Lung cancer is the leading cause of cancer death among men and the second leading cause of cancer death among women worldwide(Reference Torre, Siegel and Jemal1). Risk factors for lung cancer, based on epidemiological evidence, include tobacco smoking, exposure to second-hand smoke and radon(Reference Torre, Siegel and Jemal1). Sex, age and diet are also found to be associated with the risk of lung cancer(Reference de Groot and Munden2,Reference Dela Cruz, Tanoue and Matthay3) .
Carrot is the main source of α-carotene, and previous studies have shown that carrot may play an important role in the prevention of cancers(Reference Sharma, Karki and Thakur4,Reference Zaini, Brandt and Clench5,Reference Sevimli-Gur, Cetin and Akay6) . The association between carrot consumption and lung cancer has drawn much attention since the 1980s. Several observational epidemiological studies have reported inconsistent results about the relationship between carrot consumption and lung cancer risk. For example, in 1997, an observational study(Reference Rachtan and Sokolowski7) concluded that intake of carrots was statistically significantly associated with a lower risk of lung cancer. However, a population-based case–control study(Reference Hu, Mao and Dryer8) found that the association between the risk of lung cancer and consumption of carrots was statistically non-significant. It remains unclear about whether higher carrot consumption is associated with lower risk of lung cancer. Considering the inconsistent conclusions of existing epidemiological studies, we conducted a meta-analysis of observational studies to evaluate the association between carrot consumption and the risk of lung cancer.
Methods
Ethical approval is not required for this systematic review.
Literature search
This systematic review was conducted in accordance with the meta-analysis of observational studies in epidemiological guidelines(Reference Stroup, Berlin and Morton9). We searched PubMed and Embase databases for articles published from their inception up to April 2018 using the following terms: ‘carrot(s)’ and ‘lung cancer’ or ‘lung carcinoma’, and the medical subject headings ‘daucus carota’ and ‘lung neoplasm(s)’ or ‘lung tumo*’. In addition, reference lists of included original studies were also hand-searched and scrutinised to determine eligibility for inclusion in the present study.
Study selection
Studies in any language were included if they met all the following criteria: (i) the study had a prospective cohort or case–control study design, (ii) the exposure of interest was consumption of carrots, (iii) the end point of interest was lung cancer and (iv) the OR or relative risk with the corresponding 95 % CI of lung cancer for the highest compared with the lowest category of carrot intake was reported or could be calculated from the data provided.
Data extraction
The following data were independently extracted from eighteen included studies by two authors (H. B. X. and Y. G.): first author, country, year of publication, study design, number of study subjects, number of lung cancer cases/controls or cohort, source of controls, adjusted relative risk/OR with 95 % CI for the highest category of carrot consumption v. the lowest counterpart, level of carrot consumption, and confounding factors adjusted in the analysis. Discrepancies were resolved by discussion with the third author (Z. X. L.).
Quality assessment
The Newcastle–Ottawa Scale was used to evaluate the quality of cohort and case–control studies(Reference Wells, Shea and O’Connell10). The score of the scale was calculated premised on three factors: selection of participants, comparability of groups and exposure/outcome ascertainment. The points regarding the scale varied from 0 to 9 points, and studies scoring 0–3 points, 4–6 points and 7–9 points were rated as low, moderate and high quality of studies, respectively.
Statistical analyses
The fully adjusted OR was used as the common measure of association across studies. We combined the case–control and cohort studies in the primary meta-analysis, because OR and relative risks provide similar estimates of risk when the outcome is rare(Reference Rigby11,Reference Filardo, Adams, Ng and Lovric12) . Pooled OR and 95 % CI of the highest compared with the lowest category of carrot intake were estimated by using the random-effects model to account for anticipated heterogeneity(Reference DerSimonian and Laird13).
Heterogeneity was tested by I 2 statistic(Reference Higgins, Thompson and Deeks14). Heterogeneity was interpreted as low (I 2 < 25 %), moderate (I 2 25–50 %) and high (I 2 > 75 %). An I 2 value of ≥50 % was considered to represent substantial heterogeneity.
Heterogeneity was explored in subgroup analyses stratified by the following factors: study location, year of publication, study design, source of controls, specific dietary assessment method and adjustment for confounding factors such as residence, smoking and sex. Additionally, the differences among subgroups were tested by meta-regression analysis (using STATA ‘metareg’ command). We used the meta-regression of random effect, and characteristic variables were analysed separately for different subgroups.
Potential publication bias was assessed via using the Begg’s rank correlation and Egger’s linear regression tests(Reference Begg and Mazumdar15,Reference Egger, Davey Smith and Schneider16) , and we conducted a trim and fill analysis, which yielded an effect adjusted for funnel plot asymmetry. STATA statistical software (version 12.0) was used for all the statistical analyses. A two-tailed P < 0·05 was considered statistically significant.
Results
Study selection
The process of study selection, identification and inclusion using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram(Reference Moher, Liberati and Tetzlaff17) is shown in Fig. 1. Initially, we retrieved thirty citations from the PubMed and 24 733 citations from the Embase. After, thirty duplicates were excluded, 24 733 citations were screened through titles and abstracts, of which 24 214 were excluded because of reviews or irrelevant studies. After full-text review of the remaining nineteen articles, one article(Reference Le Marchand, Yoshizawa and Kolonel18) was excluded, since it did not report 95 % CI or provide sufficient data to calculate it. Finally, eighteen studies were included(Reference Rachtan and Sokolowski7,Reference Hu, Mao and Dryer8,Reference Brennan, Fortes and Butler19 Reference Dosil-Diaz, Ruano-Ravina and Gestal-Otero34) .
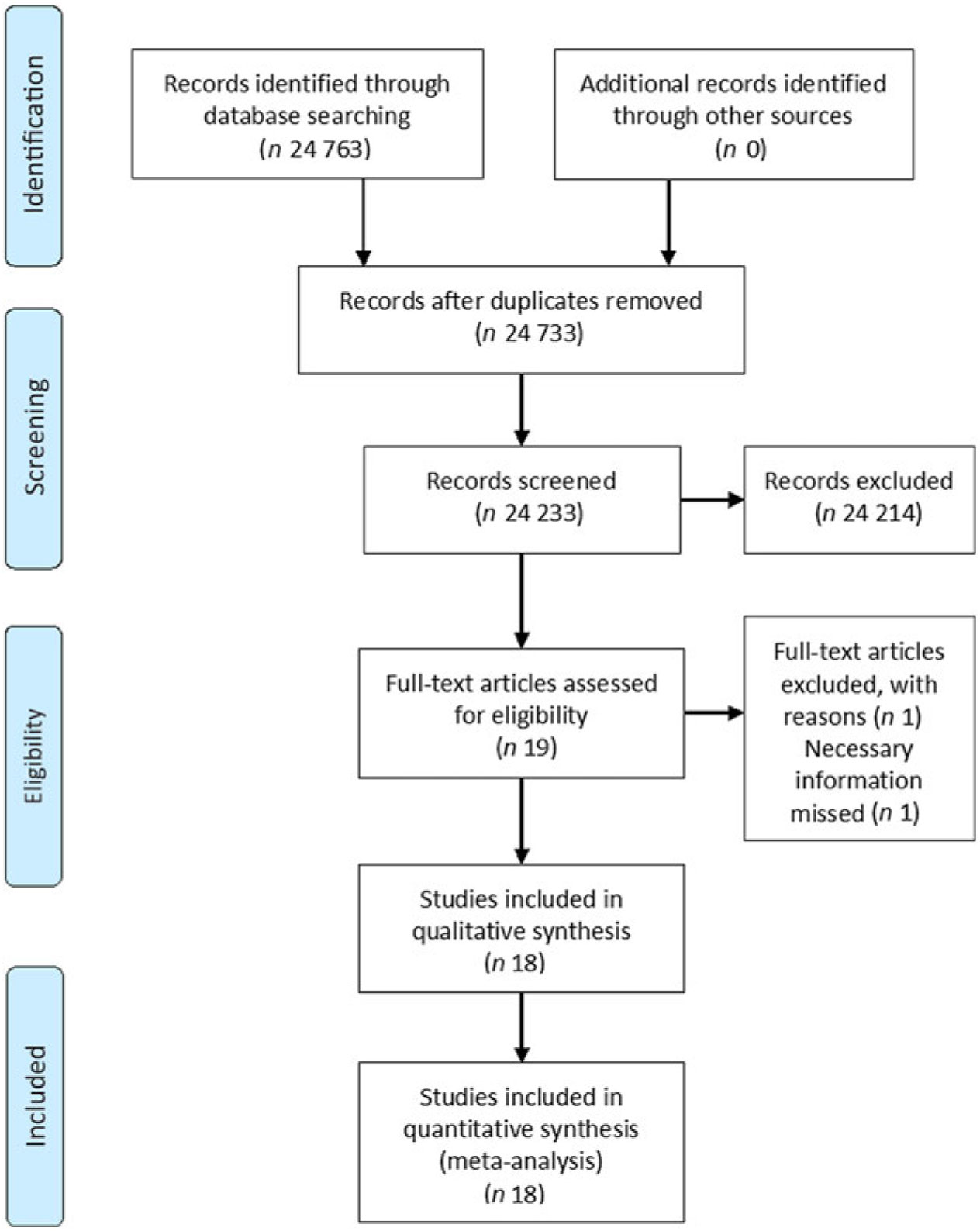
Fig. 1. Flow diagram of study selection regarding relevant observational studies.
Characteristics of the included studies
The main characteristics of the eighteen studies are summarised in Table 1. These studies were published between 1986 and 2014. Of the eighteen studies, nine were conducted in Europe, six in Asia and three in North America. Only one study was a prospective cohort study, the others were case–control studies. A total of 202 969 subjects, including 5517 lung cancer cases were included. Most studies adjusted for some potential confounders, including age, sex and smoking status. Thirteen studies were hospital-based, four studies were population-based and one study was a combination of the two types. The quality assessment scores of all the included studies ranged from 5 to 8 points, with an average score of 6·1 points. The quality assessment score of the prospective cohort study was 8 points, and the quality assessment scores concerning the fourteen case–control studies ranged from 5 to 8 points, with an average score of 6 points.
Table 1. Study characteristics of included studies on investigating the association between carrot consumption and lung cancer
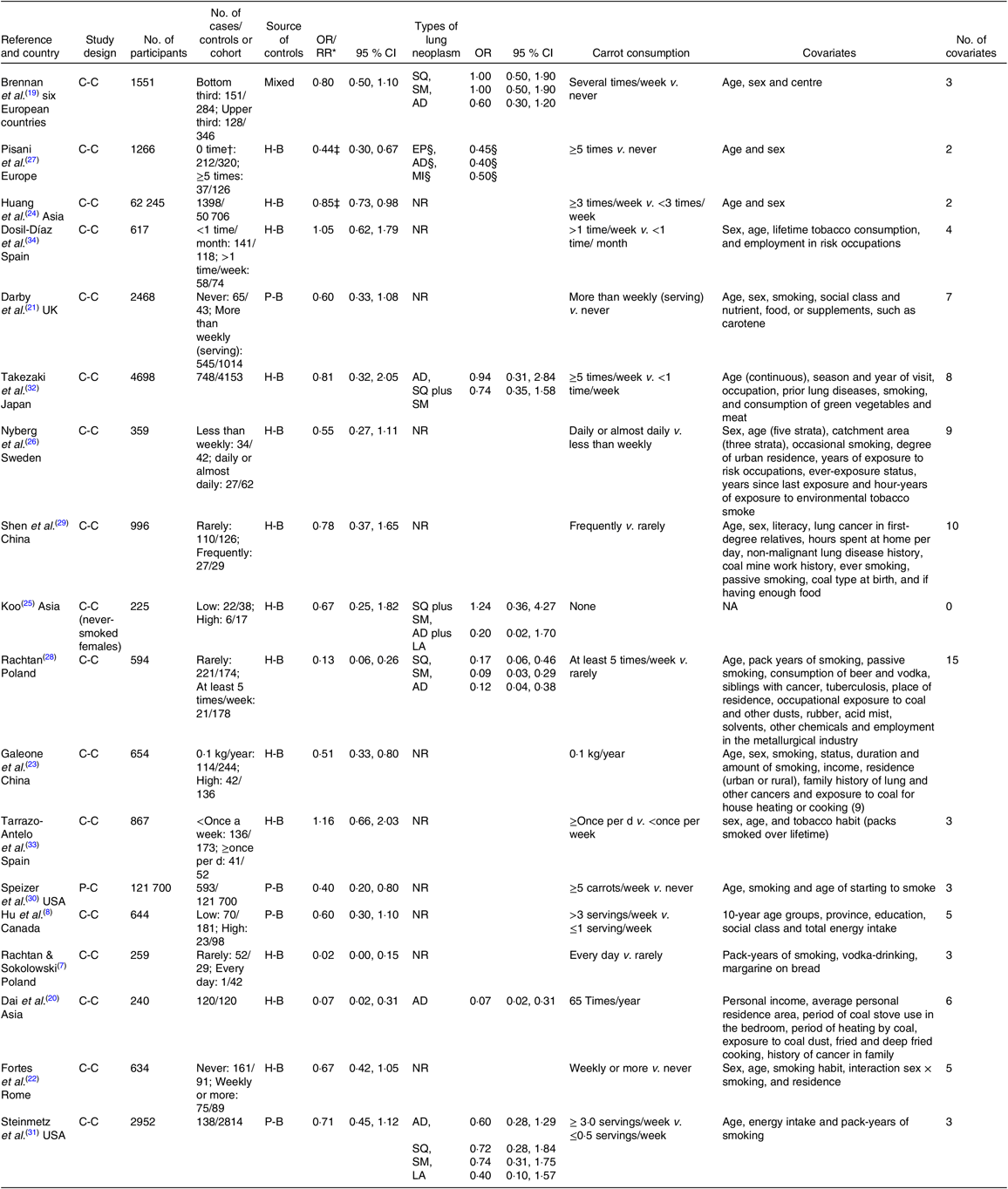
AD, adenocarcinoma; C-C, case–control study; EP, epidermoid; H-B, hospital-based; LA, large-cell carcinoma; MI, microcitomas; NA, not available; NR, not reported; P-B, population-based, P-C, prospective cohort study; RR, relative risk; SM, small-cell carcinoma; SQ, squamous cell carcinoma.
* The OR/RR of the highest category of carrot intake compared with the lowest category of carrot intake.
† Case groups and control groups included non-smokers, ex-smokers and current smokers.
‡ Crude OR.
§ Due to the limited data, 95 % CI could not be calculated for the three subtypes of lung cancer.
Association between the highest consumption of carrots compared with the lowest consumption and lung cancer risk
Fig. 2 showed the results from the random-effects model combining the OR for the risk of lung cancer in relation to carrot intake. Of the eighteen studies, seven showed a significantly inverse relationship between carrot consumption and the risk of lung cancer, whereas the others suggested that there was no statistically significant association between carrot intake and lung cancer risk. Overall, the highest category of carrot intake was associated with a significantly lower risk of lung cancer (OR 0·58; 95 % CI 0·45, 0·74). A moderate-to-high heterogeneity across studies was observed (I 2 = 72·6 %, P < 0·001).
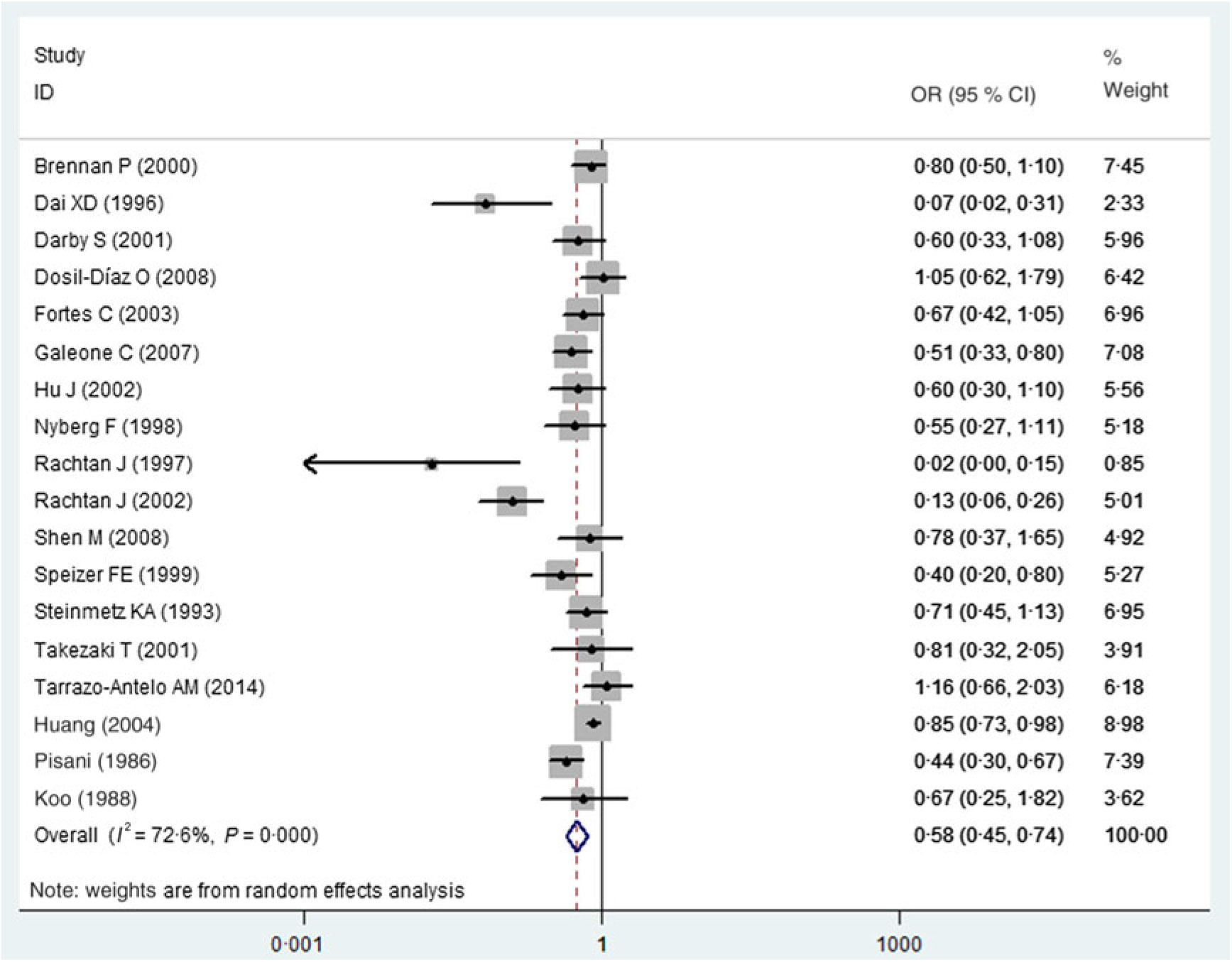
Fig. 2. Association between carrot consumption and lung cancer risk.
Subgroup analyses and sensitivity analyses
Table 2 shows the results from different subgroups. A statistically significant effect of carrot intake on lung cancer risk was observed among studies conducted in Europe (OR 0·50, 95 % CI 0·35, 0·72) and Asia (OR 0·75, 95 % CI 0·61, 0·92), but not in North America (OR 0·50, 95 % CI 0·13, 1·84). The significant inverse association between carrot intake and the risk of lung cancer was found among the following types of lung cancer: adenocarcinoma (OR 0·34, 95 % CI 0·15, 0·79) and mixed types (OR 0·61, 95 % CI 0·46, 0·81). However, there was no statistically inverse association in squamous cell carcinoma (OR 0·52, 95 % CI 0·19, 1·45), small-cell carcinoma (OR 0·43, 95 % CI 0·12, 1·59), large-cell carcinoma (OR 0·40, 95 % CI 0·10, 1·57), squamous and small-cell carcinoma (OR 0·85, 95 % CI 0·45, 1·62) and adenocarcinoma and large-cell carcinoma (OR 0·20, 95 % CI 0·02, 1·70). When we examined whether the association differed by study design, carrot intake was significantly associated with decreased risk of lung cancer in the case–control studies (OR 0·57, 95 % CI 0·45, 0·74), but not in the prospective cohort study (OR 0·60, 95 % CI 0·31, 1·15). Carrot intake was significantly associated with decreased lung cancer risk among hospital-based studies (twelve studies, OR 0·59, 95 % CI 0·45, 0·78), but not among population-based studies (four studies, OR 0·52, 95 % CI 0·21, 1·28) and mixed studies (one study, OR 0·80, 95 % CI 0·54, 1·19). Additionally, there was a significant inverse association between carrot consumption and the risk of lung cancer across studies with adjustment for smoking (OR 0·56, 95 % CI 0·42, 0·75), but was not significant among studies without adjustment for smoking (OR 0·63, 95 % CI 0·33, 1·20).
Table 2. Results of subgroup analyses concerning carrot intake and lung cancer risk
(Odds ratios and 95 % confidence intervals)
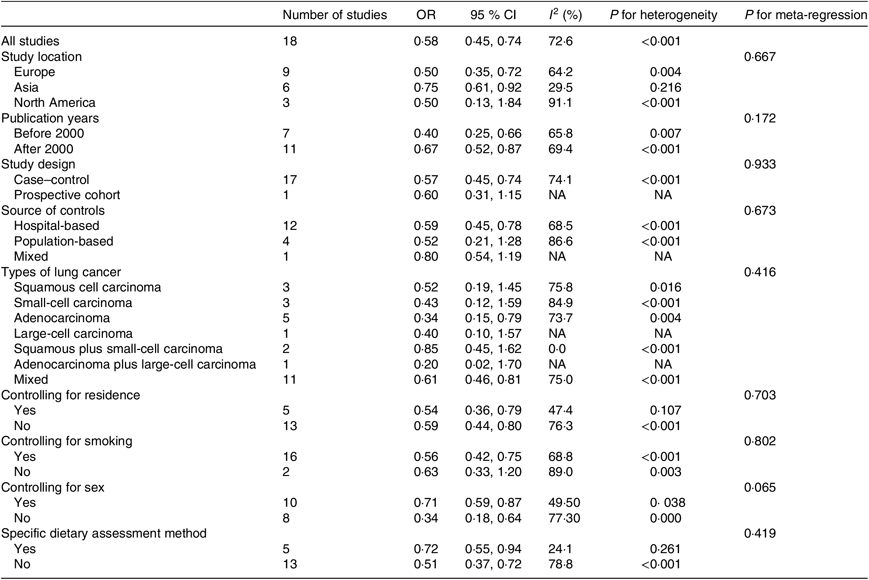
NA, not available.
To identify the potential influence of every single study on the pooled results, any single study was excluded in turn and pooled the results of the remaining studies. The overall combined OR did not materially change, with a range from 0·55 (95 % CI 0·42, 0·72) to 0·64 (95 % CI 0·52, 0·79).
Publication bias
Visual inspection of a funnel plot identified substantial asymmetry (Fig. 3). The Begg’s rank correlation test (Z = 1·52, P = 0·130) suggested no evidence of publication bias among included studies, whereas Egger’s linear regression test indicated that there was potential publication bias among these studies. The trim-and-fill method was used to evaluate the impact of any potential biases, and the results showed that two potentially missing studies were needed to obtain funnel plot symmetry for lung cancer (Fig. 4). The corrected OR was 0·52 (95 % CI 0·40, 0·67).
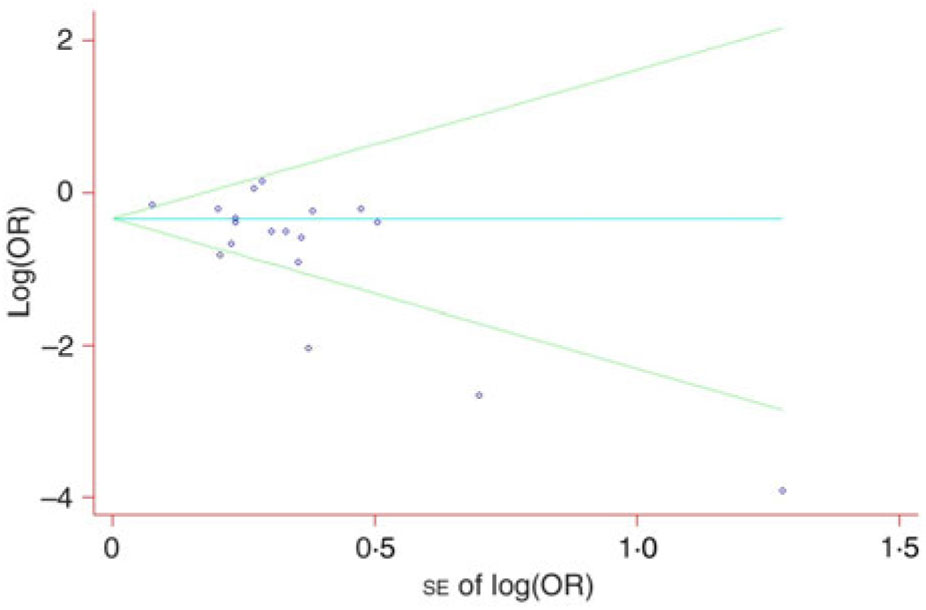
Fig. 3. Begg’s funnel plot with pseudo 95 % confidence intervals for publication bias.
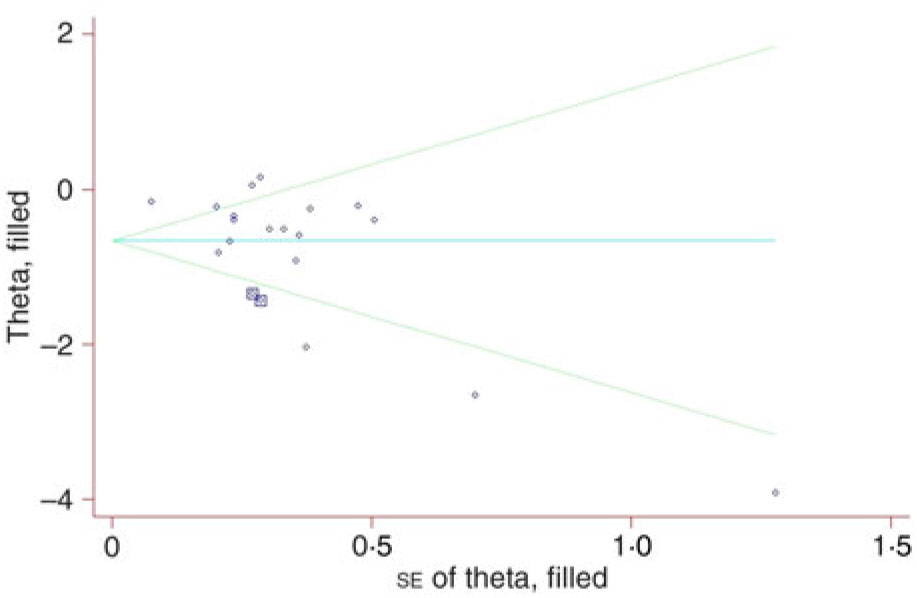
Fig. 4. Filled funnel plot of odds ratios with pseudo 95 % confidence intervals in the studies investigating risk for lung cancer associated with carrot intake.
Discussion
Findings from our meta-analysis on observational studies indicated that higher carrot intake is associated with a decrease in the risk of lung cancer, suggesting that carrot intake could reduce the risk of lung cancer by up to 42 % (OR 0·58; 95 % CI 0·45, 0·74), especially for adenocarcinoma (OR 0·34, 95 % CI 0·15, 0·79) and mixed types (OR 0·61, 95 % CI 0·46, 0·81), which was inconsistent with previous results of randomised controlled trials (RCT)(Reference Albanes, Heinonen and Taylor35). The results of previous RCT had suggested that β-carotene supplementation was associated with increased lung cancer risk (relative risk 1·16; 95 % CI 1·02, 1·33; P = 0·02), and the results of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study(36) have also shown that no reduction in the incidence of lung cancer among male smokers after years of dietary supplementation with β-carotene. Although our results were different from previous studies, as a kind of vegetables, carrots were not equivalent to carotenoids, and carrot not only contained β-carotene, but contained other nutrients such as dietary fibres. Thus, between carotenoids and carrot was a significant difference. Additionally, carrot intake could decrease the risk of lung cancer based on our results of observational studies, and additional or more prospective studies were needed to further replicate the findings in future. Besides, a few original studies have adjusted for other food items in our study. Thus, more studies confounded by other food items were needed to further confirm our results.
The pooled OR for lung cancer across studies adjustment for smoking was 0·56 (95 % CI 0·42, 0·75), while the pooled OR for lung cancer between studies without adjustment for smoking was 0·63 (95 % CI 0·33, 1·20). Thus, carrot consumption could significantly decrease the risk of lung cancer adjusted for smoking.
The study by Pisani et al.(Reference Pisani, Berrino and Macaluso27) reported that the effect of carrots on lung cancer among non-smokers, ex-smokers and current smokers, but it should be noted that cancer-decreasing effect of carrot was only found in the current smokers. Thus, in our study the cases and controls with the highest carrot intake compared with the lowest consumption among non-smokers, ex-smokers and current smokers were included to calculated OR and the corresponding CI.
The pooled OR for lung cancer that excluded the three outlier studies(Reference Rachtan and Sokolowski7,Reference Dai, Lin and Sun20,Reference Dosil-Diaz, Ruano-Ravina and Gestal-Otero34) was 0·61 (95 % CI 0·49, 0·76). The pooled OR for lung cancer that included the outlier studies was 0·58 (95 % CI 0·45, 0·74). However, after excluding the three outlier studies, the significance of association between carrot intake and lung cancer remained unchanged. Additionally, the three outlier studies contributed to a low heterogeneity. Therefore, the three outlier studies were not the source of significant heterogeneity in our study.
This inverse association was found among Europeans and Asians, but not in North Americans in the highest v. the lowest category of carrot consumption. This difference may be explained by variation in dietary habits. A previous study showed that consumption of vegetables was considerably higher among Asians and Europeans, and intakes of red meat were lower in Asians and Europeans compared with North Americans(Reference Micha, Khatibzadeh and Shi37). Notably, the null inverse association in North American participants might also result from the limited number of included studies (three studies including 2457 participants and 824 patients with lung cancer). More studies are warranted to investigate the potential difference between the different ethnic backgrounds.
Carrots were the main source of vitamin A(Reference Sharma, Sheehy and Kolonel38). Furthermore, orange carrots has in abundance α-carotene as well as β-carotene(Reference Arango, Jourdan and Geoffriau39), and were found to contain the greatest concentration of total carotenoids(Reference Surles, Weng and Simon40) among carrots with different colours. Previous studies have suggested that higher category of dietary vitamin A and β-carotene intake could reduce lung cancer risk(Reference Yu, Su and Wang41).
The inverse relationship between intake of carrots and the risk of lung cancer was generally plausible, premised on biological activities of natural substances contained in carrots, such as carotenoids, dietary fibres and folic acid(Reference Sharma, Karki and Thakur4). In addition, the presence of high concentration of carotenoids mentioned above may account for the biological properties of carrots. Carotenoids in carrots could prevent the risk of lung cancer through following biological mechanisms. First of all, carotenoids have been reported to inhibit the incidence of tumour(Reference Nishino42,Reference Bast, Haenen and van den Berg43) . A previous study showed that retinoic acid has been reported to have the effect on the inhibition of lung cancer cell. It could increase the expression of miR-512-3p in microRNA, since overexpression of miR-512-3p could not only inhibit tumour cell adhesion, migration and invasion in NSCLC A549 and H1299 cell lines, but could also decrease the DOCK3 protein(Reference Zhu, Gao and Chu44). Furthermore, some types of carotenoids are converted into vitamin A, which is related to cellular differentiation may contribute to cancer prevention(Reference Toma, Raffo and Isnardi45). Additionally, carrots are contained α- and β-carotene that may contribute to its anticancer effect. Previous studies have shown that lung hexosamine content in animals was inhibited significantly when treated with β-carotene, and the treated animals lived longer than the controlled ones(Reference Pradeep and Kuttan46). β-Carotene intake could enhance natural killer cell activity(Reference Santos, Meydani and Leka47), and decrease the growth of NCI-H69 lung cancer cells(Reference Prakash, Jackson and Gerber48), which maybe be beneficial to inhibit carcinogenesis. A study by Michaud et al. suggested that α-Carotene intake was significantly associated with decreased risk of lung cancer(Reference Michaud, Feskanich and Rimm49). Apart from that, carrots have in abundance dietary fibres(Reference Bao and Chang50), and a previous study has demonstrated that dietary fibre intake is inversely related to cancer risk(Reference Lattimer and Haub51). Finally, carrots are rich in folic acid. A previous study suggested that most types of lung cancers could strongly express folate receptor, which showed that folate-linked targeted therapy may be useful to treat the majority of lung cancers(Reference Cagle, Zhai and Murphy52).
The characteristics of individual studies may also be important in interpreting their contribution to the meta-analyses. For instance, three population-based studies Darby et al.(Reference Darby, Whitley and Doll21), Hu et al.(Reference Hu, Mao and Dryer8) and Steinmetz et al.(Reference Steinmetz, Potter and Folsom31) did not report an inverse association between carrot consumption and lung cancer. Moreover, as shown in Table 1, Steinmetz et al.(Reference Steinmetz, Potter and Folsom31) ascertained the effects of carrot intake on different types of lung cancer, whereas the other two studies did not report any association between carrot consumption and the risk of lung cancer.
Although the effect of carrot intake on lung cancer risk is biologically plausible, heterogeneity across studies was statistically significant. The heterogeneity may be owing to differences in methods for dietary assessment. Most of our included studies collected data based on self-administrated or interviewer-administrated questionnaire which were more likely to be uninformed assessment standard. This may be the potential source of heterogeneity. To explore the resources of heterogeneity, subgroup analyses based on specific dietary assessment methods (FFQ v. questionnaire/interview) were performed, and we found that the heterogeneity in these studies measuring carrot consumption using FFQ was 24·1 % (OR 0·72, 95 % CI 0·55, 0·94), which significantly decreased the heterogeneity compared with these studies using questionnaire (OR 0·51, 95 % CI 0·37, 0·72; I 2 = 78·8 %). However, the differences between the dietary assessment methods were statistically non-significant. Subsequently, to further explore the sources of heterogeneity and the stability of the pooled results, sensitivity analysis was performed by excluding one study at a time. We found that there was no significant influence on the stability of the results. In the present study, the source of heterogeneity may result from some factors, such as the number of samples, study design and the age of the patients. Overall, in our study, sensitivity analysis and consistent results from various subgroup analyses indicated that our findings were reliable and robust, although heterogeneity existed among the included studies.
The present study had several strengths. Firstly, it was the first meta-analysis to systematically analyse the association between carrot consumption and lung cancer risk. Since carrot intake could reduce the risk of lung cancer by 42 %, the results of our study could not only act as an aetiology explanation but could also increase medical and public health field awareness. Secondly, based on our subgroup analysis, a significant inverse association between carrot consumption and lung cancer risk was observed in the European and Asian populations. Furthermore, the inverse association of carrot intake on the risk of lung cancer was observed in the adenocarcinoma and mixed types. This provided an insight for future study of how the biological mechanisms of carrot consumption and lung cancer are affected by ethnicity and subtypes of lung cancer. Sensitivity and consistent results from subgroup analyses suggested that our findings were reliable, although some heterogeneity existed across the included studies.
There were some limitations in the study that should be mentioned. Firstly, seventeen studies out of the eighteen original studies used in our meta-analysis were case–control study design. This design is particularly vulnerable to potential biases (both selection and information biases). Secondly, some possible important residual confounders such as smoking and sex were not adjusted for in some studies. For example, one case–control study(Reference Rachtan and Sokolowski7) only controlled for age. However, our subgroup analyses by whether residence, smoking or sex were controlled or not suggested that there is no significant difference in OR. Thirdly, limited original studies were adjusted for other food items in our study. Thus, the results of our study might be confounded by other food items. Additionally, OR with corresponding 95 % CI for lung cancer associated with consumption of other food items were listed as a supplementary data. At last, the dose–response analysis between carrot intake and lung cancer risk was not performed because of limited data in original studies. Further studies with providing information of dose, cases and carrot consumption will be needed to assess the dose–response relationship.
In conclusion, our study combining with the most up-to-date evidence suggests that higher carrot consumption is inversely associated with the risk of lung cancer, particularly in Asian and European population, and adenocarcinoma and mixed types of lung cancer. Given the significant association strength between carrot intake and lung cancer, these findings provide practical and valuable insight for the prevention of lung cancer and a study of its aetiology.
Acknowledgements
The present study was supported by the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology, China (2016YXMS215). The funding organisation had no role in the design or conduct of this research.
H. B. X. and Z. X. L. conceived the study. H. B. X. and Y. G. searched and checked the databases according to the inclusion and exclusion criteria. H. J. helped to develop search strategies. H. B. X and G. Y. extracted the data and assessed their quality. H. B. X and Y. G. analysed the data. F. J. S. and Y. G. gave advice on meta-analysis methodology. H. B. X. wrote the draft of the paper. All authors contributed to writing, reviewing or revising the paper and read and approved the final manuscript. Z. X. L. is the guarantor of this work and had full access to all the data in the study and takes responsibility for its integrity and the accuracy of the data analysis.
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519001107.









