Non-technical Summary
Harpetids and trinucleids are two different types of trilobite. They both shared an unusual body plan, with a wide, flat brim extending from the head. Scientists once thought this must mean they were closely related, but more recently they've instead assumed that these two groups evolved their matching brims independently. We wanted to find out which of these two ideas was correct and learn more about how those unusual brims actually evolved. To do this, we studied the fossils of harpetids, trinucleids, and their relatives, and built up a detailed family tree. Our tree showed that harpetids and trinucleids most likely evolved their brims separately. What's more, both seem to have evolved their brims in the same way, following the same steps in the same order. This makes these brims a perfect example of what's called “parallel evolution”. Our detailed family tree showed a few other interesting features as well. It suggested that trinucleids actually belong to a bigger group of trilobites called Asaphida, and that liostracinidid trilobites, which some people thought were an early kind of trinucleid, are actually more distantly related to their trinucleid cousins.
Introduction
Trilobites of the order Harpetida Ebach and McNamara, Reference Ebach and McNamara2002, and the superfamily Trinucleioidea Hawle and Corda, Reference Hawle and Corda1847, make their first appearances during the Furongian (Hughes, Reference Hughes2007; Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020) and share a striking and unusual morphology: small to medium absolute body size, a vaulted cephalic chamber flanked by long genal prolongations, a thorax suspended above the sediment's surface, and greatly reduced eyes (Fortey and Owens, Reference Fortey and Owens1999; Adrain et al., Reference Adrain, Edgecombe, Fortey, Hammer, Laurie, Webby, Paris, Droser and Percival2004).
Most strikingly of all, both groups frequently share a wide, flattened cephalic brim or fringe with many pits or holes (Fig. 1). In this paper we borrow the adjective ‘harpiform’ from Hughes et al. (Reference Hughes, Ingham and Addison1975) to describe this structure, irrespective of the taxonomic group in which it appears. At one time, it was thought that these similar-looking trilobites must also be closely related (Swinnerton, Reference Swinnerton1919; Warburg, Reference Warburg1925), but more recently the assumption has been that these two trilobite groups evolved their harpiform cephalic brims independently, most likely through a process of parallel or convergent evolution (Fortey and Chatterton, Reference Fortey and Chatterton1988; Ebach and McNamara, Reference Ebach and McNamara2002; Adrain et al., Reference Adrain, Edgecombe, Fortey, Hammer, Laurie, Webby, Paris, Droser and Percival2004). However, it can be challenging to positively verify instances of such processes in extinct animal groups without close living relatives, such as trilobites, because no molecular data exist as an independent validation of morphological assessments of relatedness (Wiens et al., Reference Wiens, Chippindale and Hillis2003). This is especially true when the similar morphological structures that would imply relatedness lack clear functional analogues in modern ecosystems, as is the case for the harpiform brim.
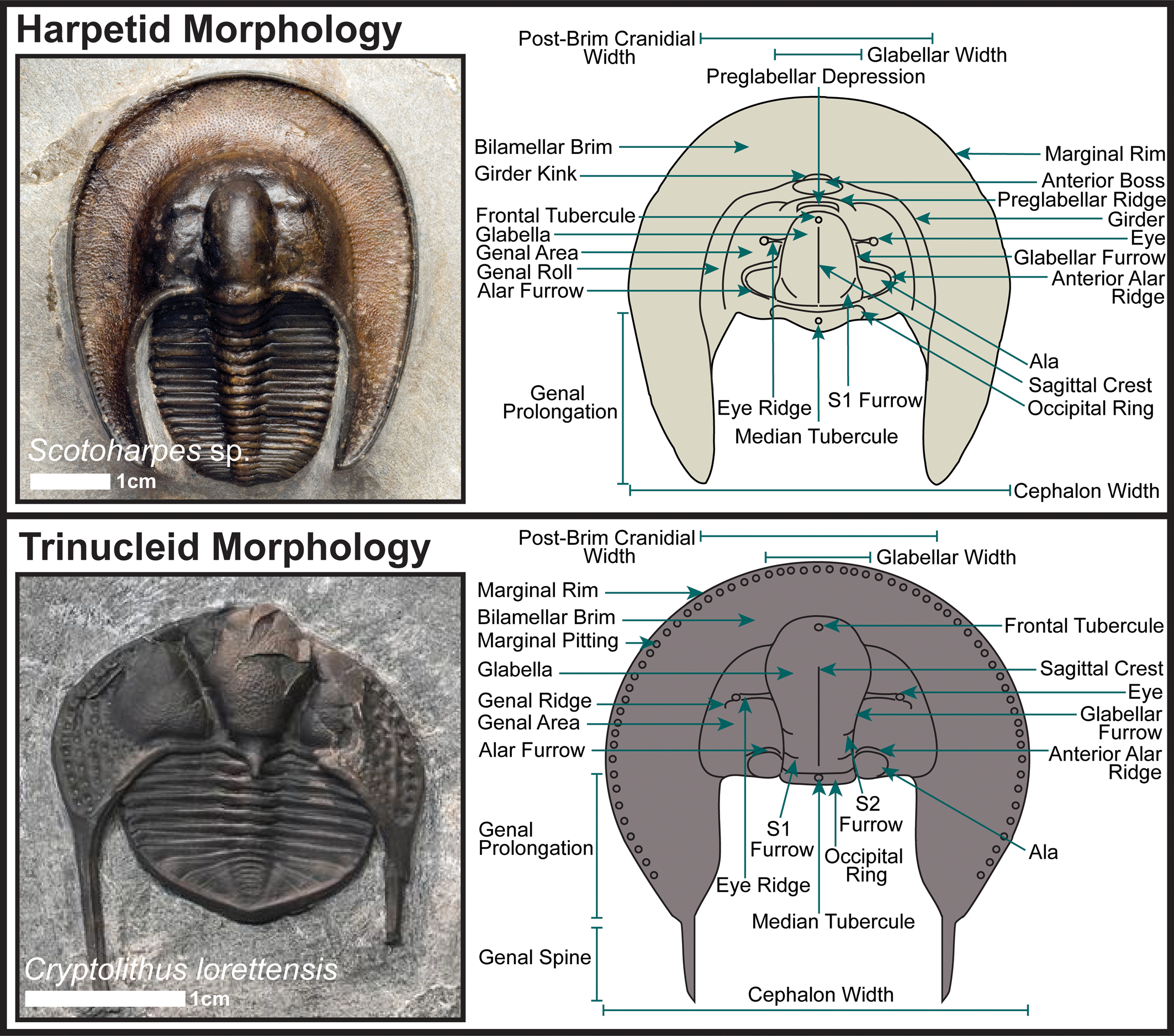
Figure 1. General dorsal cephalic morphology of harpetid and trinucleid trilobites. Adapted from Beech and Lamsdell (Reference Beech and Lamsdell2021).
It is therefore worthwhile to explicitly investigate how harpetid and trinucleid trilobites came to evolve their striking harpiform brims. This investigation, although prompted by the general similarity of the cephalic brim, offers an opportunity to test longstanding assumptions about the evolutionary relationships of these trilobites and serves as a case study in how to evaluate different kinds of morphological similarity in the fossil record.
When two taxa share a similar morphology, there are several possible scenarios that may have led to this condition (Fig. 2). On the one hand, the similarity may be the result of shared ancestry. Perhaps the two taxa diverged only recently from their common ancestor and so retain much of their ancestral morphology. Alternatively, the rate of evolutionary change may be low enough that the two have remained relatively similar despite diverging in the distant past, which can be described as evolutionary stasis (Eldredge et al., Reference Eldredge, Thompson, Brakefiled, Gavrilets and Jablonski2005; Stayton, Reference Stayton2015). On the other hand, the similarity may be homoplastic, arising separately in each taxon, so that the current condition of each lineage is quite unlike its ancestral condition. If this ancestral condition is the same or similar in both taxa, this can be described as parallel evolution (Zhang and Kumar, Reference Zhang and Kumar1997; Pearce, Reference Pearce2012; Schweizer et al., Reference Schweizer, Güntert, Seehausen, Leuenberger and Hertwig2014; Stayton, Reference Stayton2015). If the two taxa instead begin from very different ancestral conditions, but nevertheless independently evolve similar phenotypes, this can be described as convergent evolution (Zhang and Kumar, Reference Zhang and Kumar1997; Pearce, Reference Pearce2012; Stayton, Reference Stayton2015; Speed and Arbuckle, Reference Speed and Arbuckle2017). Both parallel and convergent evolution are frequently thought to be the result of different taxa adapting to similar ecological niches or responding to similar environmental pressures (Wiens et al., Reference Wiens, Chippindale and Hillis2003; Stayton, Reference Stayton2015; Moen et al., Reference Moen, Morlon and Wiens2016).
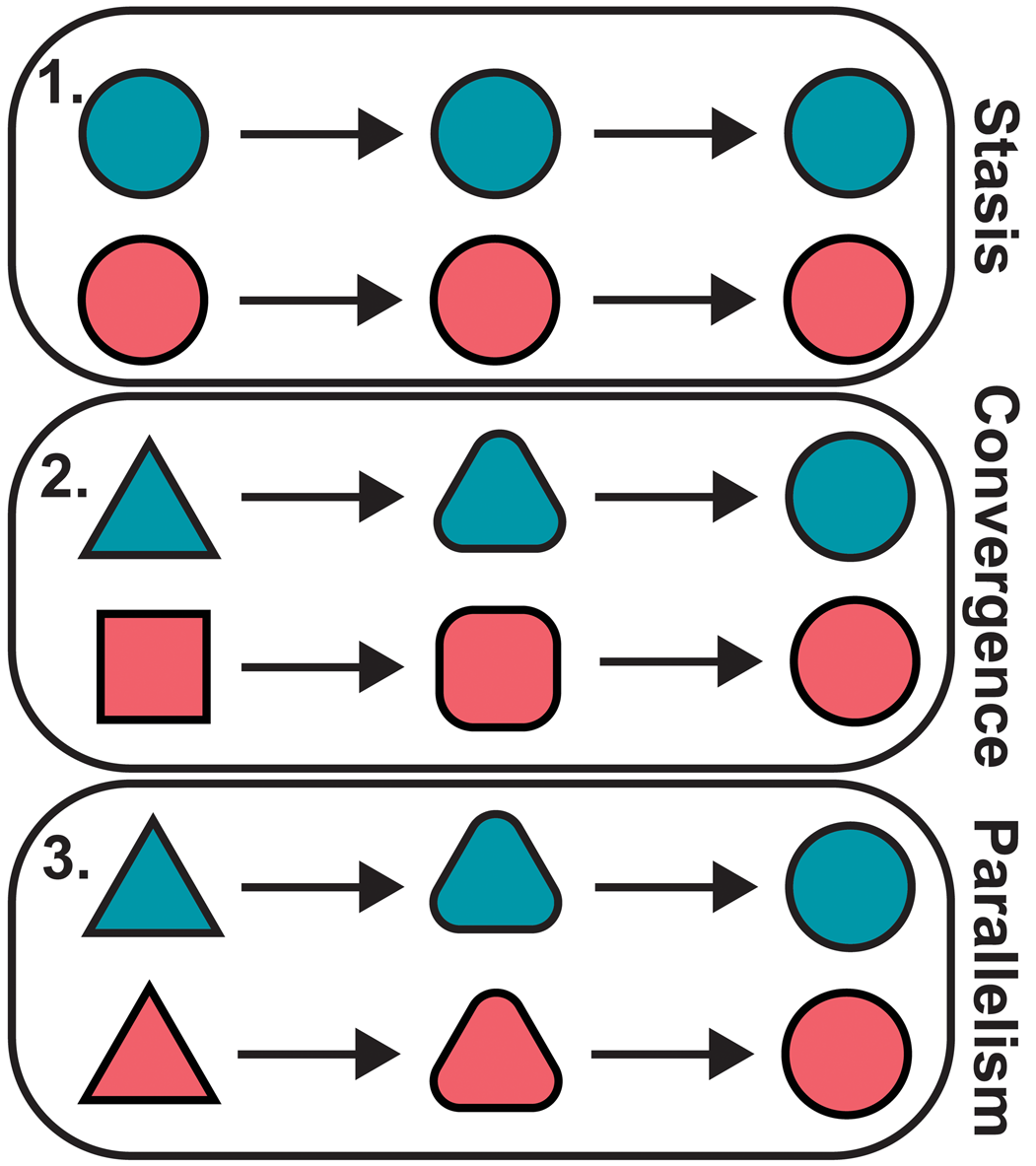
Figure 2. Alternative scenarios explaining morphological similarity: evolutionary stasis following divergence from a common ancestor (1), convergent evolution (2), and parallel evolution (3). The different colors are used to denote distinct evolutionary lineages. The different shapes indicate different phenotypes/morphotypes.
Our first task, therefore, is to confirm whether the harpiform brims of harpetid and trinucleid trilobites are the result of shared ancestry or whether these brims did indeed evolve independently in each group. Brims are first definitively seen in harpetids around 485 Myr ago in the Tremadocian genus Brachyhipposiderus Jell, Reference Jell1985, and in Tremadocian trinucleids some 6 Myr later. This gap in time of appearance is consistent with a homoplastic origin for harpiform brims, but too narrow to be conclusive. To confidently state that harpiform brims originated independently in both groups, it must be demonstrated that the two are phylogenetically distinct and that the last common ancestor of both lacked a harpiform brim. From there we can begin to test other hypotheses, such as convergence, parallelism, and stasis (Fig. 2). Because the only way to study the last common ancestor of these trilobites is through phylogenetic inference, much depends on the phylogenetic position of the trinucleids.
Until relatively recently, trinucleids were widely considered to be part of the order Asaphida Salter, Reference Salter1864, based largely on the work of Fortey and Chatterton (Reference Fortey and Chatterton1988) and on a few key, putative synapomorphies: a pre-occipital glabellar tubercle, a cephalic median suture (present plesiomorphically in trinucleids), and a broadly similar globular protaspid larval stage (Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020). This last characteristic is especially notable because Fortey and Chatterton (Reference Fortey and Chatterton1988) chose to assign extra weight to character states present early in ontogeny. However, there are no definitive a priori reasons for assigning extra relative weight to earlier ontogenetic characters. Indeed, recent work by Laibl et al. (Reference Laibl, Saleh and Pérez-Peris2023) has suggested that globular planktic protaspides may have evolved independently up to ten times in different trilobite lineages. Additionally, weighted characters have been shown to potentially propagate errors in phylogenetic analyses (Congreve and Lamsdell, Reference Congreve and Lamsdell2016) and new analyses have argued that the supposed synapomorphies linking Asaphida and Trinucleida arose convergently (Park et al., Reference Park, Kihm, Kang and Choi2014; Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020). Park et al. (Reference Park, Kihm, Kang and Choi2014) called for the superfamily Trinucleioidea to be excluded from Asaphida, and Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020) amplified this assessment, calling for the group to be recognized as its own independent order of trilobites. Meanwhile, other researchers have used new ontogenetic and morphological data to argue for continued inclusion of the trinucleids within Asaphida (Chatterton et al., Reference Chatterton, Edgecome, Speyer, Hunt and Fortey1994; Yang et al., Reference Yang, Peng, Babcock, Zhu and Liu2022), but these studies have not been supported by detailed phylogenetic analyses.
Part of the debate centers on the family Liostracinidae Raymond, Reference Raymond1937, which has been proposed as a basal-most clade of trinucleids (Fortey and Chatterton, Reference Fortey and Chatterton1988; Peng et al., Reference Peng, Babcock and Lin2004; Adrain, Reference Adrain2011; Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020). Yang et al. (Reference Yang, Peng, Babcock, Zhu and Liu2022) examined previously unknown thoracic and ventral material from Liostracina and found evidence of a rostral plate and a natant hypostomal condition. These characters, along with evidence of a non-asaphoid-type protaspis, led them to conclude that Liostracinidae did not belong either to Asaphida or to the trinucleids, instead preferring to reassign the family to the paraphyletic ‘Ptychopariida’. In this, they broadly confirmed the earlier assessment of Öpik (Reference Öpik1967).
Notably, none of these recent investigations has considered a possible relationship between trinucleids and harpetids, despite their gross morphological similarities, the individual genera (e.g., Eotrinucleus Zhou and Zhang, Reference Zhou and Zhang1978) that have occasionally been shuffled between Harpetida and Trinucleioidea, and the fact that historically (Swinnerton, Reference Swinnerton1919; Warburg, Reference Warburg1925) such a close relationship was considered plausible if not indeed probable. There are therefore at least three relevant phylogenetic hypotheses that need to be tested. The first is that trinucleids are highly derived, harpiform asaphids (Fortey and Chatterton, Reference Fortey and Chatterton1988; Chatterton et al., Reference Chatterton, Edgecome, Speyer, Hunt and Fortey1994; Yang et al., Reference Yang, Peng, Babcock, Zhu and Liu2022). The second is that trinucleids are phylogenetically independent of both Asaphida and Harpetida, potentially representing their own unique order of trilobites (Park et al., Reference Park, Kihm, Kang and Choi2014; Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020). The third hypothesis is the unexplored possibility that trinucleids and harpetids are closely related, potentially forming a single clade of harpiform trilobites.
Materials and methods
For this study, we conducted a novel phylogenetic analysis of the evolutionary relationships between Harpetida and Trinucleioidea. In this effort we built on recent phylogenetic studies of both groups, assembling a list of relevant discrete morphological characters from the character–taxon matrices of Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020) and Beech and Lamsdell (Reference Beech and Lamsdell2021). This list was further expanded with characters adapted from the diagnostic descriptions in the Treatise on Invertebrate Paleontology (Fortey and Owens, Reference Fortey, Owens and Kaesler1997). The final character list consisted of 112 discrete, unordered, and equally weighted morphological characters (Appendix).
These characters were then used to code the morphologies of a broad sampling of both harpetid and trinucleid trilobites; 21 out of 29 recognized harpetid genera were included in the analysis, and 56 out of 132 recognized trinucleid genera (including Liostracina Monke, Reference Monke1903). Five non-trinucleid asaphids were also coded. These particular asaphid taxa were chosen because of their morphological diversity, which captures much of the variation within Asaphida, and because they belong to genera previously utilized as outgroups in phylogenetic analyses of trinucleid trilobites (Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020). The analysis was rooted on the outgroup taxon Eoredlichia intermedia Lu, Reference Lu1940, from the paraphyletic order ‘Redlichiida’ Richter, Reference Richter, Dittler, Joos, Korschelt, Linek, Oltmanns and Schaum1932. Six ptychopariid trilobites were included to act as additional unconstrained outgroups. These outgroup taxa were likewise chosen because of their morphological diversity and their previous use as outgroups in relevant phylogenetic studies (Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020; Beech and Lamsdell, Reference Beech and Lamsdell2021). A total of 114 trilobite species were included in the analysis. Character states were coded from physical and digital museum specimens and from photographic figures taken from the published trilobite literature (Whittington, Reference Whittington1941, Reference Whittington1950; Cave, Reference Cave1957; Fortey, Reference Fortey1975; Hughes et al., Reference Hughes, Ingham and Addison1975; El-Khayal and Romano, Reference El-Khayal and Romano1985; Shaw, Reference Shaw1995; Hoel, Reference Hoel1999; Mansson, Reference Mansson2000; Fortey and Edgecome, Reference Fortey and Edgecome2017; Fortey and Gutiérrez-Marco, Reference Fortey and Gutiérrez-Marco2022).
We performed parsimony analysis in TNT (Tree analysis using New Technology) (Goloboff et al., Reference Goloboff, Farris and Nixon2008). The data matrix was subjected to cladistic analysis, using random addition sequences followed by tree bisection–reconnection (TBR) branch swapping with 100,000 repetitions with all characters unordered and of equal weight. An additional round of TBR branch swapping on the best trees recovered in the initial analysis was performed to check for still more parsimonious trees. Results were summarized as a strict consensus of all most parsimonious trees recovered. Three characters hypothesized to be important in the evolution and origin of the harpiform brim (the yoked condition of the librigenae [char. 51], the marginal placement of the facial suture [char. 52], and the presence or absence of a well-developed bilamellar brim [char. 9]) were mapped onto the strict consensus tree using TNT. Clade support across the tree was assessed using bootstrap and jackknife resampling analyses performed in TNT.
Several constraint analyses were also performed to compare alternative hypotheses of higher-level relationships. These analyses involved creating four different backbone constraint trees: one that constrained a single origin for the harpiform brim (Constraint 1), one that constrained a sister group relationship between Asaphida and Trinucleoidea (Constraint 2), one consistent with the higher order relationships proposed by Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020) (Constraint 3), and one that constrained Liostracina to form a clade with Trinucleoidea (Constraint 4). The constraint analyses were performed in TNT using random addition sequences followed by tree bisection–reconnection (TBR) branch swapping with 10,000 repetitions with all characters unordered and of equal weight. An additional round of TBR branch swapping on the best trees recovered in the initial analysis was performed to check for still more parsimonious trees consistent with the specified constraints.
Repositories and institutional abbreviations
Physical specimens examined for this study are held by the following institutions: the Cincinnati Museum Center (CMC), Cincinnati, USA; the Yale Peabody Museum of Natural History (YPM), New Haven, USA; the Museum of Comparative Zoology at Harvard University (MCZ), Cambridge, USA; and the Natural History Museum (NHM), London, UK. Additional specimens examined digitally are held by the Smithsonian National Museum of Natural History (NMNH), the Houston Museum of Natural Science (HMNS), the British Geological Survey (BGS), the Utah Field House of Natural History State Park Museum (FHPR), St. Albans Museums (SAM), the American Museum of Natural History (AMNH), the National Museum of Wales (NMW), the Sedgwick Museum of Earth Sciences (SEDG), and the Senckenberg Museum Frankfurt (SMF).
Results
Strict consensus
Phylogenetic analysis of the final dataset resulted in 751 most parsimonious trees (MPTs); a strict consensus of all MPTs is presented in Figure 3. These trees showed an ensemble consistency index of 0.177, an ensemble retention index of 0.657, and a tree length of 813. The strict consensus tree includes a large harpetid clade, which appears as the sister group to a clade consisting of the putative basal harpetid Baikadamaspis jikdongensis Park and Choi, Reference Park and Choi2011, the ptychopariid Maladioides coreanicus Kobayashi, Reference Kobayashi1935, a small group of liostracinidid trilobites, and a much larger group of asaphid and trinucleid trilobites. The harpetid clade is united by five unambiguous synapomorphies: small glabellar lobes (char. 48), yoked librigenae (char. 51), strongly convex genae (char. 66), a posteriorly curved occipital furrow (char. 80), and an upturned pleural field margin (char. 108).
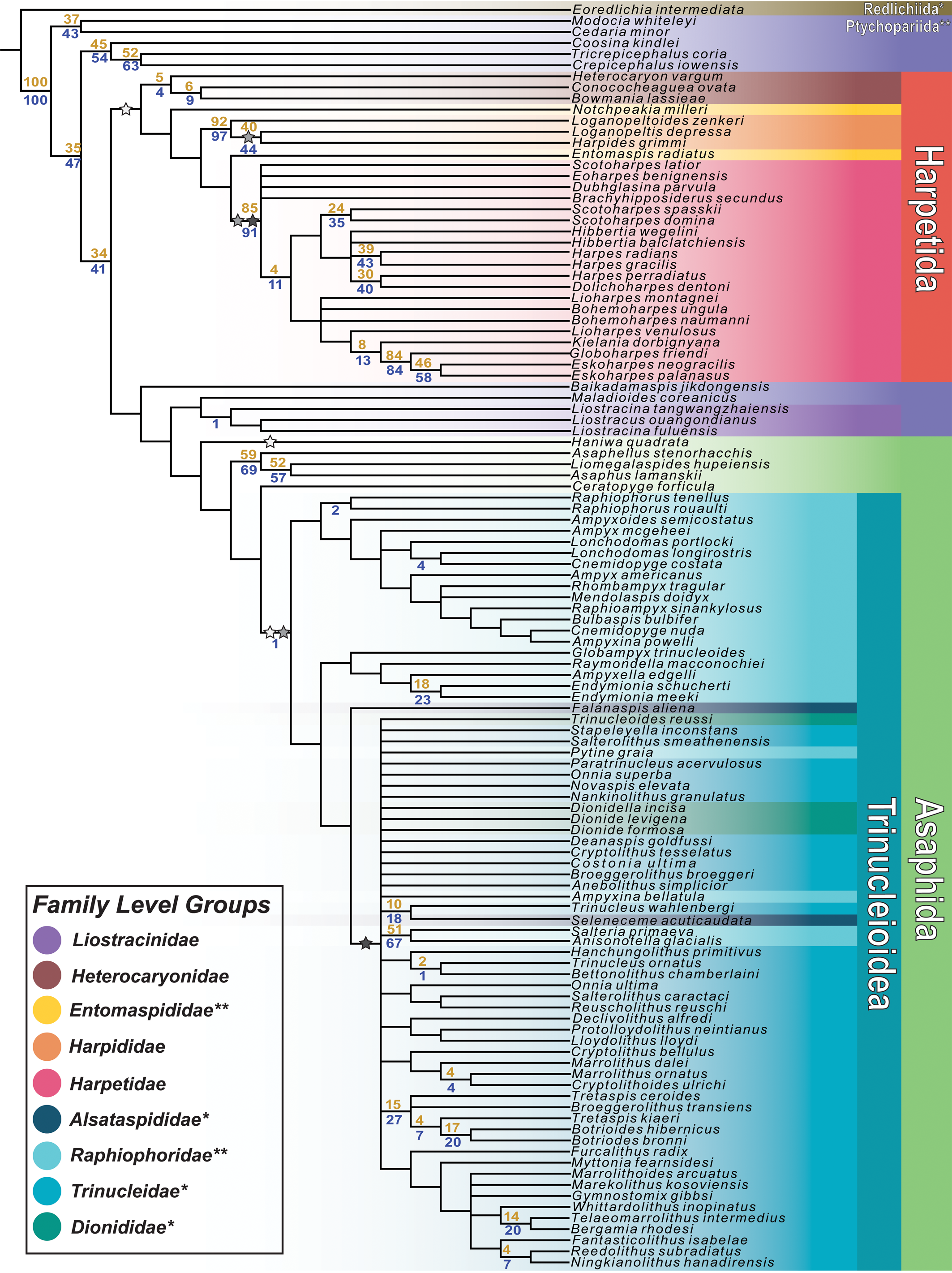
Figure 3. A strict consensus of the 751 most parsimonious trees found by TNT. Bootstrap support values of nodes are shown in gold, jackknife support values in blue. Stars indicate the appearance of characters important to the evolution of the harpiform brim. White stars correspond to yoked librigenae, gray stars correspond to marginal facial sutures, and black stars correspond to the harpiform brim itself. Secondary losses of these characters are not indicated. The colors shown in the legend are used to designate different taxonomic groups of trilobites. Groups whose names are marked with * in the legend are shown to be actually or potentially paraphyletic. Groups marked with ** in the legend are shown to be polyphyletic.
The consensus tree also includes a monophyletic Trinucleioidea preceded by a paraphyletic grade consisting of non-trinucleid asaphids; in other words, Trinucleioidea is phylogenetically nested within Asaphida. Asaphida is united by three unambiguous synapomorphies: parallel glabellar lateral margins (char. 47), small glabellar lobes (seen convergently in harpetids; char. 48), and globular or non-adult-like protaspis (char. 112). Within Asaphida, Trinucleioidea is united by three unambiguous synapomorphies: yoked librigenae (char. 51), marginal facial sutures (char. 52), and reduced or absent eyes (char. 56). Notably, the liostracinidid trilobites in this analysis do not group with the trinucleids, instead forming a clade with the putative andrarinidid trilobite ‘Liostracus’ ouangondianus Hartt, Reference Hartt and Dawson1868, which appears as the sister group to the ptychopariid Maladioides coreanicus. This liostracinidid clade is united by four unambiguous synapomorphies: a median preglabellar depression (char. 30), eyes located on the posterior of the cephalon (char. 59), a median tubercule on the occipital ring (char. 79), and a straight pygidial anterior border (char. 99).
Within the harpetid clade, our consensus tree showed support for the monophyly of three previously recognized harpetid families: Harpididae Whittington, Reference Whittington1950; Harpetidae Hawle and Corda, Reference Hawle and Corda1847; and Heterocaryonidae Hupé, Reference Hupé1953. Heterocaryonidae is united by two unambiguous synapomorphies, Harpididae by seven, and Harpetidae by eleven. Within Trinucleioidea, none of the four presently recognized families is shown to be monophyletic. The greater part of the family Raphiophoridae Angelin, Reference Angelin1854, appears in a grade that is paraphyletic with respect to the rest of Trinucleioidea. The family Trinucleidae Hawle and Corda, Reference Hawle and Corda1847, is the largest of the trinucleid families in this analysis but appears to be paraphyletic with respect to the only other brim-bearing trinucleid family, Dionididae Gürich, Reference Gürich1907, and with respect to certain members of Raphiophoridae and Alsataspididae Turner, Reference Turner1940.
Character mapping across the strict consensus tree (Fig. 3) found that all three of the key morphological characters hypothesized to be important in the evolution of the harpiform brim appeared multiple times in separate and distinct groups of trilobites. Yoked librigenae are found independently in harpetids, trinucleids, and the asaphid trilobite Haniwa quadrata Kobayashi, Reference Kobayashi1933. Marginal facial sutures are found in two families of harpetids (Harpididae and Harpetidae), and in trinucleids. Well-developed bilamellar, harpiform brims are found both in Harpetidae and in the large clade of derived trinucleids that includes the families Trinucleidae and Dionididae.
Constraint analyses
In our first constraint analysis (Table 1) we constrained a single origin for the harpiform brim, uniting brimmed harpetids with brimmed trinucleids in a monophyletic group, but not specifying any interrelationships within this clade (Constraint 1). Taxa inferred to have secondarily lost their brims or where the presence of a brim was ambiguously preserved were allowed to exist as floating taxa. One-hundred and eight most parsimonious trees were found under Constraint 1, with a tree length of 821 (eight steps longer than the unconstrained MPTs), a consistency index of 0.175, and a retention index of 0.653. The consensus of these trees shows Harpetidae as a clade within the already paraphyletic trinucleidid group, itself within a larger trinucleid-asaphid group. The other, non-brimmed harpetids form a clade, which appears as the sister group to a possibly paraphyletic Asaphida.
Table 1. The results of our constraint analyses, showing the number of most parsimonious trees recovered under each set of constraints, along with the tree scores, consistency indices, and retention indices for those trees. For Constraint 1, a single origin for the harpiform brim was enforced. In Constraint 2, Trinucleioidea was constrained to be the sister group to a monophyletic Asaphida. In Constraint 3, the tree was constrained to reflect the high-level phylogenetic relationships proposed by Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020), supporting an independent order of trinucleids. In Constraint 4, Liostracinidae was constrained to form a clade with Trinucleioidea.
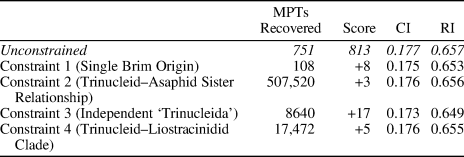
In our second constraint analysis we constrained Trincleioidea to appear as sister-taxon to a monophyletic Asaphida, rather than falling within it (Constraint 2). Under Constraint 2, we found 507,520 most parsimonious trees, with a tree length of 816 (three steps longer than the unconstrained MPTs), a consistency index of 0.176, and a retention index of 0.656. The consensus of these trees otherwise closely resembles the consensus of our unconstrained MPTs.
Our third constraint analysis used a backbone constraint tree based on the higher-level relationships proposed by Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020): a monophyletic trinucleid–liostracinidid clade, more distantly related to a monophyletic Asaphida than to a potentially paraphyletic group of ptychopariids (Constraint 3). Harpetids were allowed to group with the ptychopariids in this scenario, based on their position in our unconstrained consensus tree and on the phylogenetic work of Beech and Lamsdell (Reference Beech and Lamsdell2021). Under Constraint 3, we found 8,640 most parsimonious trees with a tree length of 830 (17 steps longer than the unconstrained MPTs), a consistency index of 0.173, and a retention index of 0.649. In the consensus of these trees, liostracinidids appear as the sister group to all other putative trinucleids. Most of the harpetids still form a familiar clade, but the heterocaryonidids now appear as a paraphyletic grade leading to the trinucleid–liostracinidid clade.
Our final constraint analysis simply constrained Liostracina to appear in a clade with the other putative trinucleids in our analysis (while allowing ‘Liostracus’ ouangondianus to exist as a floating taxon) (Constraint 4). Under Constraint 4, we found 17,472 most parsimonious trees with a tree length of 818 (five steps longer than the unconstrained MPTs), a consistency index of 0.176, and a retention index of 0.655. In the consensus of these trees, liostracinidids still form a clade with ‘Liostracus’ ouangondianus, with this clade appearing as the sister group to all other putative trinucleids.
Discussion
Parallel origins of the harpiform morphology
Our phylogenetic analysis strongly indicates that the harpiform brim—a wide, flattened bilamellar brim with many pits or holes that fringes the cephalon—evolved multiple times in distantly related groups of trilobites, namely the order Harpetida and the superfamily Trinucleioidea. These two groups are shown here to be phylogenetically independent. Both can trace their ancestry to the paraphyletic order ‘Ptychopariida’ Swinnerton, Reference Swinnerton1915; there is no convincing evidence however to suggest that harpetids and trinucleids share any closer relationship. Moreover, their last common ancestor is inferred to have had neither a harpiform brim, nor any of the morphological precursors that might have facilitated the evolution of such a brim (Fig. 4). This indicates that harpetids and trinucleids evolved their striking cephalic brims entirely independently. This homoplasy can be clearly recognized through a detailed phylogenetic analysis despite the limitations of the fossil record and their gross morphological similarity. Moreover, our constraint analyses (Table 1) confirmed that a single origin for the harpiform brim within Librostoma is less parsimonious than a scenario where harpetids and trinucleids evolved their brims independently, requiring more overall homoplasy. These findings emphasize the importance of a “total evidence” approach in phylogenetic studies of fossil taxa, because relying on a few favored synapomorphies—such as the presence of a striking and unusual cephalic brim or, equally, a larval morphology supposed to be particular to a single group—can lead to interpretations at odds with the greater bulk of the morphological evidence.
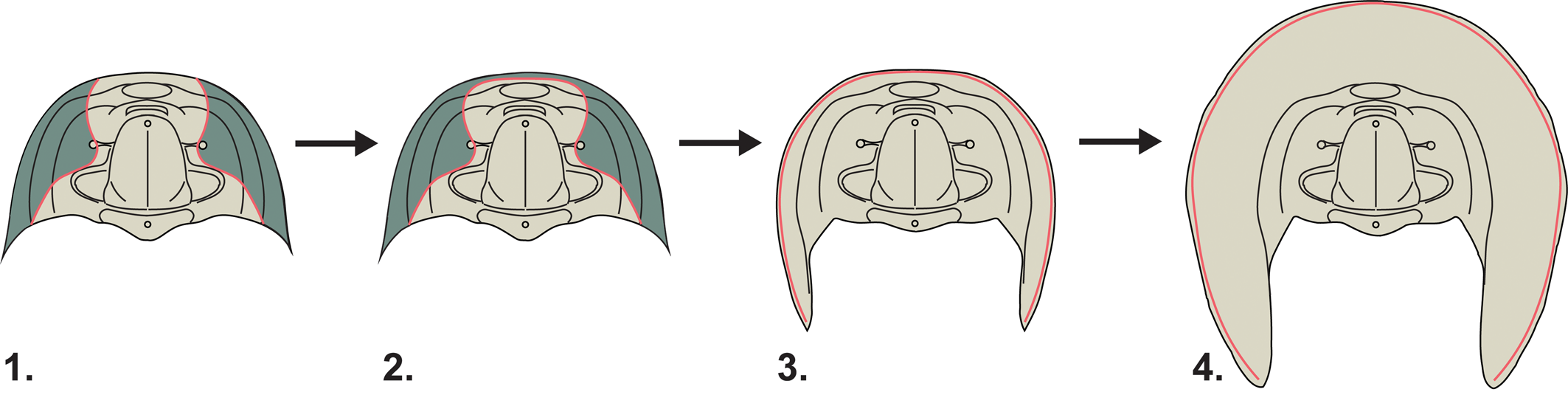
Figure 4. Inferred evolutionary sequence leading to the development of the harpiform brim. The cephalons figured here in dorsal view represent idealized morphotypes rather than specific taxa. The facial sutures are highlighted in red and the librigenae (free cheeks) in green. (1) An ancestral morphology with ptychopariid-like facial sutures and unyoked librigenae. (2) The librigenae become connected by a “yoke” running parallel to the cephalic margin. (3) The facial suture migrates to the outer margin of the cephalon. (4) The development of a wide, flattened bilamellar brim.
With the phylogenetic independence of the two groups and their brims established, we are able to eliminate evolutionary stasis (Eldredge et al., Reference Eldredge, Thompson, Brakefiled, Gavrilets and Jablonski2005; Stayton, Reference Stayton2015) as a possible explanation for similarities between harpetid and trinucleid cephalic morphology. It is tempting on this basis to conclude that harpetids and trinucleids must have independently evolved their harpiform brims as an adaptation to similar ecological niches or in response to similar environmental pressures; such processes are frequently invoked to explain patterns of homoplasy in nature (Wiens et al., Reference Wiens, Chippindale and Hillis2003; Stayton, Reference Stayton2015; Moen et al., Reference Moen, Morlon and Wiens2016).
Unfortunately for this line of reasoning, the ecological function of the harpiform brim is still not well constrained. One early explanation was that the wide brim could have acted as a kind of “snowshoe”, preventing the trilobite from sinking into soft sediments (Stubblefield, Reference Stubblefield1959). Subsequent authors have favored the idea that the brim was instead a sieve, used for filtering food particles (Fortey and Owens, Reference Fortey and Owens1999; Adrain et al., Reference Adrain, Edgecombe, Fortey, Hammer, Laurie, Webby, Paris, Droser and Percival2004), although this idea has been challenged by more recent experiments with physical models (Pearson, Reference Pearson2017; Pearson et al., Reference Pearson, Hubbard and Cruz2019). Schoenemann (Reference Schoenemann2021) focused on the possibility that the brim played a sensory function, either picking up vibrations through the substrate or housing chemosensory organs within its many pits. Still others have suggested that the pitted brim was a plough, a shovel, a hydrostatic device, a respiratory organ, or a strategy for strengthening and lightening the exoskeleton (Bergström, Reference Bergström1972; Ebach and McNamara, Reference Ebach and McNamara2002; McNamara et al., Reference McNamara, Feist and Ebach2009). With such a diversity of plausible explanations, we cannot at present rule out the idea that harpetids and trinucleids evolved their harpiform brims to perform quite different ecological functions, although the two groups often share other morphological traits (e.g., small body size, reduced eyes), which certainly suggests the possibility of a shared ecological niche. Until such time as future experiments are able to better constrain the function of the harpiform brim we cannot assume that the pattern of homoplasy seen in harpetid and trinucleid trilobites also indicates adaptation to a shared niche.
While we may not yet be able to say why the harpiform brim evolved, this phylogenetic work is able to speak to the question of how it evolved. By mapping three key morphological characters related to the harpiform brim across our consensus tree (Fig. 3), we can reconstruct a possible series of evolutionary events leading to the harpiform brim's development (Fig. 4). First, the librigenae, or free cheeks, of the trilobite become yoked, connected to each other by a thin bar running parallel to the cephalic margin so that they are shed as a single unit during ecdysis. In our phylogeny, this character state appears in all harpetids except the putative basal harpetid Baikadimaspis jikdongensis. The absence of this and other important characters, and its failure to reliably form a clade with other harpetids, may indicate that Baikadimaspis is better thought of as a ptychopariid outgroup to a unified Harpetida, and we have provisionally reclassified it as such in the summary of our results (Fig. 3). When the librigenae become yoked, the median suture (or the sutures between the cheeks and the rostral plate) becomes fused. The facial suture then migrates to the extreme margin of the cephalon. In the harpetids in our phylogeny, this character state appears to have evolved twice—once in the family Harpididae and once in the last common ancestor of the family Harpetidae—indicating a probable instance of parallel evolution (Pearce, Reference Pearce2012) within Harpetida. It may be that this marginal suture position created a wider opening in the exoskeleton during ecdysis, allowing wider cephalic brims to evolve without interfering with the molting processes. In harpidids, this widening is moderate and seen only in the more derived members of the family. In harpetidids, however, this widening gives rise to the wide, flattened, and pitted bilamellar brim that is so characteristic of this group of trilobites.
Interestingly, it seems likely that a similar sequence of morphological innovations (Fig. 4) may have occurred in early trinucleids. Looking at the trinucleid clade of our consensus tree (Fig. 3), both yoked librigenae and marginal facial sutures appear as important synapomorphies of the entire group. However, yoked librigenae also appear independently in the asaphid trilobite Haniwa quadrata, suggesting that, of the two, yoked librigenae may be the more easily evolved. Bilamellar harpiform brims appear for the first time in the common ancestor of the poorly resolved clade that includes the families Trinucleidae and Dionididae. Due to the poorly resolved nature of this clade, we cannot rule out the possibility that harpiform brims evolved independently in these two families. However, the close relationship between Dionididae and Trinucleidae recovered here is still notable, because it diverges from the findings of Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020), where dionidids are more closely allied with the raphiophoridids, and may indicate that this part of our phylogeny is being misled by morphological convergence (Wiens et al., Reference Wiens, Chippindale and Hillis2003). Alternatively, Trinucleidae may be genuinely paraphyletic with respect to Dionididae, suggesting that one or both families require revision. Also, of note is that while no brimmed harpetids appear to subsequently lose their harpiform brims, our consensus tree shows multiple examples of secondarily brimless trinucleids, possibly indicating a greater degree of morphological flexibility.
The results of all this character mapping show that, not only did brimmed trinucleids evolve the same suite of important changes to their cephalic morphology as brimmed harpetids, but they also very likely evolved them in the same sequential order. From an ancestral, ptychopariid-like condition, they first evolved yoked librigenae, then marginal facial sutures, and then finally their characteristic broad, bilamellar brims. This makes the harpiform brim a prime example of parallel evolution in the fossil record.
Of course, the dorsal side of any trilobite cannot undergo major changes without significant change to accommodate it from the ventral side. Due to the limited availability of specimens with well-preserved ventral morphology, the most we can currently say is that all of the brimmed trilobites in our sample are inferred to have a merged cephalic doublure, not bisected by any ventral median suture or rostral plate, mirroring the fusion of the facial sutures seen in the dorsal view. Ancestral character state reconstruction indicates that this condition was most likely shared by the last common ancestor of both harpetids and trinucleids, possibly indicating an isolated example of evolutionary stasis (Eldredge et al., Reference Eldredge, Thompson, Brakefiled, Gavrilets and Jablonski2005; Stayton, Reference Stayton2015), although this should be interpreted with caution due to the limited sampling of our ventral characters. This also contrasts with the suggestions of Chatterton et al. (Reference Chatterton, Edgecome, Speyer, Hunt and Fortey1994), which discussed the possibility of the rostral plate being present in early growth stages of trinucleids and raphiophorids.
The phylogenetic position of Trinucleioidea
In our consensus tree (Fig. 3), Trinucleioidea appears as a single clade within Asaphida. This aligns with the earlier assessments of Fortey and Chatterton (Reference Fortey and Chatterton1988) and Chatterton et al. (Reference Chatterton, Edgecome, Speyer, Hunt and Fortey1994) and supports Yang et al.'s (Reference Yang, Peng, Babcock, Zhu and Liu2022) call to restore Trinucleioidea to the order Asaphida. However, unlike several previous studies, this work attaches no special value to larval form or to character states expressed early in ontogeny. Rather, it uses a phylogenetic framework to consider the preponderance of the morphological data and, weighting all characters equally (Congreve and Lamsdell, Reference Congreve and Lamsdell2016), arrives independently at the same conclusion: trinucleids are highly specialized, harpiform asaphids.
To test the strength of that conclusion, we performed multiple constraint analyses (Table 1). Under Constraint 2, trinucleids were constrained to appear as the sister group to a monophyletic Asaphida. This represents the minimum modification to our unconstrained consensus tree (Fig. 3) necessary to entertain the idea that trinucleids are their own group, separate and distinct from Asaphida. This scenario was found to be less parsimonious than a monophyletic Trinucleioidea nested within a paraphyletic Asaphida, but only slightly so—the alternative MPTs found under this constraint were only three steps longer than the unconstrained MPTs. However, even supposing the relationships found under our specified constraints were to be preferred, the picture painted would be one of phylogenetic uncertainty, rather than unequivocal support for an independent order of trinucleids. It is also worth noting that the present analysis is only enforcing the monophyly of a handful of asaphids. A denser sampling of asaphids, requiring more taxa to be constrained to monophyly, might be expected to increase tree length more dramatically.
In light of these considerations, we conducted another constraint analysis, this time using constraints based on the higher order phylogenetic relationships hypothesized by Bignon et al. (Reference Bignon, Waisfeld, Vaccari and Chatterton2020) for the recognition of Trinulceida as an independent order of trilobites. A monophyletic trinucleid–liostracinidid clade was constrained and was further constrained to be more distantly related to a monophyletic Asaphida than to a potentially paraphyletic group of ptychopariids (Constraint 3). We allowed harpetids to group with the ptychopariids in this scenario, based on their position in our consensus tree (Fig. 3) and on previous phylogenetic analyses of the order (Beech and Lamsdell, Reference Beech and Lamsdell2021). Constraint 3 produced alternative MPTs a full 17 steps longer than our unconstrained MPTs, making this set of constraints the least parsimonious of the various hypotheses explored in our constraint analyses (Table 1). Although there are essentially no methods for assessing whether an alternative phylogenetic tree is a statistically significantly worse fit for the available character data within a parsimony framework (Goldman et al., Reference Goldman, Anderson and Rodrigo2000; Smith, Reference Smith2010), this nevertheless suggests that trinucleids should be considered part of the order Asaphida despite their unusual appearances.
In addition to informing the debate surrounding the position of Trinucleioidea as a whole, our findings also have a special bearing on one family in particular: Liostracinidae. The type genus for the family is Liostracina, and the family has been previously interpreted to represent a basal clade of trinucleids (Fortey and Chatterton, Reference Fortey and Chatterton1988; Peng et al., Reference Peng, Babcock and Lin2004; Adrain, Reference Adrain2011; Park et al., Reference Park, Kihm, Kang and Choi2014; Bignon et al., Reference Bignon, Waisfeld, Vaccari and Chatterton2020). Park et al. (Reference Park, Kihm, Kang and Choi2014) in particular used the variable suture types and protaspid morphology of Liostracina to argue that trinucleids should be excluded from Asaphida, an order defined by a ventral median suture and a globular protaspis. They considered liostracinidids to be basal trinucleids, which seemed to be evidence that trinucleids had evolved their globular protaspis separately from Asaphida and should be considered an independent group. However, Yang et al. (Reference Yang, Peng, Babcock, Zhu and Liu2022) examined a new and more complete series of Liostracina fossils, preserving previously unknown thoracic and ventral characters, including a rostral plate rather than the ventral median suture implied by Fortey and Chatterton (Reference Fortey and Chatterton1988), indicating a natant hypostomal condition. From this, as well as the lack of an asaphoid-type protaspis, Yang et al. (Reference Yang, Peng, Babcock, Zhu and Liu2022) concluded that liostracinidids were neither asaphids nor trinucleids. They chose to reclassify Liostracinidae as a ptychopariid family and called for Trinucleioidea to be restored to the order Asaphida. Therefore, the question of whether liostracinidids are or are not basal trinucleids has potentially important implications for larger questions about trilobite taxonomy. However, neither study offered any new phylogenetic analysis in support of their conclusions.
For our analysis, we coded the Liostracina fossils figured in both Park et al. (Reference Park, Kihm, Kang and Choi2014) and Yang et al. (Reference Yang, Peng, Babcock, Zhu and Liu2022), once again placing no special weight on any ontogenetic characters. These two species, along with one species previously assigned to the defunct genus ‘Liostracus’, form a clade that appears as the sister group to the ptychopariid Maladioides coreanicus in our consensus tree (Figure 3). It seems likely that this clade accurately represents the family Liostracinidae and suggests that this family neither belongs to the superfamily Trinucleioidea nor to the order Asaphida. Constraining our liostracinidid taxa to group with the trinucleids (Constraint 4) resulted in less-parsimonious phylogenetic trees and more overall homoplasy. This once again confirms the assessment of Yang et al. (Reference Yang, Peng, Babcock, Zhu and Liu2022) and complements their analysis with new phylogenetic data not contingent on a specially chosen character of interest. This finding also implies additional support for the idea that genuine trinucleids belong within the order Asaphida.
Conclusions
This study reevaluates the relationship between the order Harpetida and the superfamily Trinucleioidea—two trilobite groups that are made instantly recognizable and superficially similar by a number of shared morphological traits. The most notable of these is the ‘harpiform’ brim, a structure that is here shown to be the result of parallel evolution in harpetids and in trinucleids. While further study is needed to address the hypothesis that the harpiform brim arose in both groups in response to similar ecological pressures, that the brim arose independently in both groups is shown clearly by our phylogenetic analysis. Character mapping across our phylogeny reveals that the same sequence of morphological innovations likely led to the evolution of the harpiform brim in both groups. In addition, our analysis indicates support for the idea that trinucleids are highly specialized, harpiform asaphids, rather than constituting an independent order of trilobites (or grouping with the harpetids). Finally, our analysis reexamines the family Liostracinidae, which had previously been suggested to be a basal-most trinucleid clade and confirms recent assessments that this family is more distantly related to the trinucleids. Overall, this work highlights the importance of appropriate phylogenetic methods and the ways that they can be used to distinguish different kinds of morphological similarity, even in fossil species without close living relatives.
Acknowledgments
The authors would like to thank F. Corsetti, A. Hendy, D. Cameron, and J. Lamsdell for their valuable feedback on the project, and B. Hunda, C. Schwalbach, J. Utrup, S. Butts, J. Cundiff, and L. Stevens for all their help in accessing the specimens that made this work possible. We also thank B. Hunda, L. Collantes, L. Laibl, and two anonymous reviewers for their thoughtful comments and corrections. We would like to express our thanks to the Cincinnati Museum Center, the Yale Peabody Museum of Natural History, the Museum of Comparative Zoology at Harvard University, and the Natural History Museum, London for the firsthand use of their specimens. The work was made possible by funding from the Geological Society of America, the Paleontological Society, and the University of Southern California.
Declaration of competing interests
The authors declare none.
Data availability statement
Supplemental data are available from MorphoBank: http://morphobank.org/permalink/?P4115.
Appendix
Characters for phylogenetic analysis.—Illustrated examples of many of these characters may also be found in Beech and Lamsdell (2021, supplement 1). These characters are marked with a *.
1. Anterior axial spine extending from the preglabellar cephalon: absent (0); present (1).
2. Glabellar spine: absent (0); present (1).
3. Curvature of cephalic margins: straight (0); curved (1); angulate (2).
4. Angle of cephalic curvature*: greater than 90 degrees (0); 90 degrees or less (1).
5. Cephalon convexity*: low (e.g., Dionide) (0); high (e.g., Globoharpes) (1).
6. Widest point of cephalon*: posterior one-third (0); anterior one-third (1); central one-third (2).
7. Cephalic length over cephalic width: 0.43 or lower (foreshortened) (0); between 0.5 and 0.43 (proportionate) (1); 0.5 or greater (elongate) (2).
8. Marginal rim demarcated along its inner margin by enlarged pits*: inner margin of rim NOT demarcated by enlarged pits (0); row of enlarged pits demarcates inner margin of rim (1).
9. Harpiform bilamellar brim*: absent (0); present (1).
10. Harpiform bilamellar brim profile*: flat (0); concave (1); convex (2).
11. Brim width*: narrower than glabellar length (0); equal to or wider than glabellar length (1).
12. Upper lamella of brim inflated anterolaterally: absent (0); present (1).
13. Narrowing of brim along prolongations*: brim constant in width for the majority of prolongation (0); brim narrowing almost from the level of the occipital ring (1).
14. Pitting present on outer field of cephalon/brim*: absent (0); present (1).
15. Brim pitting extent*: less than 50% of the brim surface visibly pitted (0); 50% or more than of the brim surface visibly pitted (1).
16. Brim pitting extending to genal angle: absent (0); present (1).
17. Brim pitting organization: disorganized (0); organized (1).
18. Raised ridges between rows of pits: absent (0); present (1).
19. Radiating ridges at the genal roll-brim boundary*: absent (0); present (1).
20. Girder separating genal roll from brim*: absent (0); present (1).
21. Girder kink*: absent (0); present (1).
22. Deep pits along outer margin of genal roll*: absent (0); present (1).
23. Hypostome form*: maximum constriction found within the anterior one-fourth of hypostome (0); maximum constriction found at or near the midpoint of hypostome (1); maximum constriction found within the posterior one-fourth of hypostome (2).
24. Frontal lobe position: posterior to anterior border (0); overhanging anterior border (1).
25. Frontal lobe outline: subcircular (0); subovate (1); subquadrate (2); subtriangular (3).
26. Median glabellar tubercule*: absent (0); present (1).
27. Median glabellar tubercule position: anterior third of glabella (0); posterior to anterior third of glabella (1).
28. Reduced preglabellar field: absent (0); present (1).
29. Preglabellar transverse ridge*: absent (0); present (1).
30. Median preglabellar depression or furrow*: absent (0); present (1).
31. Anterior boss*: absent (0); present (1).
32. Genal roll: absent (0); present (1).
33. Vaulted inner genal roll*: absent (0); present (1).
34. Glabellar morphology*: narrowing anteriorly (0); widening anteriorly (1).
35. Glabella laterally overlaps genal field: absent (0); present (1).
36. Glabella height: less than two times the genal height (0); two times the genal height or greater (1).
37. Depth of 1st pair of lateral glabellar furrows (S1)*: deep, well defined (0); shallow, poorly defined (1).
38. Morphology of S1*: posterolaterally directed furrows (0); J-shaped furrows not continuous with S0 (1); semi-circular (2); anterolaterally directed furrows (3); parallel to long axis of the glabella (4).
39. Contact between first glabellar furrow (S1) and axial furrow: absent (0); present (1).
40. Second pair of lateral glabellar furrows (S2)*: absent (0); present (1).
41. Third pair of lateral glabellar furrows (S3)*: absent (0); present (1).
42. Length of 1st pair of lateral glabellar furrows (S1)*: shorter than 50% of glabellar width (0); 50% of glabellar width or longer (1).
43. Maximum length of S2 or S3*: long, c. 50% of glabella width (0); short, c. 25% of glabella width (1).
44. Positioning of S2 and S3*: evenly spaced (0); S2 and S3 close together, third pair of glabellar lobes (L3) expanded (1).
45. Cross-sectional morphology of glabellar furrows*: rounded (0); incised (1).
46. Curvature of glabellar furrows S2–S3*: straight (0); incurving (curve posteriorly) (1); outcurving (curve anteriorly) (2); divots (3).
47. Glabellar lateral margins*: converging anteriorly (0); parallel (1); converging posteriorly (2).
48. Relative area of first pair of glabellar lobes (L1)*: L1 less than 10% of glabellar area (0); L1 10% of glabellar area or more (1).
49. Glabellar and genal primary surface ornament/sculpture*: tuberculate (0); reticulation of pits (1); fine granulations (2); smooth (3); punctate (4); striated (5).
50. Sagittal crest*: absent (0); present (1).
51. Yoked librigenae*: absent (0); present (1).
52. Facial suture position*: cuts through outer margin of cephalon (0); skirts margin (1).
53. Ventral cephalic assembly: separated by rostral plate or rostellum (0); with median ventral suture (1); merged (2).
54. Angle of anterior facial suture and transverse line passing through both compound eyes*: 30–45 degrees (0); greater than or equal to 60 degrees (1); less than or equal to 0 degrees (2).
55. Cephalic sutures with marked inward curve just posterior to cephalic margin*: absent (0); present (1).
56. Eyes: absent (0); eyed (1).
57. Eye structure*: eye lobes (0); tubercles (1). Taxa without eyes coded as inapplicable (-).
58. Angle formed between axis of greater elongation of eye and longitudinal axis of cephalon *: diverging posteriorly (0); equal (1).
59. Anterior–posterior position of eye: on posterior of cephalon (0–48%)*: (0); on midline or anterior (more than 48%) (1); eye encompasses entire cephalon length (2).
60. Genal ridge running posterolaterally from eye*: genal ridge absent (0); genal ridge present (1).
61. Eye area in dorsal view (as percentage of cephalon)*: 20–30% (0); less than 20% (1).
62. Eye ridges*: absent (0); present (1).
63. Eye ridge direction*: anterolaterally directed (0); posterolaterally directed (1); transversely directed (perpendicular to longitudinal axis of the body) (2).
64. Ridge insertion on compound eye*: anterior point of eye (0); mid-point of eye (1).
65. Palpebral lobe: absent (0); present (1).
66. Genae curvature*: flattened/moderately convex (0); concave (1); extremely convex (2).
67. Radiating, anastomosing genal caeca*: absent (0); present (1).
68. Axial furrow depth*: shallow (e.g., Kielania) (0); deep (e.g., Cryptolithus) (1).
69. Extension of axial furrows beyond glabella onto genal roll*: absent (0); present (1).
70. Alae*: absent (0); present (1).
71. Alae strength as defined by alar furrow*: faint (shallow alar furrow) (0); strong (deep alar furrow) (1).
72. Alae morphology*: subdivided into two crescentic portions by presence of interalar furrow (0); continuous, interalar furrow absent (1).
73. Relief of alae*: depressed or sunken (0); flattened/low relief (1); inflated (2).
74. Vaulting of inflated alae*: inflated alae not exhibiting vaulting (0); inflated alae vaulted (1).
75. Alae size*: small (less than 10% glabellar volume) (0); large (10% glabellar volume or greater) (1).
76. Alar direction*: transversely/laterally directed (0); anterolaterally directed (1).
77. Large pits on genal area opposite alae*: absent or not enlarged (0); present and enlarged (1).
78. Anterior alar ridge*: absent (0); present (1).
79. Median tubercule on occipital ring*: absent (0); present (1).
80. Occipital furrow: anteriorly curved or transverse (0); posteriorly curved (1).
81. Occipital ring posterior border: straight (0); posteriorly curved (1).
82. Occipital ring height: lower than genal field (0); as high as the genal field or higher (1).
83. Orientation of occipital ring in lateral view: vertical (0); posteriorly oriented (1).
84. Genal spines or prolongations*: posterolateral margin of cephalon extending into genal spines/prolongations (0); cephalon not extending into genal spines/prolongations (1).
85. Morphology of genal spines or prolongations*: broad, flattened (prolongations) (0); narrow, rounded (spines) (1); both (2).
86. Genal spine or prolongation curvature*: medial margin straight (0); medial margin incurving (1).
87. Genal spine or prolongation angle of divergence*: high (greater than or equal to 30 degrees) (0); moderate (11–29 degrees) (1); reduced (less than or equal to 10 degrees) (2).
88. Curvature of genal spines or prolongations*: straight (rapidly flattens from cephalon) (0); concave (twisted along length of prolongation) (1).
89. Genal spine or prolongation length*: longer than post-brim cranidium (0); equal to or shorter than post-brim cranidium length (1).
90. Genal spines or prolongations in cross-section: circular to subcircular (0); subquadrate to sub-rectangular (1).
91. Thoracic width: constant (0); posteriorly reducing only (1); anteriorly and posteriorly reducing (2); anteriorly reducing only (3).
92. Maximal thoracic width: much wider than maximal pygidial width (0); similar to maximal pygidial width (1).
93. Maximal thoracic length: similar to pygidial length (0); much longer than pygidial length (1).
94. Thoracic axis width (at broadest point)*: less than glabellar width (0); equal to glabellar width (1); greater than glabellar width (2).
95. Thoracic tergite count*: less than or equal to 15 (0); more than or equal to 16 (1).
96. Form of pleural spine terminations on thoracic tergites*: acute, spinous (0); blunt (1).
97. Thoracic pleural furrow strength: faint (0); deep (1).
98. Thoracic pleural furrow curvature: straight (0); forward curving (concave front) (1); backward curving (concave back) (2); sinuous (3).
99. Pygidial anterior border: straight (0); curved (1).
100. Pygidial outline: rounded to subcircular (0); triangular to subtriangular (1).
101. Pygidial width over pygidial length: 1.25 or lower (0); 1.26 to 1.99 (1); 2 or more (2).
102. Extremely transverse pygidium: pygidial width 2 to 3 times pygidial length (0); pygidial width more than 3 times pygidial length (1).
103. Pygidium compared to cephalon: much smaller (0); subisopyous (1); isopygous (2).
104. Pygidium with medial posterior indentation*: absent (0); present (1).
105. Number of axial rings in pygidium*: 4–6 (0); 3 (1); 2 (2); 7+ (3).
106. Pygidial pleural furrows: faint (0); well defined (1).
107. Pygidial border width: constant (0); posteriorly wider (1).
108. Pygidial border surface: smooth (0); bearing terrace lines (1).
109. Pleural field margin*: downturned (0); upturned (1); flat (2).
110. Pygidial axis extremity in lateral view: not overhanging posterior border (0); overhanging posterior border (1).
111. Terminal lappets*: absent (0); present (1).
112. Protaspis type: adult-like (0); non-adult-like/globular (1).








