INTRODUCTION
Severe sepsis and septic shock are the most common causes of morbidity and mortality in critically ill patients [Reference Angus1–Reference Harrison3]. Angus et al. [Reference Angus1] reported an incidence of severe sepsis of 2·26 cases/100 hospital discharges with 51·1% requiring intensive care. The overall mortality rate was 28·6%, and this increased in patients with comorbidities and more organ failure [Reference Angus1]. More recently data from a large European study, Sepsis Occurrence in Acutely Ill Patients (SOAP), showed that severe sepsis accounted for 29·6% of intensive care unit (ICU) admissions and mortality rates in ICU of patients with severe sepsis and septic shock were 32·2% and 54·1%, respectively [Reference Vincent4]. However, despite dramatic improvements in our knowledge of pathogenesis, diagnosis and therapeutic and supportive care, the mortality of septic patients remains unacceptably high with the overall rate in the range of 30–50% in severe sepsis cases and increasing to 50–87% in septic shock patients [Reference Harrison3–Reference Tanriover16].
Several studies have explored the epidemiology, outcomes and risk factors of mortality in severe sepsis and septic shock from different populations and different times [Reference Harrison3–Reference Tanriover16]. However, these features may be subject to change as a result of the larger numbers of comorbidities in these patients, e.g. increase in age, higher frequency of infections caused by antibiotic-resistant organisms, more frequent use of invasive procedures or devices, and new adjuvant therapies for severe sepsis and septic shock. In addition, the epidemiological picture and surveillance data can differ significantly between ICUs and between countries.
We conducted this study to describe the demographic characteristics, microbiological, outcome and risk factors of hospital mortality in ICU patients admitted due to severe sepsis or septic shock in an emerging country such as Thailand.
METHODS
The study was conducted in the ICUs of Songklanagarind Hospital, an 854-bed tertiary referral university teaching hospital at Prince of Songkla University in southern Thailand. The adult ICU comprised two units: a 10-bed surgical unit and a 10-bed mixed medical and coronary care unit. Analysis of data collected prospectively between 1 July 2004 and 30 June 2006 was performed. All new admissions aged >15 years to any of these units during this period with severe sepsis or septic shock were included. Those admitted to the ICU for <24 h duration for routine post-operative care were excluded. The study subjects were followed up until death or hospital discharge. If a patient was discharged and then readmitted during the study period, only the first admission was included. Approval for the project was obtained from the Faculty Ethics Committee.
Clinical definitions
Infection was identified based on clinical history, physical examination, laboratory findings and administration of antibiotics (excluding antimicrobial prophylaxis) and defined according to the International Sepsis Forum Consensus Conference [Reference Calandra and Cohen17]. Sepsis was defined according to the American College of Chest Physicians/Society of Critical Care Medicine consensus as infection plus at least two systemic inflammatory response syndromes [18]. Severe sepsis was defined as sepsis plus failure of at least one organ. Organ failure was defined as a Sequential Organ Failure Assessment (SOFA) score of >2 for each involved organ [Reference Vincent4, Reference Vincent19]. Septic shock was defined as sepsis-induced hypotension [systolic blood pressure (SBP) <90 mmHg or mean arterial pressure (MAP) <65 mmHg] for at least 1 h despite adequate fluid resuscitation (central venous pressure >8 mmHg or pulmonary artery occlusion pressure >12 mmHg) or use of a vasopressor (dopamine >5 μg/kg per min or norepinephrine, epinephrine any dose) for >1 h in an attempt to maintain SBP >90 mmHg or MAP >65 mmHg. Community-acquired infection was defined as manifestation of infection before or within 48 h after admission whereas hospital-acquired infection was manifest later than 48 h after hospital admission. Mixed infections referred to infections that were considered to have affected more than one type of organism per patient. Acute respiratory distress syndrome (ARDS) was defined by acute onset, presence of bilateral infiltrates on a CXR, PaO2/FiO2 ratio <200 mmHg regardless of the level of positive end-expiratory pressure, and absence of clinical evidence of left atrial hypertension.
Data collection on admission included demographic data and comorbidities as defined according to Knaus et al. [Reference Knaus20] (liver cirrhosis, metastatic cancer, severe chronic obstructive pulmonary disease, AIDS, haematological malignancy, chronic renal failure requiring renal replacement and immunocompromised host). Clinical and laboratory data for the Acute Physiology and Chronic Health Evaluation II (APACHE II) [Reference Knaus20] and SOFA scores [Reference Vincent19] were reported as the worst value within 24 h after admission. The Logistic Organ Dysfunction score (LOD) was calculated on admission and every 24 h until discharge from the ICU. The worst physiological values of each organ failure in the 24 h following ICU admission and those subsequent were used for our calculations as outlined in the original literature [Reference Le Gall21]. Mean fluid balance was calculated as the total fluid balance during the ICU stay divided by the duration of the ICU stay in days. Procedures during ICU stay such as central venous and pulmonary artery catheter (PAC) placement and continuous renal replacement therapy (CRRT) were recorded.
Data were entered on the computer using EpiData 3.21 software (The EpiData Association, Denmark) and analysed using Stata 7 software (Stata Corporation, USA). Normal and non-normal distribution were reported as mean±s.d. and median with interquartile range, respectively. Student's t test and Wilcoxon's rank sum test were used to compare normally distributed continuous variables and non-parametric data, respectively. χ2 test was used to test for the statistical significance of categorical variables. A backward elimination logistic regression multivariate analysis with hospital mortality as the dependent factor was performed. Variables include the potentially relevant variables of age, gender and those found to be significant to P<0·2 on univariate analysis. All statistics were two-tailed and a P value <0·05 was considered to be statistically significant.
RESULTS
During the study period 390 patients were diagnosed with severe sepsis or septic shock in a total of 2057 patients admitted to the ICUs (18·9/100 ICU admissions). The incidence of severe sepsis and septic shock increased significantly from 16·6 to 21·6/100 ICU admissions during the first and second year of the study but hospital mortality did not decrease significantly during the same period (51·2% vs. 48%, respectively).
Severe sepsis and septic shock were identified in 87 (22·3%) and 303 (77·7%) patients, respectively and the overall ICU and hospital mortality rate were 39·2% and 49·7%. The ICU mortality rate of patients with severe sepsis was 21·8% and 44·2% in those with septic shock. However, the hospital mortality of severe sepsis and septic shock patients was 34·5% and 54·1%. The most common sources of ICU admission were the general ward (70%), emergency room (20%) and operative/recovery rooms (10%). Mortality rates did not differ between patients admitted from the general ward or emergency room but the mortality rate of patients admitted from operative/recovery rooms was lower than patients admitted from general ward (30·8% vs. 52·8%, P=0·01).
Comorbidities were reported in 157 (40·3%) patients (Table 1); these were older than patients without comorbidities (60·5 vs. 50 years, P<0·001). ARDS was identified in 80 patients (20·5%) and was more frequent in septic shock than in those with severe sepsis (68·8% vs. 31·3%, P=0·03). Septic patients with ARDS had significant higher mortality than those without ARDS (68·8% vs. 31·3%, P<0·001). PAC was more commonly used in septic patients with ARDS than in non-ARDS patients (64·5% vs. 35·5%, P<0·001).
Table 1. Demographic data, severity and organ dysfunction scores, procedures in the ICU and length of hospital stay
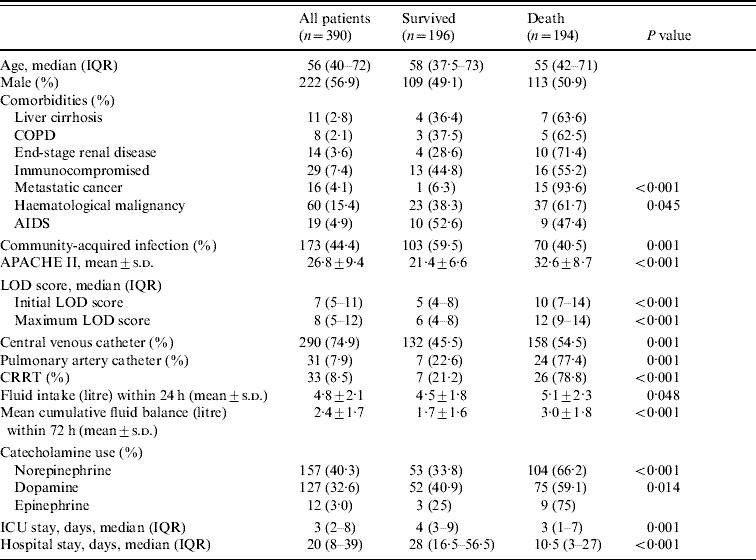
ICU, Intensive care unit; IQR, Interquartile range; COPD, chronic obstructive pulmonary disease; AIDS, acquired immune deficiency syndrome; APACHE II, Acute Physiology and Chronic Health Evaluation II; LOD, Logistic Organ Dysfunction score; CRRT, continuous renal replacement therapy.
Table 2 shows that community-acquired infections occurred in 173 (44·4%) of patients and that patients with hospital-acquired infections had higher hospital mortality than those with community-acquired infections (57·1% vs. 40·5%, P=0·001). The respiratory tract was the most common site for both community- and hospital-acquired infections and septic patients with a primary bloodstream infection were more likely to die than patients with other sites of infection. Patients who survived had significantly more urinary tract and obstetrics/gynaecological infections. Other sites of infection were not significantly different between the survivor and non-survivor groups. Multiple sites of infection were involved in seven hospital-acquired infection patients. Blood cultures were positive in 106 (27·5%) patients but the incidence of bacteraemia was not different between the community- and hospital-acquired infection groups (47·2% vs. 52·8%, respectively). Microorganisms were isolated from 241 patients (61·8%), including 66·7% of community-acquired infection cases and 58% of hospital-acquired infection cases. The most frequent organisms were Klebsiella pneumoniae (19·9%), Escherichia coli (14·1%) and Pseudomonas aeruginosa (9·1%) (Table 3). Gram-negative bacteria predominated in 68% of cases whereas Gram-positive species were found only in 19·9% of samples. Mixed infections accounted for 56 (23·2%) patients and those with hospital-acquired infection had a higher incidence of mixed infections than patients with community-acquired infection (18·3% vs. 9·4%, P=0·013).
Table 2. Demographic data, severity and organ dysfunction scores, length of hospital stay and infection foci for the source of infection

IQR, Interquartile range; APACHE II, Acute Physiology and Chronic Health Evaluation II; LOD, Logistic Organ Dysfunction score; ICU, intensive care unit.
Table 3. Microorganisms isolated from patients with severe sepsis and septic shock stratified according to source and type of infection
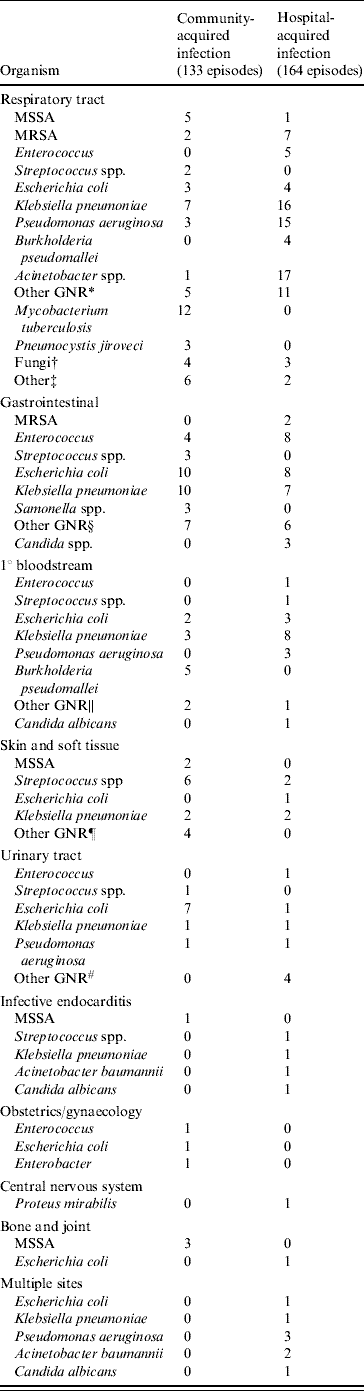
MSSA, Methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; GNR, Gram-negative rod.
* Other GNR: Aeromonas, Enterobacter, Haemophilus, Morganella, Morexella, Serratia, Sphingobacterium, Xanthomonas, anaerobes.
† Fungi: Aspergiilus, Candida, Histoplasma.
‡ Other: Cytomegalovirus, Leptosira, Nocardia.
§ Other GNR: Aeromonas, Acinetobacter, Bacteroides, Enterobacter, Proteus.
∥ Other GNR: Aeromonas, Acinetobacter.
¶ Other GNR: Acinetobacter, Morganella, Pseudomonas.
# Other GNR: Aeromonas, Enterobacter, Proteus.
The microorganism was considered once per patient even if present in more than one site. The numbers of episodes are defined solely for patients with microbiological documentation.
Multiple organ failures occurred in 242 (62·1%) patients within 24 h of ICU admission, the most common being cardiovascular, pulmonary and neurological (78·9%, 52·1% and 25·1%, respectively). The frequency of organ failure and the correlation of hospital mortality rate are shown in Figure 1. Patients with single organ failure during the first 24 h after admission had a hospital mortality of 24·3% which increased to 82·9% in those with more than four organ failures.
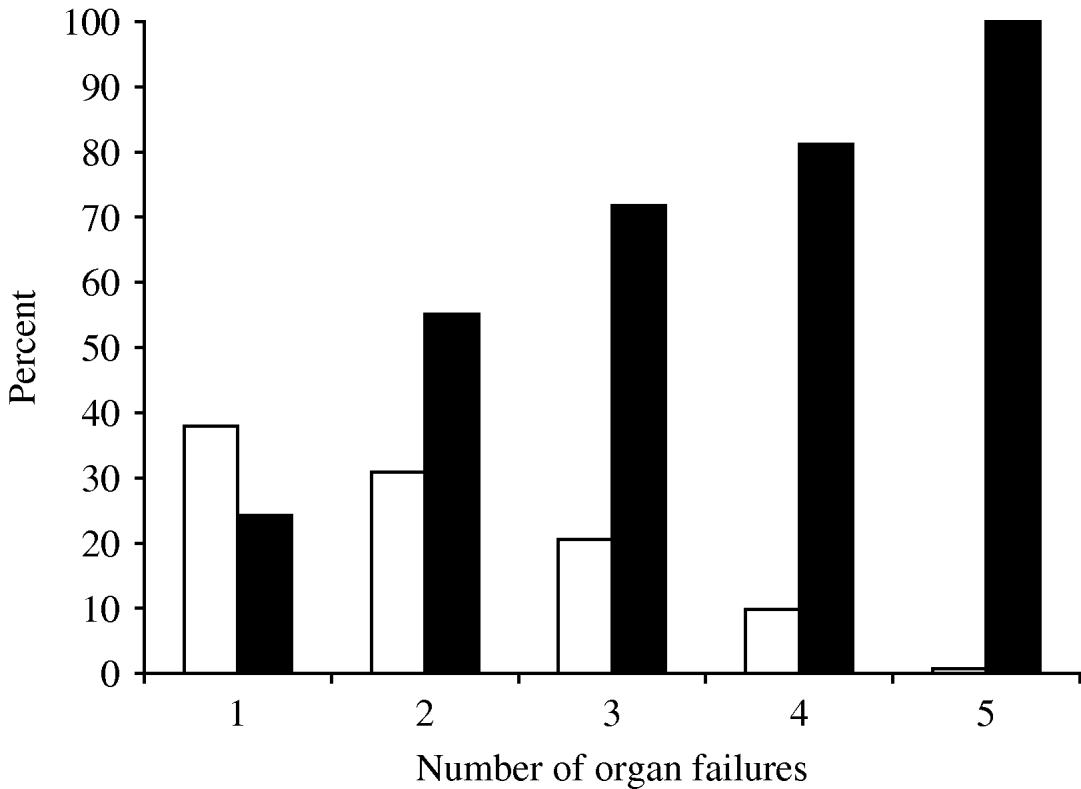
Fig. 1. Frequency of initial organ failure and the correlation with hospital mortality rate. □, Patients; ![]() , mortality.
, mortality.
Univariate analysis showed that several factors were associated with a significantly higher hospital mortality in patients with severe sepsis: comorbidities (P<0·001), ARDS (P<0·001), central venous catheter placement (P=0·001), PAC placement (P=0·001), CRRT (P<0·001), vasopressor use prior to ICU admission (P=0·019), hospital-acquired infection (P=0·001), melioidosis (P=0·017), primary bloodstream infection (P=0·04), dopamine use (P=0·014), norepinephrine use (P<0·001), mean cumulative fluid balance within the first 72 h (P<0·001), admission APACHE II score (P<0·001), admission LOD score (P<0·001), maximum LOD score (P<0·001), number of organ failure during admission (P<0·001), ICU length of stay (P=0·001) and hospital length of stay (P<0·001). Other factors associated with a trend towards higher mortality included fluid intake within the first 24 h (P=0·05), epinephrine use (P=0·06), central nervous system infection (P=0·181) and leptospirosis (P=0·102). However, urinary tract infection (P=0·023) and obstetrics/gynaecological infection (P=0·014) were associated with a lower hospital mortality. However, after multivariate analysis using logistic regression only ARDS, PAC placement, hospital-acquired infection, patients with comorbidities, APACHE II score on admission and maximum LOD score were independent predictors of increased risk of hospital mortality (Table 4).
Table 4. Multivariate logistic regression analysis with hospital mortality as the dependent factor
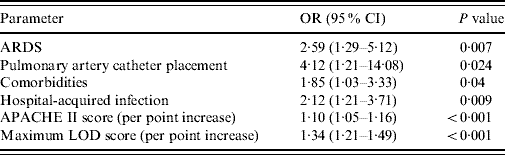
OR, Odds ratio; CI, confidence interval; ARDS, Acute respiratory distress syndrome; APACHE II, Acute Physiology and Chronic Health Evaluation II; LOD, Logistic Organ Dysfunction score.
DISCUSSION
This study found a high admission rate of severe sepsis in the ICUs of the major tertiary hospital in southern Thailand, with an overall hospital mortality rate approaching half of all patients. The respiratory tract was the most common sites of infection with a predominance of Gram-negative bacteria as the major cause. Comorbidities, ARDS, PAC placement, hospital-acquired infection, initial severity score and multiple organ dysfunctions during the ICU stay were risk factors for hospital mortality in patients with severe sepsis or septic shock.
The number of admissions for severe sepsis or septic shock (18·9%) to the ICU in this study is higher than most previous reports [Reference Alberti7–Reference Silva14] but still significantly lower than some studies such as Adrie et al. [Reference Adrie5] in France (42%) and Padkin et al. [Reference Padkin15] in England (27·1%). It is, of course, difficult to compare the epidemiologies of severe sepsis in different settings due to different populations, methodologies and types of ICU enrolment. The prevalence of severe sepsis increased significantly over the 2 years of the study in line with previous reports of increasing incidence of ICU admission with severe sepsis and associated mortality rates [Reference Angus1–Reference Harrison3].
This study had a high proportion of septic shock patients (77·7%) [Reference Vincent4, Reference Brun-Buisson8, Reference Silva14] with a higher mean severity score than previous studies [Reference Vincent4, Reference Adrie5, Reference Brun-Buisson8–Reference Silva14]. However, the ICU and hospital mortality rates from severe sepsis and septic shock here were slightly lower than previous reports [Reference Vincent4, Reference Annane6, Reference Brun-Buisson8, Reference Engel11, Reference Silva14–Reference Tanriover16]. The SOAP study reported an ICU mortality rate of 32·2% for patients with severe sepsis and 54·1% for septic shock [Reference Vincent4]. Annane et al. [Reference Annane6] documented a hospital mortality rate of 61·2% in septic shock patients and Brun-Buisson et al. [Reference Brun-Buisson8] reported 59% in severe sepsis in France. Nevertheless, the sepsis mortality rate in our study was similar to [Reference Cheng10] or higher than some other studies [Reference Karlsson13, Reference Finfer22]. Several factors may explain these differences, for example the criteria used for diagnosis of severe sepsis and septic shock, the ratio of septic shock to severe sepsis patients, associated comorbidities, type of infection, severity of sepsis and number or severity of organ failures and standard of care of the patient.
The respiratory tract was the most common site of infection, as in other reports [Reference Vincent4, Reference Alberti7, Reference Brun-Buisson8, Reference Engel11–Reference Silva14], and the frequency of recovery of microorganisms (61·8%) falls within the range of 45–70% described in other studies [Reference Vincent4, Reference Alberti7–Reference Karlsson13]. Similarly, Gram-negative bacteria predominated as was found in a tertiary care hospital in Turkey (65·9%) [Reference Tanriover16], and in 53·8% of severe sepsis cases in critically ill surgical patients in China [Reference Cheng10]. However, microbiological patterns in the present study were quite different from the reports that Gram-positive bacteria were predominant in Western countries [Reference Angus1, Reference Vincent4, Reference Alberti7, Reference Brun-Buisson8, Reference Engel11–Reference Karlsson13]. Moreover, fungi were less frequently isolated (4·4%), which differs markedly from other reports of fungal infections ranging from 5·9–28·3% [Reference Vincent4, Reference Cheng10–Reference Finfer12]. The reasons for the differences in microbiological patterns of severe sepsis and septic shock may be the result of differing characteristics in patient populations, differing comorbidities, less extensive use of broad-spectrum antibiotics, or less use of invasive interventions or procedures. Nevertheless, microorganisms, bacteraemia and site of infection were not the significant risk factors for death in severe sepsis patients in our study.
Several factors related to an increased mortality in severe sepsis and septic shock cases were consistent with the literature such as comorbidities [Reference Brun-Buisson8–Reference Cheng10, Reference Alberti23], hospital-acquired infection [Reference Alberti23], higher severity [Reference Vincent4, Reference Brun-Buisson8–Reference Cheng10, Reference Alberti23, Reference Pittet24] and organ dysfunction scores [Reference Vincent4, Reference Brun-Buisson9, Reference Cheng10, Reference Alberti23, Reference Pittet24]. However, we found that only the maximum LOD score was an associated prognosis factor for septic patients. Several studies have found that maximum organ dysfunction scores correlated better with mortality than an initial score [Reference Ferreira25]. Indeed previous studies in our population have shown that the APACHE II and LOD are better correlated with mortality prediction than other scores [Reference Khwannimit26, Reference Khwannimit and Geater27], hence the reason for using these scores here.
The importance of ARDS and PAC as a predictor of hospital outcome is also interesting. Although sepsis has previously been identified as a mortality risk factor in ARDS patients [Reference Estenssoro28, Reference Zilberberg and Epstein29], it is a new finding that ARDS can be associated with a greater mortality in severe sepsis and septic shock cases. Despite adjustment with severity scores, PAC was one of the independent risk factors for hospital death in severe sepsis in this study. Yu and colleagues [Reference Yu30] found that PAC placement was not associated with mortality in severe sepsis but was an increased risk for renal failure in a case-control study. Moreover, several studies have shown PAC insertion to be associated with a higher complication rate and no improvement in survival [Reference Sandham31–Reference Harvey33]. Importantly, however, none of these studies used PAC-derived variables to attempt to define therapies of proven benefit. Thus, a prospective randomized controlled trial to attempt to ascertain the effectiveness of PAC, including a strict protocol treatment in severe sepsis patients could be a useful future investigation.
Age has been associated with an increased mortality in severe sepsis patients [Reference Vincent4, Reference Adrie5, Reference Cheng10, Reference Engel11]. However, we were unable to support this finding as in other studies [Reference Brun-Buisson8, Reference Brun-Buisson9, Reference Alberti23, Reference Pittet24]. A possible explanation is that the effect of age has probably been erased by the presence of comorbitidies and APACHE II score. Patients with comorbidities were found in the older group and age is used to derive the APACHE II score.
Our study had some limitations. First, as a single centre there may have been bias concerning ICU admission, quality of ICU care and management. Second, there was no attempt to evaluate ICU-acquired infections and finally, we did not evaluate the Surviving Sepsis care bundles. A multicentre study examining the epidemiology and outcomes of severe sepsis with sepsis bundle treatment should be conducted in Thailand.
In conclusion, severe sepsis and septic shock are a frequent cause of ICU admission and have a high mortality rate. Comorbidities, ARDS, PAC placement, hospital-acquired infection, APACHE II score and maximum LOD score are the risk factors for hospital death. Patients with these factors should be closely monitored. Careful assessment in sepsis patients is needed to ensure effective ICU utilization and care strategies, early aggressive treatment and also stratification of patients in clinical trials or accounting for confounding factors in subgroup analysis.
ACKNOWLEDGEMENTS
This study was supported by a research grant from the Faculty of Medicine, Prince of Songkla University. We thank Alan Geater of the Epidemiology Unit, Faculty of Medicine, Prince of Songkla University, for his statistical review and comments.
DECLARATION OF INTEREST
None.







