Introduction
Cannabis use is common in the general population in many high-income countries. Some cannabis policy regimes have become more liberal, including legalization and regulation of adult use and supply in some instances (Fischer, Daldegan-Bueno, & Boden, Reference Fischer, Daldegan-Bueno and Boden2020; Hall et al., Reference Hall, Stjepanovic, Caulkins, Lynskey, Leung, Campbell and Degenhardt2019). Non-medical cannabis use has been legalized in Canada, Uruguay, and Mexico, and in fifteen US states, with other jurisdictions considering such a step.
An essential component of public health-oriented approaches to cannabis policy is how to best reduce health harms among – the mostly young – populations of users (Fischer, Rehm, & Hall, Reference Fischer, Rehm and Hall2009; Hoch & Lorenzetti, Reference Hoch and Lorenzetti2020; Melchior et al., Reference Melchior, Nakamura, Bolze, Hausfater, El Khoury, Mary-Krause and Da Silva2019). The main risks for adverse health outcomes of non-medical cannabis use include acute and chronic neurocognitive functioning impairments; mental health problems (psychosis/schizophrenia, depression); cannabis use disorder (CUD), cannabis-impaired driving and motor-vehicle crashes resulting in injuries/death, and pulmonary problems associated with cannabis smoking (Duperrouzel, Granja, Pacheco-Colon, & Gonzales, Reference Duperrouzel, Granja, Pacheco-Colon and Gonzales2020; Leung, Chan, Hides, & Hall, Reference Leung, Chan, Hides and Hall2020; National Academies of Sciences Engineering & Medicine, 2017; Preuss et al., Reference Preuss, Huestis, Schneider, Hermann, Lutz, Hasan and Hoch2021; Sánchez-Gutiérrez et al., Reference Sánchez-Gutiérrez, Fernandez-Castilla, Barbeito, González-Pinto, Becerra-García and Calvo2020; Volkow et al., Reference Volkow, Swanson, Evins, DeLisi, Meier, Gonzalez and Baler2016).
Recent reviews have confirmed a moderately significant association between cannabis use and psychosis (Kiburi, Molebatsi, Ntlantsana, & Lynskey, Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; Polkosnik, Sorkhou, & George, Reference Polkosnik, Sorkhou and George2021; van der Steur, Batalla, & Bossong, Reference van der Steur, Batalla and Bossong2020). The association between cannabis use and mental health problems, most notably psychosis, receives prominent attention in policy debates, even though other adverse outcomes (e.g. cannabis-impaired driving, CUD) are more common and make greater contributions to the cannabis-attributable burden-of-disease (DeVylder, Mittal, & Schiffman, Reference DeVylder, Mittal and Schiffman2021; Hall & Degenhardt, Reference Hall and Degenhardt2008; Imtiaz et al., Reference Imtiaz, Shield, Roerecke, Cheng, Popova, Kurdyak and Rehm2016; Leyton, Reference Leyton2019). Psychotic disorders, such as schizophrenia, are severe clinical and usually chronic events, entailing extensive health and societal costs (de Oliveira, Cheng, Rehm, & Kurdyak, Reference de Oliveira, Cheng, Rehm and Kurdyak2016; DeVylder et al., Reference DeVylder, Mittal and Schiffman2021; Hasin & Walsh, Reference Hasin and Walsh2021). The relationship between cannabis use and psychosis is multi-directional, with psychosis caused by cannabis use being just one possible pathway. The exact mechanisms underlying this relationship continue to be debated (Murray & Hall, Reference Murray and Hall2020; Wright, Cather, Gilman, & Evins, Reference Wright, Cather, Gilman and Evins2020). The proportion of first-episode psychosis (FEP) attributable to cannabis use was estimated to be 12% in five national European sites (Di Forti et al., Reference Di Forti, Quattrone, Freeman, Tripoli, Gayer-Anderson, Quigley and van der Ven2019). Current evidence however, suggests that the legalization of non-medical cannabis use may lead to an increased incidence of psychosis (Hamilton & Monaghan, Reference Hamilton and Monaghan2019; Ksir & Hart, Reference Ksir and Hart2016; Murray & Hall, Reference Murray and Hall2020). Therefore, evidence-based methods to reduce cannabis-attributable psychosis outcomes are crucially important for individual users and public health protection, especially in liberalized policy environments for cannabis.
While evidence for cannabis use as a causal contributor to psychosis development exist (Polkosnik et al., Reference Polkosnik, Sorkhou and George2021; Wright et al., Reference Wright, Cather, Gilman and Evins2020), additional cannabis use-specific characteristics that function as moderating factors for this relationship have been identified. These factors include the age-of-use onset, high potency cannabis use, and the frequency of use (Matheson & Le Foll, Reference Matheson and Le Foll2020; Sideli, Quigley, La Cascia, & Murray, Reference Sideli, Quigley, La Cascia and Murray2020; van der Steur et al., Reference van der Steur, Batalla and Bossong2020). Frequent cannabis use, specifically, has been shown to function as a strong predictor of psychosis and other adverse outcomes (e.g. cognitive functioning, psychosocial outcomes and CUD) (Fischer et al., Reference Fischer, Robinson, Bullen, Curran, Jutras-Aswad, Medina-Mora and Hall2022; Kroon, Kuhns, Hoch, & Cousijn, Reference Kroon, Kuhns, Hoch and Cousijn2020; Leung et al., Reference Leung, Chan, Hides and Hall2020; Lorenzetti, Chye, Silva, Solowij, & Roberts, Reference Lorenzetti, Chye, Silva, Solowij and Roberts2019).
Systematic reviews have consistently found a high risk of psychosis associated with frequent cannabis use. An umbrella review (2020) involving four meta-analyses found a dose–response relationship between cannabis use and the risk of psychosis (Hasan et al., Reference Hasan, von Keller, Friemel, Hall, Schneider, Koethe and Hoch2020). A meta-analysis focusing on adolescents (Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021) showed a 2.5-fold increase in the odds of psychosis onset in frequent v. infrequent cannabis users [odds ratio (OR) 2.7, 95% confidence interval (CI) 1.65–3.71]. Another meta-analysis showed a four-fold increase in odds of psychosis (OR 3.90, 95%CI 2.84–5.34) for the most frequent cannabis use and a two-fold increase (OR 1.97, 95% CI 1.68–2.31) for moderate as compared to non-use (Marconi, Di Forti, Lewis, Murray, & Vassos, Reference Marconi, Di Forti, Lewis, Murray and Vassos2016). Elsewhere, a two-fold increase (AOR = 2.09, 95% CI 1.54–2.84) in the odds of psychosis had been found in a comparison of frequent to no cannabis use (Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). Other reviews, however, have been limited in their level of detail about the relationship between cannabis use frequency and psychosis risk where, for example, frequent use is commonly compared to only infrequent or non-use (Campeny et al., Reference Campeny, Lopez-Pelayo, Nutt, Blithikioti, Oliveras, Nuno and Gual2020; Hasan et al., Reference Hasan, von Keller, Friemel, Hall, Schneider, Koethe and Hoch2020; Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; Marconi et al., Reference Marconi, Di Forti, Lewis, Murray and Vassos2016; Polkosnik et al., Reference Polkosnik, Sorkhou and George2021; van der Steur et al., Reference van der Steur, Batalla and Bossong2020). Binary categorizations between frequent use v. no use cannot distinguish possibly different risk-by frequency of use levels (e.g. daily, weekly, monthly)(Hoch & Lorenzetti, Reference Hoch and Lorenzetti2020; Kraan, Velthorst, Koenders, & Zwaart, Reference Kraan, Velthorst, Koenders and Zwaart2016), and therefore cannot indicate possible thresholds of cannabis use frequency where the risk of psychosis development may significantly change.
Other illustrations of substance use-related risk-thresholds for health outcomes exist. For example, systematic reviews have quantified exposure levels of alcohol use (e.g. by standard drinks/week) that represent risk-thresholds for stroke, cardio-vascular disease and atrial fibrillation (Samokhvalov, Irving, & Rehm, Reference Samokhvalov, Irving and Rehm2010; Tu et al., Reference Tu, Gallagher, Elliott, Linz, Pitman, Hendriks and Wong2021; Wood et al., Reference Wood, Kaptoge, Butterworth, Willeit, Warnakula and Bolton2018), which have been translated into ‘Low Risk Drinking Guidelines’ (Holmes, Angus, Meier, Buykx, & Brennan, Reference Holmes, Angus, Meier, Buykx and Brennan2019). Similarly, recent ‘Lower-Risk Gambling Guidelines’ have quantified risk-thresholds for gambling exposure toward problem incidence (Young et al., Reference Young, Hodgins, Brunelle, Currie, Dufour, Flores-Pajot and Nadeau2021). Conversely, recently updated ‘Lower-Risk Cannabis Use Guidelines’ were largely unable to list risk-thresholds for main adverse outcomes due to a lack of adequately quantified exposure data (Fischer et al., Reference Fischer, Robinson, Bullen, Curran, Jutras-Aswad, Medina-Mora and Hall2022). If risk-thresholds for cannabis use frequency and psychosis can be identified, these may inform prevention/educational messages toward reducing cannabis-related harm among users.
In this context, the aim of this systematic review and meta-analysis was to determine whether significant risk thresholds exist between different levels of frequency of non-medical cannabis use and the development of psychosis.
Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 framework (online Supplementary eTable 1) (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021). The study protocol was registered pre-initiation with the International Register of Systematic Reviews (PROSPERO)(#CRD42021234708). Study data used in the analyses, and other related details are available upon request.
Search strategy
Searches were conducted in Embase, MEDLINE, PsycINFO, CINAHL, and Web of Science. The search strategy (online Supplementary eMethods 1) was initially developed for Embase and subsequently modified for other databases. Search terms included a mixture of Medical Index Subject Headings (MeSH) and keywords related to the main search topics (e.g. cannabis, use frequency/dose–response relationships, and psychosis). Databases were searched from 1 January 2010, through 26 April 2021. Reference lists of included articles were manually searched for potential additional studies of relevance.
Inclusion and exclusion criteria
Studies were included in this review if they: (1) investigated the relationship between cannabis use and risk of psychosis development, (2) were of case–control or cohort design, (3) included hazard ratios (HRs), odds ratios (ORs), or risk ratios (RRs) with 95% confidence intervals (95% CI) or the data required to calculate them, and (4) included quantified information on the frequency of cannabis consumption, and specifically allowed attribution to at least three frequency categories (i.e. ‘monthly’, ‘weekly’, ‘daily/near-daily’) because at least three categories were required to complete the dose–response analysis.
Studies were excluded if: (1) they included participants with a pre-existing psychotic condition prior to the initiation of cannabis use, diagnosed according to Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013) or International Classification of Diseases 11th Edition (ICD-11) (World Health Organization, 2018)criteria, (2) investigated cannabis use primarily for medicinal purposes, and (3) included synthetic cannabinoids in their scope.
Only studies published in 2010 onward were considered for inclusion. This cut-off was chosen in part because cannabis potency as a known predictor of use-related psychosis outcomes and has steadily increased in recent years (Sideli et al., Reference Sideli, Quigley, La Cascia and Murray2020). For example, the potency of cannabis in Europe has been reported to have doubled from 8.9% tetrahydrocannabinol (THC) in 2008 to over 17% THC in 2017, while cannabidiol (CBD) levels remained stable (Chandra et al., Reference Chandra, Radwan, Majumdar, Church, Freeman and ElSohly2019). No restrictions were placed on participants age or publication language. Grey literature, such as conference abstracts and dissertations were not excluded from database searches (e.g. Web of Science Core Collection). Additional sources of grey literature, such as government websites and clinical trial repositories, were not considered for this review due to the fact that randomized trials and organizational reports would not contain the type of data required for this analysis. Case–control studies with less than ten matched-pairs were excluded due to the increased risk of bias (RoB) encountered in studies with sample sizes below this threshold (Dekkers et al., Reference Dekkers, Vandenbroucke, Cevallos, Renehan, Altman and Egger2019).
Study selection
All citations identified from the searches were uploaded into Covidence systematic review software (Veritas Health Innovation, 2021) and duplicates removed. Citations were screened initially by title and abstract in duplicate by two independent reviewers, with full-text screening completed in the same manner. Translations were obtained for articles published in non-English languages. Disagreements related to both title/abstract and full text screening were resolved through consensus, and inter-rater reliability (IRR) was calculated. A PRISMA flow diagram presents the results of the study screening and inclusion process (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021).
Data extraction
Study data were extracted by two independent reviewers, using consensus discussions for disagreements, and final data verifications conducted by the study statistician. Basic study characteristics were extracted, including study type and location, sample size, sex and age details. The primary outcome of interest was the risk of psychosis development according to DSM-IV or ICD-11 criteria or validated questionnaires. The exposure measure was the frequency of cannabis use, which was categorically pre-defined based on the frequency of use categories commonly used in the cannabis literature (Callaghan, Sanches, & Kish, Reference Callaghan, Sanches and Kish2020; Goodman, Leos-Toro, & Hammond, Reference Goodman, Leos-Toro and Hammond2019; Steeger et al., Reference Steeger, Hitchcock, Bryan, Hutchison, Hill and Bidwell2021). The categories utilized were as follows: (1) never/no use, (2) 1–11 days a year (‘yearly’), (3) 1–3 days a month (‘monthly’), (4) 1–4 days a week (‘weekly’), and (5) 5–7 days a week (‘daily/near-daily’), with study data standardized into these categories for analysis. For studies that presented use frequency information for more than one-time point, the most current frequency of use data were recorded. Effect estimates were obtained from individual studies.
Data analysis
Relative risk (RR) was used as the measure of association between cannabis use frequency and psychosis development (Hogue, Gaylor, & Schulz, Reference Hogue, Gaylor and Schulz1983). Where reported, ORs were converted to RRs [formula: RR = OR/[(1-Po) + (PoxOR), where Po is the outcome incidence] (Zhang & Yu, Reference Zhang and Yu1998). RRs were calculated from raw data for studies without effect estimates. Two-stage dose–response multi-variate meta-analytic models were conducted to estimate the relationship between the cannabis use frequency and psychosis development using the RR data (Crippa & Orsini, Reference Crippa and Orsini2016). First, the dose–response associations between log-relative-risk and levels of cannabis use according to frequency categories were analyzed within each study (Greenland & Longnecker, Reference Greenland and Longnecker1992). Second, study-specific estimates were combined across studies using multi-variate random effect modeling [REM] (Jackson et al., Reference Jackson, Law, Barrett, Turner, Higgins, Salanti and White2016). Sensitivity analyses were performed using both quadratic and flexible non-linear models with restricted cubic splines with three knots (10th, 50th, and 90th percentiles) of the distribution (Liu, Cook, Bergström, & Hsieh, Reference Liu, Cook, Bergström and Hsieh2009; Orsini, Bellocco, & Greenland, Reference Orsini, Bellocco and Greenland2006). Goodness-of-fit statistics (Akaike information criteria ‘AIC’, deviance test ‘D’, and the coefficient of determination ‘R 2’) were assessed to select the best-fitting model (Discacciati, Crippa, & Orsini, Reference Discacciati, Crippa and Orsini2017). A hierarchical multi-level multivariate meta-analytical approach for data plotting was used to account for statistical dependence in effect sizes from multiple within-study comparison arms (cannabis use frequencies) to a common control group (non-use) (Assink & Wibbelink, Reference Assink and Wibbelink2016; Gleser & Olkin, Reference Gleser, Olkin, Cooper, Hedges and Valentine2009; Pastor & Lazowski, Reference Pastor and Lazowski2017). Study-level variables in the analyses included the categories of cannabis use frequency, number of psychosis cases in each exposure group, and the natural logarithm and the standard error for the relative risk logarithms (Liu et al., Reference Liu, Cook, Bergström and Hsieh2009). All data-analyses were completed using STATA v.16 (IPDFC module) (StataCorp, 2019) and R (dosresmeta and Metafor packages) (R Core Team, 2020; Wei & Royston, Reference Wei and Royston2020).
Statistical heterogeneity was assessed using the I2 statistic, with thresholds assessed according to standard recommendations (Deeks, Higgins, & Altman, Reference Deeks, Higgins, Altman, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019). Publication bias was assessed by funnel plots, whereby regression/mixed-effects models and variance/standard errors assessed funnel plot asymmetry (Sterne & Egger, Reference Sterne, Egger, Rothstein, Sutton and Borenstein2005).
Methodological quality and certainty-in-findings assessments
The RoB of included studies was assessed using the Newcastle Ottawa Scale versions for both cohort and case–control studies (Wells et al., Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2013). Bias for case selection, comparability of cases and controls, and exposure were examined. RoB assessments were completed in duplicate by two independent reviewers and disagreements resolved through consensus discussions. All studies assessed were included in this review, regardless of their methodological quality. Certainty-in-findings for the primary review outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluations (GRADE) approach (Schunemann, Brozek, Guyatt, & Oxam, Reference Schunemann, Brozek, Guyatt and Oxam2013) and reported through the online GRADEPro tool (https://gradepro.org/).
Results
Study selection (see Fig. 1)
The database searches returned 5253 results. After duplicate removal, 2847 records underwent title and abstract screening; 2706 records were excluded, and 141 retrieved for full-text review (online Supplementary eMethods 2). A total of ten original studies (three cohort and seven case–control studies) were included in this review (Arranz et al., Reference Arranz, Monferrer, Algora, Cabezas, Sole, Vilella and Sanchez-Gistau2018; Buchy et al., Reference Buchy, Cadenhead, Cannon, Cornblatt, McGlashan, Perkins and Addington2015; Bugra et al., Reference Bugra, Studerus, Rapp, Tamagni, Aston, Borgwardt and Riecher-Rössler2013; Castañeda et al., Reference Castañeda, Alliende, Iruretagoyena, Nachar, Mancilla, Diaz and Crossley2020; Di Forti et al., Reference Di Forti, Marconi, Carra, Fraietta, Trotta, Bonomo and Murray2015, Reference Di Forti, Quattrone, Freeman, Tripoli, Gayer-Anderson, Quigley and van der Ven2019; Núñez et al., Reference Núñez, Ochoa, Huerta-Ramos, Baños, Barajas, Dolz and Usall2016; Rössler, Hengartner, Angst, & Ajdacic-Gross, Reference Rössler, Hengartner, Angst and Ajdacic-Gross2012; Sideli et al., Reference Sideli, Fisher, Murray, Sallis, Russo, Stilo and Di Forti2018; Valmaggia et al., Reference Valmaggia, Day, Jones, Bissoli, Pugh, Hall and McGuire2014). Inter-rater reliability was 96% agreement (κ = 0.60) for title and abstract screening and 92% agreement (κ = 0.62) for full-text screening, respectively, indicating substantial agreement.

Fig. 1. PRISMA flow diagram for study selection process.
Study characteristics (see Table 1)
Studies included a total of 7390 participants ranging in age from 12 to 65 years. The primary outcome was FEP in 9/10 studies. In one cohort study, there were two primary outcomes, schizotypal signs and schizophrenia nuclear symptoms, which were included as two separate samples (Rössler, 2012-A and Rössler, 2012-B) in the meta-analysis (Rössler et al., Reference Rössler, Hengartner, Angst and Ajdacic-Gross2012). Diagnoses of psychosis in the included studies were based on DSM-IV/ICD-10 criteria (5/10) or commonly utilized questionnaires/tools (5/10). The quality of included studies was assessed as moderate (3/10) or high (7/10; see online Supplementary material eTable 2 for RoB assessments).
Table 1. Characteristics of cohort and case–control studies included in a meta-analysis
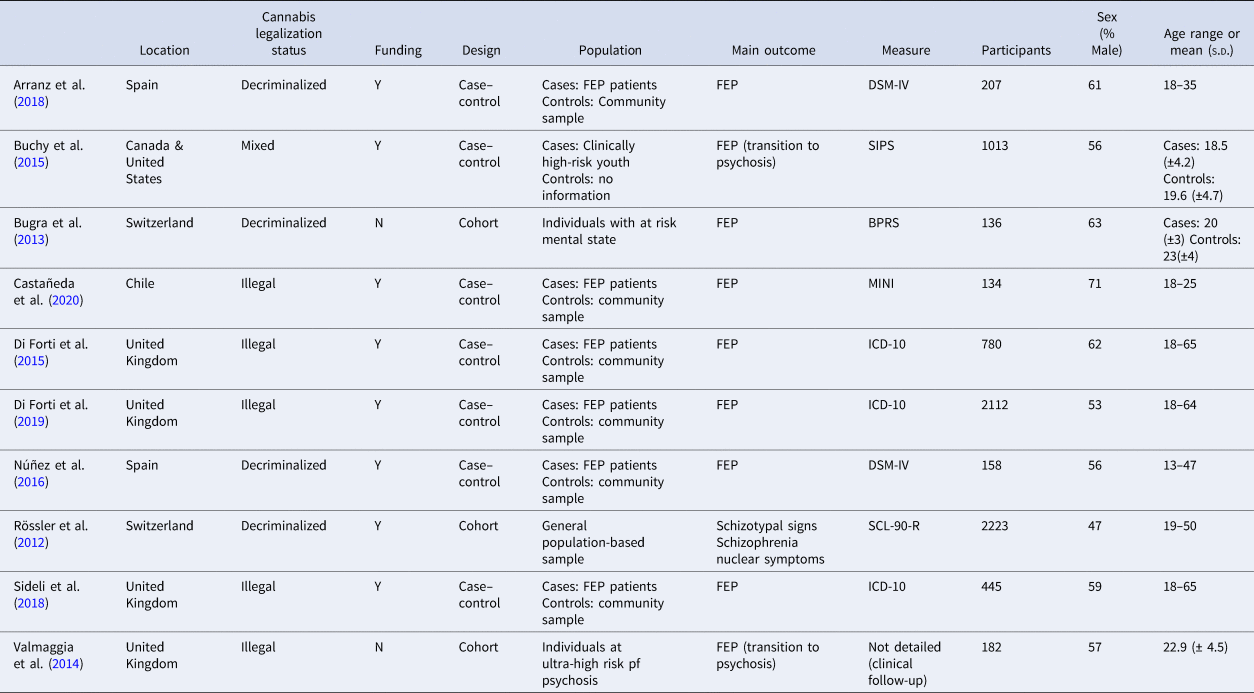
BPRS, Brief Psychiatric Rating Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; FEP, First episode psychosis; ICD-10, International Classification of Disease; MINI, Mini International Neuropsychiatric Interview; SCL-90-R, Symptom Checklist 90 Revised; SIPS, Structured interview for Prodromal Symptoms
Random effect model
The REM showed a significant log-linear dose–response association between the frequency of cannabis use category and risk of psychosis development (p < 0.0001). The risk of psychosis development increased with greater cannabis use frequency (Table 2), from RR:1.25 (95% CI 1.10–1.20) for yearly use, to RR:1.32 (95% CI 1.21–1.44) for monthly use, RR:1.51 (95% CI 1.32–1.72) for weekly use, and RR:1.71 (95% CI 1.45–2.06) for daily/near-daily use. Each category increase in cannabis consumption was associated with a 1.15-times (14.7%; 95% CI 9.8%-19.8%) increase in the risk of psychosis development.
Table 2. Results of linear dose–response model for the association between a category of cannabis use frequency and sensitivity analyses for potential non-linearity of associations using alternate models

Sensitivity analysis
Sensitivity analyses tested for potential non-linearity of associations. The log-linear assumption between cannabis use and risk of psychosis development was relaxed using a quadratic-trend and flexible non-linear model with restricted cubic-splines. In the quadratic-trend model, the risk of psychosis development varied according to cannabis use frequency; it increased from RR:1.01 (95% CI 0.95–1.07) for ‘yearly’ and RR:1.11 (95% CI 1.03–1.20) for monthly use, to RR:1.35 (95% CI 1.22–1.48) for ‘weekly’, and RR:1.76 (95%CI 1.29–2.13) for ‘daily/near-daily’ use (Table 2). The deviation from log-linearity was significant (Wald test p < 0.05, χ2 = 42.48).
The restricted cubic-splines-model demonstrated that the most conservative model had the best data-fit, with similar RRs to the quadratic-trend model. In this model, the risk of psychosis increased from RR:1.01 (95% CI 0.93–1.11) for ‘yearly’ and RR:1.10 (95% CI 0.97–1.25) for ‘monthly’ use, to RR:1.35 (95% CI 1.19–1.52) for ‘weekly’ and RR:1.76 (95% CI 1.47–2.12) for ‘daily/near-daily’ use. The deviation from log-linearity was statistically significant (p < 0.05, χ2 = 37.79).
Heterogeneity, publication bias, and certainty-in-findings
Multi-level-modeling was used to plot the data (Fig. 2), displaying significant heterogeneity (I 2 = 87.2%; p < 0.001). A large proportion of the variance was explained by within-study-differences (86.67%), with between-study variance contributing to 0.56% of heterogeneity (online Supplementary eFig. 1). There was some funnel plot asymmetry when assessing publication bias, but the majority of estimates were clustered around the summary-effect-estimate. The counter-enhanced funnel plot did not reveal studies to be missing in areas of low statistical significance (online Supplementary eFig. 2) (Peters, Sutton, Jones, Abrams, & Rushton, Reference Peters, Sutton, Jones, Abrams and Rushton2008). Certainty-in-findings was rated as moderate according (GRADE), largely due to the observational nature of study designs and heterogeneity (online Supplementary eTable 3).

Fig. 2. Risk of developing psychosis associated with frequency of cannabis use. Data are shown for three cohorts and seven case–control studies, according to categories of cannabis use frequency.
Discussion
This systematic review and meta-analysis found a dose–response relationship between the frequency of cannabis use and the development of psychosis, as shown by previous reviews (Hasan et al., Reference Hasan, von Keller, Friemel, Hall, Schneider, Koethe and Hoch2020; Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; Marconi et al., Reference Marconi, Di Forti, Lewis, Murray and Vassos2016; Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007; Polkosnik et al., Reference Polkosnik, Sorkhou and George2021; van der Steur et al., Reference van der Steur, Batalla and Bossong2020). Prior meta-analyses specifically documented a higher risk among daily users, mostly as compared to no or lower frequency use, with ORs ranging from 2.09 to 3.90 (Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; Marconi et al., Reference Marconi, Di Forti, Lewis, Murray and Vassos2016; Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). One systematic review and meta-analysis (2016) failed to find a statistically significant relationship between cannabis use and the odds of developing psychosis (Kraan et al., Reference Kraan, Velthorst, Koenders and Zwaart2016) but only considered lifetime cannabis use, likely entailing associations too weak for detection.
Importantly, this review identified discernable risk-thresholds by cannabis use at a higher frequency for psychosis development. Specifically, weekly cannabis use was associated with a 35% (RR 1.35) increase in risk, and daily/near-daily use was associated with a 76% (RR 1.76%) increase in the risk of psychosis development compared to no use, according to the model of best-fit (restricted cubic-splines). Conversely, there were no significant increases in risk associated with monthly and yearly use in this model, suggesting an absence of increased risk for lower frequency use.
The role of cannabis use in the incidence of psychotic outcomes as a causal contributor is complex, because their causes are known to be multifactorial, being influenced by the environment, genes and their interactions (Ben Amar & Potvin, Reference Ben Amar and Potvin2007; Hall & Degenhardt, Reference Hall and Degenhardt2008; Hamilton, Reference Hamilton2017; Wright et al., Reference Wright, Cather, Gilman and Evins2020). Recent research has confirmed the importance of genetic influences, specifically polymorphisms on specific genes (e.g. COMT, AKT1) that make affected individuals more vulnerable to the psychogenetic effects of cannabis (Misiak et al., Reference Misiak, Stramecki, Gaweda, Prochwicz, Sasiadek, Moustafa and Frydecka2018; Polkosnik et al., Reference Polkosnik, Sorkhou and George2021; van der Steur et al., Reference van der Steur, Batalla and Bossong2020). The present review further compounds the evidence that specific (frequent) cannabis exposure patterns appear to significantly contribute to the multifactorial interplay toward psychosis outcomes.
Beyond the role of use frequency, epidemiological data have identified the use of high potency cannabis, early age-of-use onset (e.g. pre-25), childhood trauma, and concurrent other substance use as contributing factors to psychosis development (Di Forti et al., Reference Di Forti, Marconi, Carra, Fraietta, Trotta, Bonomo and Murray2015; Hall & Lynskey, Reference Hall and Lynskey2020; Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; van der Steur et al., Reference van der Steur, Batalla and Bossong2020). Multiple risk-factors may interact to increase the risk for cannabis use-related psychosis more than the contributions of individual factors alone (Ben Amar & Potvin, Reference Ben Amar and Potvin2007; Hosseini & Oremus, Reference Hosseini and Oremus2019). For example, an early age-of-use in adolescence combined with frequent use was found to increase the risk significantly as compared to either risk-factor alone, with genetic pre-dispositions as likely additional contributors (Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021). Similarly, the combination of daily use with high potency cannabis confers almost double the odds of developing psychosis (Di Forti et al., Reference Di Forti, Marconi, Carra, Fraietta, Trotta, Bonomo and Murray2015).
The present review's findings have implications for evidence-based, public health-oriented targeted prevention messaging for cannabis use. While existing prevention content commonly convey categorical warnings regarding the link between cannabis use and risk for psychosis, our findings suggest that such a risk is significantly clearest for more frequent – i.e., ‘weekly’ or higher – patterns of cannabis use (Ghonaim, Reference Ghonaim2018; Ladegard, Thurstone, & Rylander, Reference Ladegard, Thurstone and Rylander2020; Murray, David, & Ajnakina, Reference Murray, David and Ajnakina2021). Correspondingly, the data suggest a low risk for less frequent or occasional-only (e.g. less-than-weekly) cannabis use. These results translate into the consequential refinement of prevention/education messages, for example as offered by the LRCUG (Fischer et al., Reference Fischer, Robinson, Bullen, Curran, Jutras-Aswad, Medina-Mora and Hall2022) or other pertinent recommendation sources.
While more frequent cannabis use to be appears associated with an elevated risk of psychosis, this risk is likely further influenced by other risk-factors (e.g. genetics, family history, cannabis potency) that require additional consideration for and incorporation into prevention messaging beyond mere frequency-of-use factors (Di Forti et al., Reference Di Forti, Marconi, Carra, Fraietta, Trotta, Bonomo and Murray2015; Hamilton & Sumnall, Reference Hamilton and Sumnall2021; Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; van der Steur et al., Reference van der Steur, Batalla and Bossong2020). For example, persons who have a first-degree relative (parent or sibling) with a psychosis condition have a 10-fold higher risk of psychosis development; if the risk of daily cannabis use is multiplicative, their risk would increase 20-fold.
Cannabis users, however, should be explicitly advised by evidence-based prevention messaging to keep their cannabis use infrequent in order for their risks for cannabis-attributable psychosis development to be meaningfully lowered. Such exposure-stepped, evidence-based prevention messaging about risk-thresholds for cannabis use and psychosis may be controversial in some realms. However, it is analogous to evidence-based alcohol-related health guidelines where the risk for specific adverse (e.g. cardio-vascular) outcomes is low/absent below certain consumption levels and ‘moderate’ use is recommended (Furtwaengler & de Visser, Reference Furtwaengler and de Visser2013; Holmes et al., Reference Holmes, Angus, Meier, Buykx and Brennan2019; Wood et al., Reference Wood, Kaptoge, Butterworth, Willeit, Warnakula and Bolton2018). Beyond, evidence-based, ideally quantified, risk-thresholds for cannabis use exposure (e.g. frequency, a potency of use) toward other key adverse outcomes (e.g. CUD) should be assessed toward informing public health-oriented prevention efforts (Fischer et al., Reference Fischer, Robinson, Bullen, Curran, Jutras-Aswad, Medina-Mora and Hall2022).
Limitations
This review includes some limitations. First, most of the included studies did not report data on potential contributor factors for outcomes, which therefore could not be controlled for. For example, the use of high-potency cannabis also increases the risk of a psychotic disorder (van der Steur et al., Reference van der Steur, Batalla and Bossong2020), yet many studies do not report information on the potency of cannabis used by participants, which can differ greatly across products (e.g. skunk, concentrates) and by geographical location. Additionally, half of the included studies either did not control for the possible co-use of alcohol, tobacco, or other recreational substances in their analysis or it was unclear whether these factors were controlled for. All included studies utilized self-report measures of cannabis use frequency, which may be prone to recall bias. While including only studies with multiple use frequency categories, classification of risk into categories may lead to information loss by treating every within-category participant as equal for risk (Wynants et al., Reference Wynants, van Smeden, McLernon, Timmerman, Steyerberg and Van Calster2019). The identification of risk-thresholds was limited to the a-priori defined frequency categories. Differences in design and number of (e.g. numbers of use frequency categories) of included studies also limited our ability to conduct sub-group analyses, such as by year of study or study design. While included studies were rated as having a low RoB according to the Newcastle Ottawa Scale, it is important to note that all included studies are observational designs and therefore overall study quality is lower and the potential RoB higher than if randomized trials were utilized.
Conclusions/future directions
Psychosis as a possible adverse outcome of cannabis use remains a major public health concern. This review found that the risk of psychosis is significantly elevated with frequent, i.e., at least weekly, cannabis use while not significantly elevated with infrequent consumption. These evidence-based insights require effective translation into public health-oriented cannabis prevention efforts. As cannabis use prevalence continues to grow, including in policy reform settings, and use patterns are evolving, the evidence-base on this issue should be regularly updated. Specifically, future reviews should seek to integrate the role of possible contributing factors (e.g. cannabis potency, genetics, age of exposure) to better define risk-thresholds for psychosis development and extend these to other adverse outcomes toward improved public health-oriented interventions for cannabis use.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722000502
Acknowledgements
None.
Author contributions
Tessa Robinson: conceptualization, methodology, investigation, writing-original draft, project administration; Muhammad Usman Ali: methodology, data curation, formal analysis, writing- original draft; Bethany Easterbrook: methodology, investigation, writing- review and editing; Wayne Hall: conceptualization, methodology, writing – review and editing; Didier Jutras-Aswad: conceptualization, methodology, writing – review and editing; Benedikt Fischer: conceptualization, methodology, writing – original draft, supervision, funding acquisition
Tessa Robinson and Muhammad Usman Ali had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The authors would like to thank Stephanie Coronado-Montoya (Université de Montréal) for her assistance with data collection for this review.
Conflict of interest
The authors make the following declarations of relevant financial activities outside of the submitted work: BF has received general research support from the Hugh Green Foundation Chair in Addiction Research, held at the Faculty of Medical and Health Sciences, University of Auckland, New Zealand. In the past 3 years, he has held research grants and contracts in the areas of substance use, health and policy from public funding and government (i.e. public-only) organizations. DJA has received investigational products (last in 2018) from Insys Therapeutics for a clinical trial funded by the Canadian Institutes of Health Research (CIHR). In the last 36 months he has received grants/contracts funding for substance use-related research from public and governmental agencies and has expert-consulted on related issues with public/government agencies.
Financial support
This research was funded in part by Health Canada. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.







