Serum Zn levels tend to decrease with advancing ageReference Maes, deVos and Wauters1, Reference Seiler, Itin and Stahelin2, possibly because of lower consumption of Zn-rich foods, or foods that adversely affect the bioavailability of Zn, by older individualsReference Cid-Ruzafa, Caulfield, Barron and West3, Reference Kant and Schatzkin4. Taste acuity also appears to decline in normal ageingReference Sanders, Ayers and Oakes5–Reference Bartoshuk, Rifkin, Marks and Bars8. Previous taste threshold studies have indicated that the extent of decline in taste acuity differs for each of the basic tastes. Consistent evidence implies an age-related decline in salt taste acuityReference Mojet, Christ-Hazelhof and Heidema6, Reference Bartoshuk, Rifkin, Marks and Bars8–Reference Murphy10, which may be associated with putative indicators of Zn statusReference Stewart-Knox, Simpson and Parr11. Zn is present in salivaReference Watanabe, Asatsuma, Ikui, Ikeda, Yamada, Nomura and Igarashi12 as well as in the salivary glandReference Tanaka13 and is linked to gustatory nerve activityReference Yokoi, Egger, Ramanujam, Alcock, Dayal, Penland and Sandstead14. Zn is bound to gustin, which is a protein and, like Zn, a component of salivaReference Thatcher, Doherty, Orvisky, Martin and Henkin15. Zn deficiency can be associated with a lack of gustinReference Pluhator, Thomson and Fedorak16 and can potentially lead to impaired taste acuityReference Bales, Steinman, Freelandgraves, Stone and Young7. Zn may be involved in the control of appetite through interaction with leptinReference Konukoglu, Turhan, Ercan and Serin17. Depleted Zn can be associated with reduced appetite generally and may result from, or lead to, decreased intake of Zn-rich foods by older individualsReference Seiler, Itin and Stahelin2, Reference Shay and Mangian18–Reference Little, Castellanos, Humphries and Austin20. Zn deficiency, therefore, could be both a cause and an effect of age-related decline in taste acuity.
Taste impairment has been observed in cases of Zn deficiencyReference Kettaneh, Fain, Stirnemann and Thomas21, Reference Tomita and Yoshikawa22 and supplemental Zn has been shown to both preventReference Yamagata, Nakamura, Yamagata, Nakanishi, Matsunaga, Nakanishi, Nishimoto, Minakata, Mune and Yikawa23 and treatReference Stoll and Oepen24 taste disorder and enhance taste acuity in Zn-depleted individualsReference Heckmann, Hujoel, Habiger, Friess, Wichmann, Heckmann and Hummel25–Reference Ripamonti, Zecca, Brunelli, Fulfaro, Villa, Balzarini, Bombardieri and de Conno27. It is important to consider the degree to which age-impaired taste acuity is responsive to Zn supplementation given the potentially detrimental effect upon food preference and choice and, ultimately, overall dietary qualityReference Rikkert and Rigaud28, Reference Bloomfield, Graham, Schiffman and Killenberg29. Older individuals are at risk of sub-optimal Zn intake for physiological, psychosocial and economic reasonsReference McClean, McClain, Barve and Boosalis30. Age-related decline in taste acuity, therefore, could potentially result from failure to meet Zn requirement. There is convincing evidence that would lead us to assume that a large proportion of individuals over the age of 60 years experience some degree of age-related decline in taste acuityReference Mojet, Heidema and Christ-Hazelhof31–Reference Cooper, Bilash and Zubek33. The present study, therefore, meets a need to establish whether supplemental Zn can improve taste acuity in normal ageingReference Schiffman9. The aim of the present double-blind, randomised controlled intervention trial was to compare taste acuity for the four basic tastes (sweet, sour, bitter and salt) using a signal detection approach, in response to elemental Zn supplementation (placebo, or 15 mg or 30 mg Zn/d) in healthy older European adults aged 70–87 years recruited in Grenoble (France) and Rome (Italy).
Methods
The research was carried out from centres in Grenoble (University de Joseph Fourier) and Rome (Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione; INRAN). Ethical approval was obtained independently by both research centres to conduct the present study. All volunteers gave informed written consent to take part in the screening and research study. A randomised, placebo-controlled, double-blind clinical controlled trial design was employed in which taste acuity (detection thresholds) for the four basic tastes was assessed in response to elemental Zn (15 mg and 30 mg) or placebo over a 6-month period. Because the present study aimed to determine the impact of Zn upon healthy ageing, it was necessary to employ dosages that would not be toxic to healthy individuals and available without prescription.
Sampling
Older individuals were recruited through community groups and organisations. Prospective volunteers then initiated contact with the research group. Approximately 10–15 % of those initially approached through leaflets, community groups, etc volunteered to take part in the study. Volunteers underwent screening provided they met none of the following exclusion criteria: undergoing treatment for acute or chronic disease (including cancer, diabetes, renal or hepatic disease, malabsorption and chronic inflammatory pathologies); having a BMI of < 20 and >33 kg/mReference Seiler, Itin and Stahelin2; consuming a special prescribed diet or having unusual dietary habits (vegetarian or vegan); consuming >30 g alcohol/d (more than twenty-one units/week) for men or >20 g alcohol/d (more than fourteen units/week) for women; smoking more than ten cigarettes per d (>10 g tobacco/d); taking more than four prescription drugs; using hormone replacement therapy; taking medication that could affect Zn absorption; taking nutritional supplements containing minerals or trace elements within 6 months of commencement of the studyReference Polito, Meunier and Andriollo-Sanchez34. Zn at doses of 30 mg and above can be toxic, especially if Cu intake is sub-optimalReference Milne, Davis and Nielsen35, Reference Solomons36. Individuals with chronic intestinal disorders and any who were prescribed medication, nutritional supplements or dietary regimes that could affect Zn absorption or inhibit Zn bioavailability were also excluded from taking part in the study. Full medical screening was provided which included liver and kidney function tests, full blood profiles, and blood pressure and heart rate measures. Qualified phlebotomists and nurses took blood for these tests. Volunteers were assessed for depression by means of the Geriatric Depression ScaleReference Yesavage, Brink, Rose, Lum, Huang, Adey and Leirer37 and for dementia by means of the Mini Mental State ExaminationReference Folstein, Folstein and McHugh38. Those with cognitive deficit or mental health problems were subsequently excluded from the study. There was some sample attrition subsequent to recruitment (n 23). This was because a number of volunteers did not arrive for initial testing, while others refused supplementation. The eventual sample subjects were apparently healthy community-dwelling older adults aged 70–87 years (n 199). Approximately equal numbers of males and females were randomly allocated to either the placebo, or 15 mg or 30 mg Zn-supplemented groups.
Putative indicators of zinc status
Both serum and erythrocyte Zn determinations were carried out at the Laboratoire Nutrition, Vieillissement et Maladies Cardiovasculaires (NVMC), Grenoble, France. Details of sample storage and transport have been reported elsewhere by Andriollo-Sanchez et al. Reference Andriollo-Sanchez, Hininger-Favier and Meunier39. Serum, erythrocyte and urinary Zn concentrations were determined by flame atomic absorption spectrometry using a PerkinElmer 560 (PerkinElmer Life And Analytical Sciences, Inc., Waltham, MA, USA) and employing a previously described methodReference Arnaud, Bellanger, Bienvenu, Chappuis and Favier40. Seronorm® trace element serum was used as an internal quality control (Sero®, Billingstad, Norway). Dietary Zn intake was assessed by means of a semi-structured standardised 4 d food diary. The information in the food diaries was analysed using the NetWISP (version 3.0; Tinuviel Software, Anglesey, UK) database.
Taste threshold assessment
Taste acuity refers to the range of taste stimuli that an individual is able to detectReference Schiffman and Graham41 and is usually assumed from taste threshold assessment. Taste acuity is reflected in ability to detect and/or recognise the four to five basic tastes – sweet, sour, salty, bitter, and umami (monosodium glutamate) – and can be assessed in a variety of different waysReference Bischmann and Witte42. The reported study adopts a signal detection theory approachReference Green and Swets43 adapted for sensory taste threshold measurementReference O'Mahony44 for which it has been shown to produce repeatable resultsReference O'Mahony45. Signal detection theory views sensory taste threshold detection as the outcome of a cognitive decision-making processReference Delwiche and O'Mahony46. The procedure adopted required the panellist to decide whether ‘noise’ (i.e. water) or ‘noise and signal’ (i.e. water and tastant) was present in each trial comprised of three samples (one signal and two noise stimuli)Reference Goldstein47. As a measure of decision criteria and to control for guessing, panellists were also required to indicate whether they were sure or unsure of their decision.
Test stimuli
The taste solutions were made up using glucose (sweet), sodium chloride (salt), citric acid (sour) and quinine hydrochloride (bitter). Both research centres acquired equipment and ingredients for the taste solutions from the same suppliers. All chemicals were ‘food’ grade. Sodium chloride and glucose were both supplied by Provincial Butchers Supplies Ltd (Lisburn, County Antrim, UK). Sigma Aldrich, UK, supplied the citric acid and quinine hydrochloride. Each tastant was suspended in a series of six solutions of increasing concentration using ultra-purified water (deionisation and reverse osmosis to purity greater than 15 MΩ). With exception of glucose, these initial solutions were based on concentrations previously presented to older adults and reported by Mojet et al. Reference Mojet, Christ-Hazelhof and Heidema6. A pilot study (n 4) employing three females and one male, aged over 55 years, was carried out to establish that the range of strengths required for each basic taste was adequate to prevent ceiling or floor effects. This resulted in the final ranges (g/l): sweet (glucose), 4 × 10− 1 − 51; sour (citric acid), 1 × 10− 3 − 39 × 10− 2; salty (sodium chloride), 6 × 10− 3 − 1·75; bitter (quinine hydrochloride), 1 × 10− 4 − 7·5 × 10− 2.
The solutions were prepared weekly. For each taste concentration, the compounds were weighed out, added to 800 ml purified water in a Pyrex beaker, stirred until dissolved, then poured into a volumetric 1 litre flask, to which further purified water was added to make up 1 litre of the solution and then stirred well. Solutions were stored at 5°C for use within 3–4 d. Polypropylene cups were used to contain each tastant and/or purified water, the volume of which presented was 2·5 ml. The solutions were then presented at room temperature for the purpose of the sensory trials.
Procedure
The intervention commenced in February 2003 and completed in September 2004. Subsequent to a 12 h overnight fast, blood samples (5 ml) for serum and erythrocyte Zn assay were collected via antecubital venepuncture into EDTA and serum tubes by a qualified phlebotomist at baseline and again 3 months and 6 months into the intervention period. For Zn status determination, blood was collected using trace element-free vacutainer® tubes (Becton Dickinson, Le Pont de Claix, France). Samples were kept on ice immediately after drawing. Trace element-free pipettes and vials were also used for the preparation of samples. Fasting venous blood samples were allowed to sit at room temperature and clot for 30 min before serum was separated from blood cells by centrifugation (15 min; 1000 g) and then sampled into Eppendorfs. EDTA tubes were centrifuged immediately at 1500 rpm for 15 min and then sampled into Eppendorfs. Erythrocytes were then washed three times in PBS (pH 7·4). Serum samples were diluted 1:5 in 0·1 m-HCl. Erythrocytes were diluted 1:100 in deionised water. Serum and erythrocytes were stored at − 80°C. Carriage conditions for the frozen samples were identical across the two regions.
Dietary Zn was assessed at baseline and 6 months later at the conclusion of the intervention using 4 d food diaries that included estimates of portion size. Full standardised written and verbal instructions were provided. All food and drink consumed, including snacks, was recorded in the food diary over 4 consecutive days comprising two weekdays (Thursday and Friday) and two weekend days (Saturday and Sunday).
Participants were required to take elemental Zn (either 15 or 30 mg) per d (as zinc gluconate) or placebo over a period of 6 months. Supplements were supplied by E-Pharma and issued in 7 d pill organiser boxes (Carepac, Farringdon, Oxon, UK). The study required that all participants ingested two tablets daily. Those in the 15 mg Zn group received two capsules of 7·5 mg daily and those in the 30 mg Zn group received two 15 mg capsules daily. Written instructions as to when and how to take the tablets were provided. The pill-boxes were checked for compliance and replenished every 6 weeks. Participants also kept a diary to record any supplements that they forgot to take.
Taste acuity (threshold) assessment also took place initially at baseline and was repeated at 3 and 6 months during the intervention period. Both centres carried out tasting at the same time of day, in the early morning (09.00 hours) and employed the same method throughout. Participants were asked to attend the food sensory testing laboratory early in the morning having fasted overnight to control for individual differences in satiety.
The order in which the basic tastes were presented was standardised as follows: citric acid (sour); glucose (sweet); sodium chloride (salt); quinine (bitter). Both verbal and written standardised instructions were given on how to carry out the test. The aqueous solutions (2·5 ml) were presented in the cups on a tray in six ascending concentrations for each taste quality. For each basic taste, eighteen cups were set out in six rows each of three cups, one cup per row contained the taste solution (signal plus noise) and the other two cups contained purified water (noise). A comparison of results of ‘sip and spit’ and ‘sip and swallow’ tests have indicated that sip and spit tests do not relate well to consumptionReference Lucas and Bellisle48. This suggests that results of sip and spit tests should be interpreted with caution especially when assessments have been made by untrained panellists. Accordingly, ‘natural’ consumption-based tasting methods were deemed appropriate for the present study. In an effort to mimic normal taste conditions as far as possible and in doing so to maximise the chances that the taste be detected, participants were instructed to ‘sip and swallow’ each solution ‘in their normal way’.
Participants were instructed to sample, in an ascending series, the contents of each of the three cups (2·5 ml in each cup) in each row once, decide which solution was different and to record their decision on a standardised response sheet before moving on to taste the next row containing solutions of greater strength. The task was to distinguish the ‘signal’ (tastant plus purified water) from the ‘noise’ (purified water) in each row. In addition, for each row (triad) the participants were required to indicate whether they were sure or unsure as to their decision. No rinsing was advocated between taste samples, merely to sip and swallow purified water following presentation of each taste (sour, sweet, salt and bitter) and before moving on to the next, to minimise habituation to the different taste qualities. No conferring on decisions was permitted. On completion of the research study, a light breakfast was provided.
Data analysis
The R index is a data-handling technique developed by BrownReference Brown49 for the purpose of discrimination studies and is amenable to parametric analysisReference O'Mahony44. The R index is the predicted probability of the correct choice of a signal over noiseReference O'Mahony, Gardner, Long, Heintz, Thompson and Davies50 taking into account individual differences in decision-making criteria. For the purpose of the present study, the R index was derived from the information recorded during signal detection trials regarding the detection of a signal or noise also taking into account whether the respondent was sure or unsure about each decision. Each response was quantified and tabulated into one of the following categories: correct-signal-sure; correct-noise-sure; correct-signal-unsure; correct-noise-unsure; incorrect-signal-sure; incorrect-noise-sure; incorrect-signal-unsure; incorrect-noise-unsure. Data were then converted to R indices using a macro statistical equation created in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). The higher the R index the lower the taste threshold and the greater the taste acuity. R indices were computed for citric acid, glucose, salt and quinine and were entered separately as dependent variables in each analysis. Data were then transferred into Statistical Package for the Social Sciences (version 11; SPSS Inc., Chicago, IL, USA).
Factorial ANOVA was carried out to determine any change in serum and erythrocyte Zn levels in response to the Zn intervention (placebo, or 15 mg or 30 mg Zn). Repeated-measures ANOVA was undertaken to compare dietary Zn and salt intake between centres (Rome and Grenoble) and by condition (placebo, or 15 mg or 30 mg Zn) over time (baseline and 6 months). Factorial ANOVA with repeated measures (baseline, 3 months and 6 months) was carried out separately for each region (Rome and Grenoble) and for each basic taste (sweet, sour, salt and bitter) to establish differences in taste acuity in response to Zn (placebo, or 15 mg or 30 mg Zn). Baseline serum and erythrocyte Zn status values were entered as covariates to control for differences in Zn status before intervention. Baseline taste acuity was also entered into the analysis as a covariate to control for individual differences in taste acuity.
Results
Sample description
Approximately equivalent proportions of each sex were recruited in Rome, Italy (fifty-six males and fifty-two females; mean age 74·5 years) and Grenoble, France (forty-seven males and forty-four females; mean age 74·2 years). A total of 189 individuals (ninety-one in Grenoble and ninety-eight in Rome) completed the taste tests. Owing to difficulties in classifying social class by occupation across cultures, social class has been collapsed into three groupings (professional, skilled and unskilled). Grenoble had a higher proportion of professional participants (41 %) than Rome (27 %). Educational background was evaluated in terms of the percentage that completed tertiary education in Rome (20 %) and Grenoble (15 %).
Putative indicators of zinc status
There were no differences in serum Zn between regions or treatment group at baseline. Mean serum Zn levels were within the normal range (11–18 μmol/l)Reference Andriollo-Sanchez, Hininger-Favier and Meunier39 for the placebo (13·20 (sd 1·69) μmol/l), 15 mg (13·28 (sd 1·84) μmol/l) and 30 mg (13·13 (sd 1·63) μmol/l) supplemented groups at baseline and remained so throughout the intervention period. Serum Zn increased post-intervention (F (4,197) = 11·021; P = 0·000) in both the 15 mg (13·99 (sd 2·47) μmol/l; P = 0·018) and 30 mg (15·03 (sd 3·17) μmol/l; P = 0·000) supplemented groups compared with placebo (13·05 (sd 1·66) μmol/l) (Fig. 1). Serum Zn levels in response to 30 mg Zn were higher among those recruited in Rome (16·26 (sd 3·41) μmol/l) than Grenoble (13·64 (sd 2·21) μmol/l) post-intervention (F (4,197) = 3·526; P = 0·008).
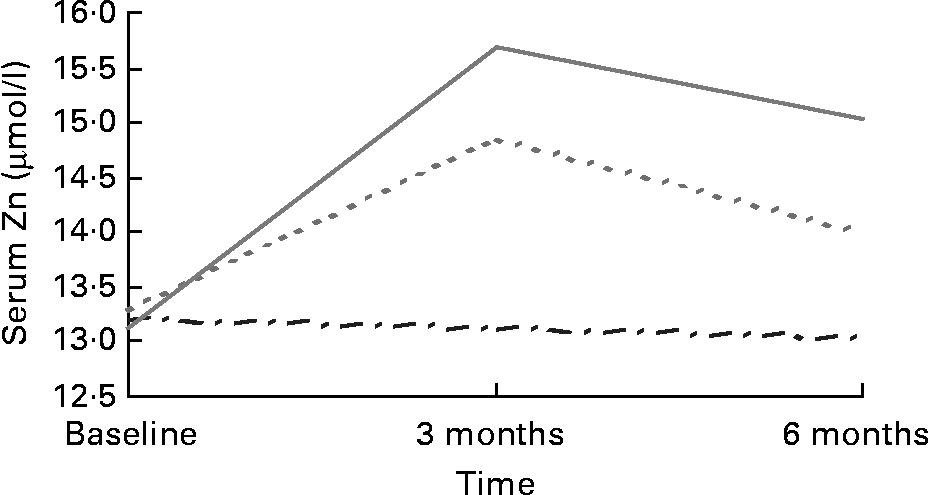
Fig. 1 Serum Zn in response to Zn supplementation in older adults (70–87 years) from Rome and Grenoble (n 197). (![]() ), Placebo; (
), Placebo; (![]() ), 15 mg Zn; (
), 15 mg Zn; (![]() ), 30 mg zinc. Values are means.
), 30 mg zinc. Values are means.
Dietary assessment
Dietary Zn intake decreased between baseline (12·33 (se 0·528) mg/d) and 6 months (10·52 (se 0·349) mg/d) among those in Grenoble (F (1,186) = 4·196; P = 0·042) but not Rome. Na intake was higher among those in Grenoble (2433·50 (se 61·07) g/d) than in Rome (1285·22 (se 55·73) g/d) at both baseline and 6 months (F (1,186) = 192·874; P = 0·000). There were no differences in Zn or Na intake between treatment groups at either centre.
Mean erythrocyte Zn levels were also within normal limits (120–250 μmol/l) at baseline and remained normal throughout the intervention period. The sample recruited in Grenoble (188·54 (sd 49·38) μmol/l) had lower erythrocyte Zn levels than those in Rome at baseline (213·79 (sd 60·28) μmol/l) (F (2,189) = 15·579; P = 0·000). Erythrocyte Zn did not alter in either sample (Rome or Grenoble) in response to the intervention.
Zinc and taste acuity
Acuity for salt taste was greater in the 30 mg supplemented group (0·84 409 (sd 0·13 349)) than the placebo group (0·75 045 (sd 0·210)) post-intervention in the Grenoble sample (F (2,91) = 3·632; P = 0·031) (Fig. 2). There were no apparent differences in taste acuity in response to the 30 mg Zn intervention for sweet, sour or bitter tastes among those recruited in Grenoble or for any of the four basic tastes in the Rome sample. There were no apparent differences in acuity for any of the tastes in response to 15 mg Zn.
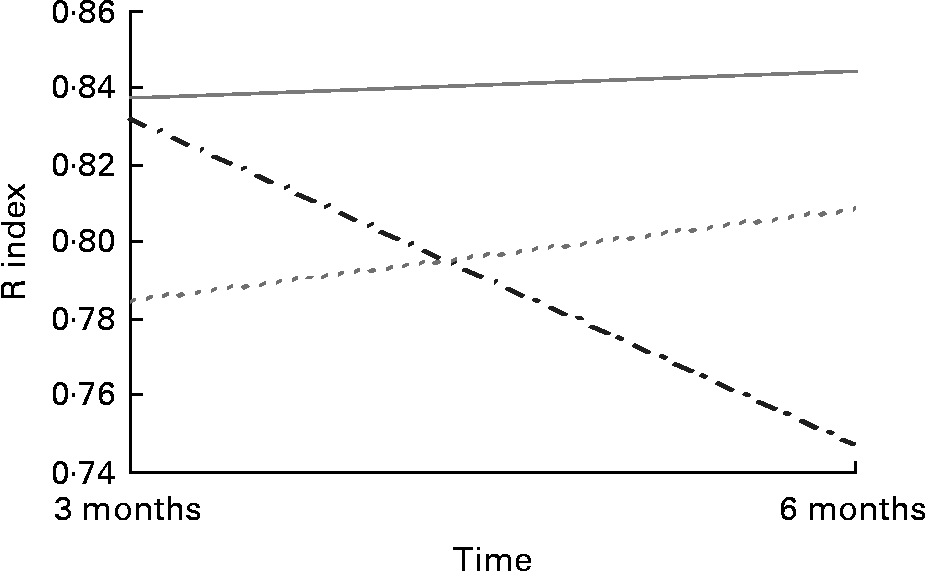
Fig. 2 Salt taste acuity in response to Zn supplementation in older adults (70–87 years) from Grenoble (n 91). (![]() ), Placebo; (
), Placebo; (![]() ), 15 mg Zn; (
), 15 mg Zn; (![]() ), 30 mg zinc. Values are means. Baseline taste acuity was entered into the analysis as a covariate.
), 30 mg zinc. Values are means. Baseline taste acuity was entered into the analysis as a covariate.
Discussion
Salt taste acuity increased in response to 30 mg Zn in older adults recruited in Grenoble. Many factors may influence salt taste acuity. Sensory threshold for salty tastes can become elevated with ageReference Mojet, Christ-Hazelhof and Heidema6–Reference Bartoshuk, Rifkin, Marks and Bars8, Reference Murphy10, Reference Schiffman and Graham41. That Zn enhanced salt taste acuity was, therefore, not surprising. The finding that salt taste acuity increased in response to Zn among those recruited in Grenoble but not in Rome is more difficult to explain. Although mean erythrocyte Zn was within normal limits in both groups throughout the study, participants recruited in Grenoble, on average, had lower levels of erythrocyte Zn than those in Rome at baseline. This apparent baseline difference in long-term putative erythrocyte Zn status could explain why salt taste acuity improved subsequent to 30 mg Zn among those in Grenoble but not Rome. Given that differences in putative measures of Zn status (serum and erythrocyte Zn) and taste acuity at baseline were controlled for in the analysis, however, suggests that this baseline difference in long-term putative Zn status was unlikely to have influenced the findings.
Differences in reported Zn and Na intake between those recruited in Grenoble and Rome may provide clues as to the disparate response of the two centres to the Zn intervention. Reported dietary Zn intake decreased between baseline and 6 months into the intervention among those recruited in Grenoble but not Rome. That a similar reduction in dietary Zn intake occurred between baseline and 6 months in the placebo and the treatment groups, however, suggests that the decrease in reported dietary Zn was not a response to the Zn supplementation and may reflect seasonal variation in dietary habits. Whether the apparent decrease in Zn intake over the intervention period among those in Grenoble was enough to bring about the improvement in salt taste acuity observed exclusively in this sample is a matter for further study.
Sodium intake was reportedly higher among those in Grenoble than in Rome. Previous research has suggested that higher dietary Zn intake is associated with better taste acuity for salt because Na and Zn intake are relatedReference McDaid, Stewart-Knox, Parr and Simpson51. It is unfortunately difficult to draw firm conclusions on the basis of food records. Not only are diaries subject to reporting biasReference Bingham, Cassidy and Cole52–Reference Livingstone and Black54 but dietary information gathered over 4 d may not be representative of habitual intakesReference Nelson, Black, Morris and Cole55. Na intake is especially difficult to assess with any degree of accuracyReference Rodriguez-Palmero, Castellote-Bargallo, Lopez-Sabater, de la Torre-Boronat and Rivero-Urgell56, Reference Moon, Zhang, Shimbo, Hokimoto, Shimazaki, Saito, Shimizu, Imai, Watanabe and Ikeda57. Improved salt taste acuity in response to the Zn intervention among those in Grenoble could, nevertheless, be associated with higher Zn and Na intake reported at baseline by this group.
Inadequate Zn intake and potentially Zn status is more common among socio-economically deprived groupsReference Maret and Sandstead58, Reference Ma and Betts59. The apparent improvement in taste acuity subsequent to intervention with Zn (30 mg) in Grenoble but not Rome cannot, however, easily be explained by socio-demographic-related differences in Zn status between the two groups. The two samples recruited to the present study, although derived from different European regions, were proportionally similar in terms of age, sex and only marginally different in terms of education and social class. The finding that supplemented Zn only benefited salt taste acuity among those in Grenoble and not Rome, therefore, provides further evidence to imply that differences in taste response to Zn were to some degree dependent upon culturally determined differences in dietary habits between the two countriesReference MacDonald, Watts and Fitzpatrick60 and, accordingly, taste response to Zn. Future research should consider culturally determined lifestyle differences including dietary Zn intake in relation to taste acuity, putative measures of Zn status and response to Zn supplementation.
Serum Zn, as expected, increased in the treated groups over the course of the study, indicating good compliance with the intervention. Erythrocyte Zn, however, did not increase in either group during the intervention period. Expressing erythrocyte Zn concentrations in absolute values rather than in terms of μg per g Hb increased the possibility that any rise in erythrocyte Zn levels in response to the intervention could have been missed if changes in the Hb content of the blood samples occurred between baseline and 6 months of supplementation. Unfortunately, no interpretative criteria for erythrocyte Zn have been established and there are no standardised units for the expression of Zn concentration in erythrocytes, making any comparison between different methods and studies difficult.
Another limitation of the present study is that, on the basis of the biochemical measures taken (serum, erythrocyte and dietary Zn), it has not been possible to establish conclusively that the participants in the present study were Zn replete. Various methods are available to assess Zn status. Diagnosis of Zn deficiency, nevertheless, is hampered by the lack of a single, specific and sensitive biochemical index that reflects the entire spectrum of Zn status from deficiency through adequacy to excess and toxicity. Besides serum and erythrocyte Zn, leucocyte, neutrophil, urine, hair and salivary Zn levels could have been assessed, none of which have proven useful in identifying marginal Zn deficiency in manReference Pluhator, Thomson and Fedorak16, Reference Garrow, James and Ralph61. Given the lack of a single valid biochemical measure of Zn statusReference Davis, Milne and Nielsen62 we cannot establish conclusively that the participants in the present study were Zn replete.
The dietary records indicate that the subjects in the study group were Zn replete. Reported mean daily dietary Zn intake in our sample of older adults was estimated at 10·53 (sd 5·24) mg/d for females and 12·04 (sd 5·01) mg/d for malesReference Andriollo-Sanchez, Hininger-Favier and Meunier39. Recommended daily intake for Zn varies between countries. Based on the Nutrition Board and Institute of Medicine63 figures (8 mg/d for females and 11 mg/d for males), according to 4 d food diaries, the proportion of those recruited to the present study who were consuming below two-thirds of the RDA for Zn was small (0 % of females and 3·55 % of males)Reference Andriollo-Sanchez, Hininger-Favier and Meunier39. These dietary Zn intake levels appear higher than those reported in other studies, for example, 10·9 (sd 0·21) mg/d for men and 7·8 (sd 0·14) mg/d for women in the Third National Health and Nutrition Examination Survey (NHANES III) population aged >60 yearsReference Briefel, Bialostosky, Kennedy-Stephenson, McDowell and Wright64, 12 (sd 6·4) mg/d for males and 8·0 (sd 4·0) mg/d for females observed in the Continuing Survey of Food Intakes by Individuals (CSFII) study of those aged 60+Reference Ma and Betts59 and 8·4 mg/d for the Iowa 65+ Rural Health Study (IRHS)Reference Marshall and Yanik65. The 4 d dietary intake records indicate that our sample of healthy older adults were dietary Zn replete.
Assuming that the sample was healthy and dietary Zn replete and in view of the negative finding in response to 15 mg Zn, it could be argued that the observed effect for 30 mg Zn was pharmacological rather than nutritional. The highest daily nutrient intake level likely to pose no risk of adverse health effects for adults is 25 mg/d, including dietary and supplemental Zn66. It has been suggested that higher than recommended doses of Zn supplemented over prolonged periods can induce toxicity by inhibiting Cu bioavailabilityReference Maret and Sandstead58, Reference Sandström67. Other research involving postmenopausal women, however, has suggested that inadequate intake of Zn is more likely than high intake of Zn to be associated with decreased Cu statusReference Milne, Davis and Nielsen35. In view of this, it was not surprising that putative indices of Cu status did not appear to alter in response to Zn intervention in the healthy older adults recruited to the present studyReference Hininger-Favier, Andriollo-Sanchez and Arnaud68, suggesting that it was unlikely that they experienced toxic effects as a consequence of any decrease in Cu status as a result of the Zn intervention.
The present study is unusual in having explored taste acuity in response to Zn in a healthy, apparently Zn-replete, group of older individuals. Most previous studies that have observed an improvement in taste acuity in response to supplemented Zn have tended to employ a small number of Zn-depleted individuals derived from clinical populationsReference Yamagata, Nakamura, Yamagata, Nakanishi, Matsunaga, Nakanishi, Nishimoto, Minakata, Mune and Yikawa23–Reference Heckmann, Hujoel, Habiger, Friess, Wichmann, Heckmann and Hummel25, Reference Ripamonti, Zecca, Brunelli, Fulfaro, Villa, Balzarini, Bombardieri and de Conno27, Reference Ryan-Crowe69. The present research is also novel in that it has investigated the impact of Zn supplementation upon taste acuity in doses that reflect recommended daily amounts. Other studies have tended to intervene with higher doses than the 15 and 30 mg Zn used for the purpose of the present study. Sweet, sour and bitter taste thresholds have been apparently enhanced subsequent to 45 mg zinc sulfateReference Ripamonti, Zecca, Brunelli, Fulfaro, Villa, Balzarini, Bombardieri and de Conno27 and all four basic tastes observed to improve in response to 66 mgReference Ryan-Crowe69 and 140 mg zinc sulfate per dReference Heckmann, Hujoel, Habiger, Friess, Wichmann, Heckmann and Hummel25. Although the present research study investigated the impact of lower doses of Zn upon taste acuity in healthy ageing, making it difficult to compare findings across studies, these findings agree with those of previous studies using higher doses in clinical populationsReference Heckmann, Hujoel, Habiger, Friess, Wichmann, Heckmann and Hummel25, Reference Ripamonti, Zecca, Brunelli, Fulfaro, Villa, Balzarini, Bombardieri and de Conno27, Reference Ryan-Crowe69 in suggesting that Zn is beneficial to taste acuity in doses of 30 mg or above.
The specific protocol employed for the sensory testing in the present study is also different in some respects from that employed in other studies. Participants were instructed to sip and swallow rather than sip and spit the taste stimuli. Given that the panel were untrained, it was considered important that the solutions be tasted in the normal way, lending greater validity to the findings. Sip and spit would not have reflected ‘normal’ tasting conditions. The present study is also unusual in employing a signal detection approach to taste threshold assessment in older adults. Signal detection theory has been previously applied to the detection of saltReference O'Mahony45, Reference O'Mahony, Gardner, Long, Heintz, Thompson and Davies50 in younger but not older individuals. Signal detection is perceived to be more robust than other sensory threshold techniques in that it enables individual differences in the way individuals make decisions to be taken into account. Signal detection theory could, therefore, be considered ideal for studies employing diverse samples. The use of the ‘basic taste’ paradigm to study taste acuity could also be perceived as controversialReference Schiffman and Graham41. The method has nevertheless provided a well-controlled model that is replicable and which enables comparison across different studies and that appears to be particularly useful for clinical intervention trials looking at taste acuity in qualitatively different study populations.
Conclusion
The present study appears to be the first randomised clinical controlled intervention trial that has considered taste acuity in response to Zn supplementation in healthy ageing. Supplementation with 30 mg Zn may have potential to enhance salt taste acuity in those over the age of 70 years in certain cultures. Meanwhile, further research is required to determine if Zn supplementation, in improving salt taste acuity, can stimulate appetite or enhance the eating experience in older individuals. Although above the upper limit of 25 mg, 30 mg Zn is generally available to the consumer ‘over the counter’; however, in view of potential adverse effects on Cu status of prolonged daily intake of Zn, we should refrain from recommending such high doses to the consumer public.
Acknowledgements
ZENITH is supported by the European Commission ‘Quality of Life and Management of Living Resources’ Fifth Framework Programme, contract no. QLK1-CT-2001-00 168.




