcal infection was diagnosed in 30 patients. Four patients were excluded from the study because they did not have a positive Cryptococcus culture during ICU stay. The remaining 26 patients (8·2/10 000 ICU admissions), comprising 16 males and 10 females, were included in the study. Of these, six patients were admitted to surgical ICUs and the other 20 were admitted to medical ICUs. The median age of the overall group was 58 years (range 24–87 years). The ICU admission indications are summarized in Table 1 and included respiratory failure (16/26, 61·5%), septic shock (8/26, 28·6%) and acute neurological change (6/26, 21·4%). Underlying conditions associated with immunocompromised status included liver cirrhosis (10/26, 38·5%), diabetes mellitus (7/26, 26·9%), HIV infection (5/26, 19·2%) and rheumatic disease (4/26, 15·4%). Ten (38·5%) patients received immunosuppressant therapy before ICU admission. Table 2 shows the underlying conditions and events during ICU stay. Two (7·7%) patients had no evidence of immunocompromised status before or after ICU admission and both of these had cryptococcal infection in the airways only.
Table 1. Indication for ICU admission in patients with cryptococcal infection diagnosed during ICU stay
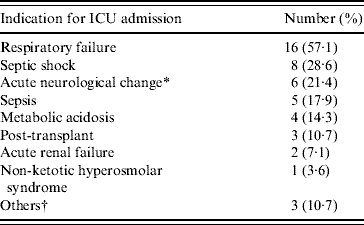
ICU, Intensive care unit.
* There were four patients with acute conscious change and two patients with increased intracranial pressure.
† Included suspected pulmonary embolism, post-resuscitation, and pulmonary oedema.
Table 2. The clinical characteristics of 2-week ICU survivors and non-survivors with cryptococcal infection
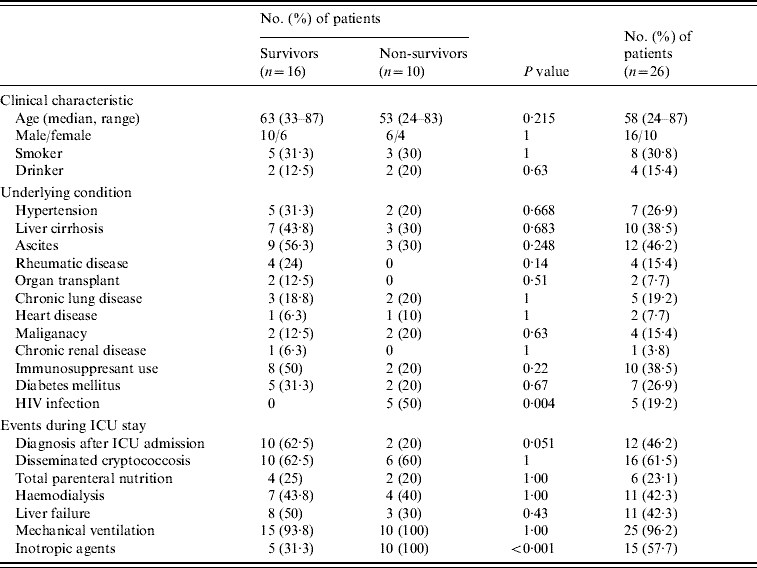
ICU, Intensive care unit.
All cryptococcal isolates tested negative on the CBG plate and exhibited var. neoformans I pattern which was consistent with the identification as serotype A (C. neoformans var. grubii [Reference Hazen, Howell, Murray, Baron, Jorgensen, Pfaller and Yolken18, Reference Meyer19]). Body sites most frequently positive for C. neoformans were blood (16/26, 61·5%), cerebrospinal fluid (10/26, 38·5%), airways (8/26, 34·6%) and pleural effusion (5/26, 19·2%) (Table 3). Six patients had a positive sputum culture and three patients yielded C. neoformans from broncheoaveolar lavage; one patient had a positive culture from both of these specimen types. Time to diagnosis of Cryptococcus identification ranged from 1–76 days after ICU admission. The patient identified at 76 days was admitted after a liver transplant operation and died before any antifungal treatment. Four patients had a diagnosis of cryptococcosis after 7 days of ICU stay.
Table 3. Identified sites of cryptococcal infection by clinical samples of 2-week ICU survivors and non-survivors
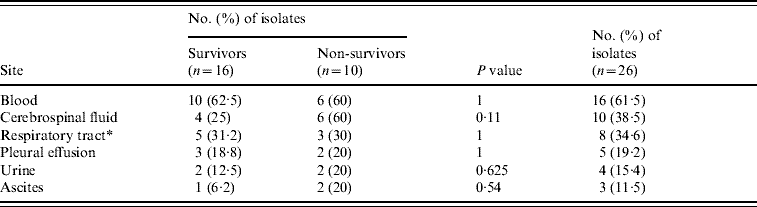
* Including six from sputum, three from broncheoaveolar lavage and one patient with both types of specimens.
Clinical course during ICU stay
The initial characteristics of the patients admitted to the ICU are summarized in Table 4. The mean APACHE II score on ICU admission was 22·46, with predicted mortality of 44·1%. The mean MOD score was 7·38, with predicted ICU mortality of 3–5% and hospital mortality of 16%. However, the actual ICU mortality rate of this cohort was 73·1% (19/26) and the overall hospital mortality rate was 80·8% (21/26). All except one patient received mechanical ventilation during the first day of ICU stay. The median duration of ventilator use was 8 days with a range of 1–74 days. Prominent elevations of white blood cell (WBC) count (10·88×109/l, range 0·14–37·11×109/l), blood urine nitrogen level (32·8 mmol, range 8·2–123·8 mmol) and decreased albumin level (2·77 g/l, range 1·5–3·95 g/l) were observed in this study cohort. Mild elevations of total bilirubin level (1·6 μmol/l, range 0·28–27·35 μmol/l) and creatinine level (1·6 μmol/l, range 0·5–9·5 μmol/l) were also seen.
Table 4. Disease severity index and laboratory data at ICU admission and comparison between 2-week ICU survivors and non-survivors with cryptococcal infection
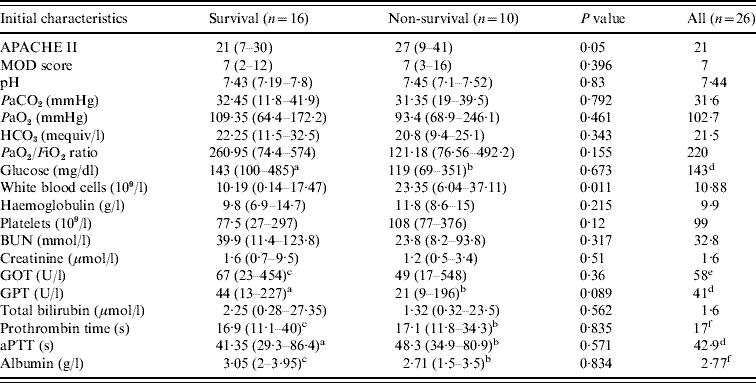
ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II scores; MOD, Multiple Organ Dysfunction; BUN, Blood urea nitrogen; GOT, aspartate aminotransferase; GPT, alanine aminotransferase; aPTT, activated partial thromboplastin time.
Data are presented as median and range.
a n=14, bn=9, cn=15, dn=23, en=25, fn=24.
Antifungal therapy
Two patients did not receive antifungal therapy due to mortality prior to identification of the pathogen and six patients were prescribed fluconazole as the only antifungal agent; the latter patients were HIV negative and three had disseminated cryptococcal infection. Five of them were alive 2 weeks after ICU admission and three died within 4 weeks of ICU admission. All six died during the study period. The other patients (18/26, 69·2%) received either amphotericin B or liposomal amphotericin B during their treatment course and 11 (61·1%) patients survived >2 weeks but use of amphotericin B-containing medication was not associated with 2-week survival or long-term survival.
Outcome and prognostic factors
Four patients died within 3 days of ICU admission (4/26, 15·4%), and 16 patients survived >2 weeks after ICU admission (61·5%). The overall ICU survival rate was only 26·9% (7/26). Five (19·2%) patients were able to be discharged from the hospital during the study period and four had long-term survival. The results of comparison of clinical features, identified sites and initial presentation between 2-week survivors and non-survivors are shown in Tables 1–4. Factors significantly associated with 2-week mortality included HIV infection (P=0·004) and use of inotropic agents on the day of ICU stay (P<0·001). Diagnosis of Cryptococcus infection before ICU admission had a borderline significant relationship to 2-week ICU prognosis (P=0·051). Increased WBC count (P=0·011) was the only laboratory parameter significantly associated with 2-week mortality. Decreased APACHE II score was significantly associated with better 2-week ICU prognosis (P=0·05).
Univariate analysis of the effects of clinical characteristics, initial ICU laboratory data and coagulatory factor levels on survival were performed; factors with a P value <0·1 are listed in Table 5. Patients with HIV infection, low WBC count during ICU stay, cryptococcosis identified before ICU admission and infection confirmed in ascites at any time during ICU stay were all significantly associated with decreased survival. Younger age, lower initial ICU albumin level and diabetes mellitus had borderline associations with long-term survival. After adjusting for the variables with P value <0·1, factors associated with hospital mortality by the Cox regression test model were patients without diabetes, HIV infection, and low WBC count during initial ICU stay and with cryptococcosis diagnosed before ICU admission (Table 6).
Table 5. Univariate analysis of factors associated with hospital survival
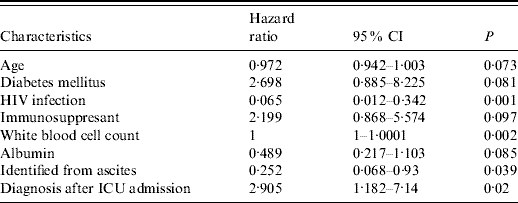
CI, Confidence interval; ICU, intensive care unit.
Table 6. Multivariate analysis of factors associated with ICU survival by Cox regression method
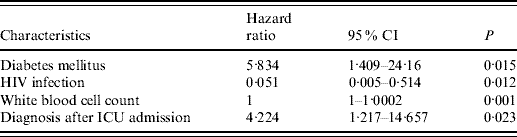
CI, Confidence interval; ICU, intensive care unit.
DISCUSSION
The aetiological agent of cryptococcosis is classified into two species; they are C. gattii (serotypes B and C) and C. neoformans, with two varieties: C. neoformans var. grubii (serotype A) and C. neoformans var. neoformans (serotype D) as well as a serotype AD hybrid [Reference Hazen, Howell, Murray, Baron, Jorgensen, Pfaller and Yolken18]. All our isolates belonged to serotype A. This is consistent with the current literature that var. grubii is the most prevalent in cryptococcal isolates worldwide [Reference Meyer19].
The most frequent underlying conditions were ascites (12/26), immunosuppressant use (10/26), and liver cirrhosis (10/26). Ascites and immunosuppressant use were related to other conditions, including liver cirrhosis, rheumatic disease and organ transplantation. The most frequent underlying immunocompromised conditions were liver cirrhosis, diabetes mellitus and HIV infection. Previous studies of cryptococcosis have highlighted liver cirrhosis as an important underlying disease [Reference Chuang20, Reference Franca21] and this work confirms its importance as an indicator of immunocompromised status in cryptococcal infection, especially in non-HIV-infected patients. The 2-week ICU survival was worse if patients had HIV infection (P=0·004). Other conditions were not related to the 2-week ICU prognosis.
Recently published guidelines have suggested treatment with amphotericin B plus flucytosine followed by consolidation therapy with fluconazole for 10–12 months in immunocompetent patients with CNS involvement or in immunocompromised HIV-negative patients [Reference Saag22]. Other studies, however, reported a good clinical response with the use of fluconazole alone, even in non-AIDS patients [Reference Yamaguchi23, Reference Kokturk24]. Combinations of agents, including terbinafine and ravuconazole with 5-fluorocytosine, were effective in vitro but remain understudied in clinical trials [Reference Zhu, Gil-Lamaignere and Muller25]. In this study, differences of antifungal regimens used for cryptococcosis were not significantly correlated with ICU survival or long-term survival. Single use of fluconazole was not related to short-term prognosis despite the fact that all patients prescribed fluconazole alone died before discharge from hospital.
In this study, the most common indications for ICU admission were respiratory failure, septic shock and acute neurological change. During ICU stay, all but one patient were mechanically ventilated and respiratory compromise was the most frequent condition of ICU patients with cryptococcal infection. Fifteen patients received inotropic agents during ICU stay and the use of these agents was related to high 2-week ICU mortality. In this study, the ICU mortality rate was marginally lower than the hospital mortality rate (73·1% vs. 80·8%). Compared to similar APACHE II scores with an estimated mortality rate of 44·1% at the time of ICU admission, the study patients had worse survival, and compared to a previous study of candidemia in the ICU [Reference Ruan26], our patients had higher death rates even for similar APACHE II scores [Reference Dimopoulos9]. Here, the mean value of the MOD scores was only 7·38 with an estimated hospital mortality rate of 16%, but our patients' mortality rate was significantly higher than this estimate. Thus, the prognosis of ICU patients with cryptococcosis was worse than that of ICU patients with similar disease severity.
Previous studies have suggested that the prognosis for C. neoformans infection is related to the progress of underlying disease and the outcome of concomitant infections [Reference Chuang20, Reference Perfect, Durack and Gallis27]. The long-term survival of cryptococcosis was not significantly different between non-HIV and HIV-infected patients [Reference Jongwutiwes, Sungkanuparph and Kiertiburanakul16] and previous ICU admission or mechanical ventilation were important prognostic factors in HIV-infected patients [Reference Darras-Joly17]. We found HIV infection status was associated with both 2-week ICU survival and long-term survival. After adjusting for other factors, HIV infection proved to be an important underlying condition and an independent risk factor for mortality. Casalino et al. [Reference Casalino28] reported that HIV-infected patients had similar ICU mortality with similar underlying conditions supporting the conclusion that HIV infection status might be an important prognostic factor in ICU patients with cryptococcosis but not necessarily in patients with other underlying diseases.
Diabetes mellitus and cryptococcosis diagnosed after ICU admission were also associated with good prognosis in the multivariate analysis. In our previous study of disseminated cryptococcosis, diabetes was not associated with survival [Reference Chuang20]. A possible explanation for this finding is that the immunodeficient status of patients with diabetes was not worse than that of the other patients in this study. In our study, patients with a diagnosis of cryptococcosis after ICU admission had better 2-week ICU survival and this relationship was more prominent in long-term survival after adjusting for other conditions. The timing of cryptococcal infection may depend on, and thus be indicative of, the time point at which immunocompromisation develops or worsens. Therefore delay in the diagnosis of cryptococcal infection after ICU admission may indicate a delay in the development of immunocompromisation and signal a better prognosis. At the same time, an elevated WBC count at ICU admission may also play a role in outcome even if it carries a small hazard ratio. These results illustrate the importance of underlying immune status for cryptococcal infection in the ICU and underline the need for further studies to confirm the pathogenesis of immunodeficiency during ICU stay and its effect on the outcome of fungal infections.
There were several limitations in this study. First, many factors could have contributed to outcome. It is difficult to clarify the actual contribution of cryptococcal infection to mortality, even in a multivariate analysis. Second, due to the limited case number, our sample of patients may not have adequately represented the clinical characteristics of cryptococcal infection in the ICU. Third, due to the retrospective nature of the study, the treatment protocol was not consistent and the APACHE II score at the time of diagnosis was not available. Nevertheless, we conclude that the prognosis of patients with C. neoformans infection in the ICU was worse than that for patients with other conditions of similar disease severity at ICU admission. HIV infection was a poor prognostic predictor and diabetes was associated with better hospital prognosis than other underlying conditions of similar severity.
DECLARATION OF INTEREST
None.








