Introduction
One of the most important daily challenges that birds must face is the discovery, consumption and use of energy sources. To do so, they must keep a balance between the costs generated to obtain energy and the energy gain (Pianka Reference Pianka1985, Gutiérrez Reference Gutiérrez, Ardila, López, Pérez, Quiñones and Reyes1998). One of the strategies used by animals to reduce the costs of competition is to occupy different ecological niches. In this respect, using different strategies to avoid competition, they differ in what to eat, where to get food and when to seek food. If these strategies overlap, resource competition may occur; this is detrimental for both parties and can eventually trigger the extinction of one of them (Pianka Reference Pianka1985).
Traditionally, food web studies have not considered scavengers (Wilson and Wolkovich Reference Wilson and Wolkovich2011). Carrion is available episodically, as a pulsating resource that can vary seasonally and spatially, playing a dynamic role in the stability of food webs, and the movements and distribution of species that feed on it (Wilson and Wolkovich Reference Wilson and Wolkovich2011, Barton et al. Reference Barton, Cunningham, Lindenmayer and Manning2013). The stable coexistence of different scavengers on the same carcass is only possible if there is a partition of the resource, allowing the different species to be segregated in space and time when they feed from the same source. To maintain coexistence in equilibrium the intervening species may need to adapt anatomically and behaviourally to reduce food-niche overlap (Houston Reference Houston1988, Arjo and Pletscher Reference Arjo and Pletscher1999, Prior and Weatherhead Reference Prior and Weatherhead2004, Blázquez et al. Reference Blázquez, Sánchez-Zapata, Botella, Carrete and Eguía2009, Moreno-Opo et al. Reference Moreno-Opo, Trujillano and Margalida2016). Even so, if the resource is scarce, competition can allow more aggressive species to monopolise food and possibly to increase, displacing the other species (Hiraldo et al. Reference Hiraldo, Blanco and Bustamante1991a, Stolen Reference Stolen1996).
In north-western Patagonia, carrion is used by several avian scavengers of different sizes (Del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1994). The three most important scavengers in the area are representatives of the Cathartidae family (Order Cathartiformes): the Andean Condor Vultur gryphus, Turkey Vulture Cathartes aura and American Black Vulture Coragyps atratus. Other birds which also use this type of food belong to the family Falconidae (Order Falconiformes, e.g. Milvago chimango and Caracara plancus), but the latter only do so facultatively (Del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1994). Therefore, at least among obligate scavengers, there could be competition for such a fleeting and random resource as carrion, especially if an imbalance in abundance occurs which favours one species over others (Cortés-Avizanda Reference Cortés-Avizanda2010, Cortés-Avizanda et al. Reference Cortés-Avizanda, Jovani, Carrete and Donázar2012). The variables that could have an impact on this balance are: the location of the roosts (Hiraldo et al. Reference Hiraldo, Blanco and Bustamante1991a, Ballejo and De Santis Reference Ballejo and De Santis2013, Novaes and Cintra Reference Novaes and Cintra2013), topography (Carrete et al. Reference Carrete, Lambertucci, Speziale, Ceballos, Travaini, Delibes, Hiraldo and Donázar2010), proximity to human structures (Lambertucci et al. Reference Lambertucci, Speziale, Rogers and Morales2009a, Novaes and Cintra Reference Novaes and Cintra2013), weather conditions that facilitate access to carrion (Shepard and Lambertucci Reference Shepard and Lambertucci2013) and hierarchy (Houston Reference Houston1988). As there is a hierarchy in access to the carrion by size, the largest avian scavenger Andean Condor (c.12 kg) displaces smaller scavenging birds (Wallace and Temple Reference Wallace and Temple1987). However, it has been documented that the number of condors feeding on a carcass decreases relative to the abundance of Black Vultures (Carrete et al. Reference Carrete, Lambertucci, Speziale, Ceballos, Travaini, Delibes, Hiraldo and Donázar2010).
Food availability resulting from human activities such as garbage dumps, slaughterhouses and fishing discards are a source of predictable resources in space and time that Black Vultures take advantage of, leading to increases in population size (Houston Reference Houston1988, Campbell Reference Campbell2014, Barbar et al. Reference Barbar, Werenkraut, Morales and Lambertucci2015). Previous work with Old World vultures has shown that, in areas where human activities generate a temporal and spatial predictability of carcass disposal, there is a decrease in the diversity of scavengers, as opposed to areas where carrion remains unpredictable. In these scenarios species richness remains constant, but not taxonomic diversity, so individuals of dominant species are present in greater numbers. (Wilmers et al. Reference Wilmers, Stahler, Crabtree, Smith and Getz2003, Cortés-Avizanda et al. Reference Cortés-Avizanda, Jovani, Carrete and Donázar2012, Oro et al. Reference Oro, Genovart, Taveccia, Fowler and Martínez Abraín2013).
The Black Vulture and the Andean Condor feed mainly on domestic livestock (Lambertucci et al. Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b, Ballejo and De Santis, Reference Ballejo and De Santis2013), whereas the Turkey Vulture has a wider diet (Hiraldo et al. Reference Hiraldo, Delibes, Bustamante and Estrella1991b). However, there have been no comparative studies on their diet overlap in the same area. Therefore, our aim is to analyse and compare the diet of three obligate scavenger species from north-western Patagonia, by analysing the overlap of trophic niche from their pellets. We expect a greater degree of trophic niche overlap between Black Vulture and Andean Condor, compared with the Turkey Vulture, mainly in places far from the urban development, which will be further support for the scenario of competition between those species.
Materials and methods
Study area
The study area lies in north-western Argentine Patagonia, southern tip of South America (36°–41°S and 71°–68°W) (Figure 1). The climate is cold-temperate, with frequent snowfall in winter and with summer being the driest season (Paruelo et al. Reference Paruelo, Beltran, Jobbagy, Sala and Golluscio1998). The study area encompasses almost 100,000 km2 and includes a mosaic of woodlands and steppes that forms a heterogeneous landscape. The geological and climatic history, along with more recent erosive processes, have created a large number of cliffs that are used as communal roosts by condors. Cliffs and forests are used by vultures as communal roosts (Del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1994). The area has been used for extensive livestock ranching since the last century, and is also one of the regions in Argentina that received the greatest amount of exotic mammal introductions, including Red Deer Cervus elaphus, Wild Boar Sus scrofa, European Hare Lepus europaeus, and Rabbit Oryctolagus cuniculus (Novillo and Ojeda Reference Novillo and Ojeda2008, Speziale et al. Reference Speziale, Lambertucci, Carrete and Tella2012). Those species have been reported as part of the scavengers’ diet (Lambertucci et al. Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b, Ballejo and De Santis Reference Ballejo and De Santis2013).
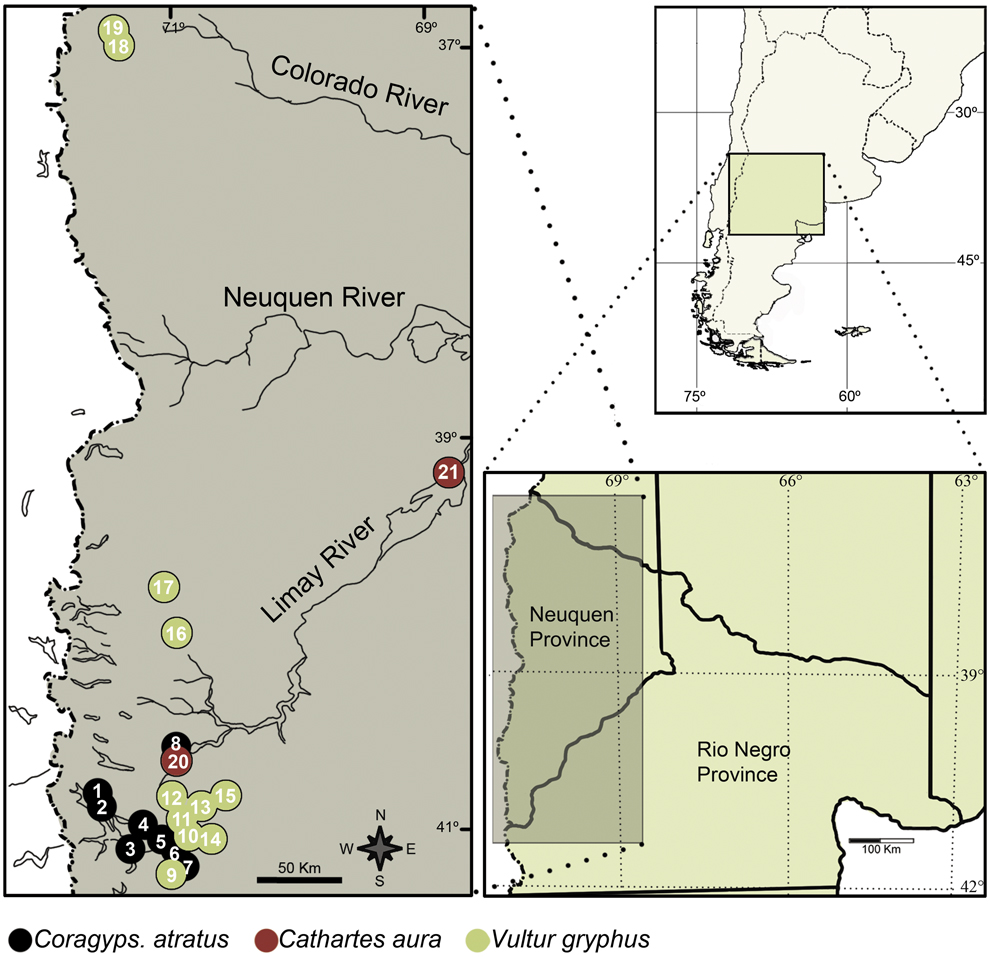
Figure 1. Diagram of the study area and location of each species roost found. 1. Estacas, 2. Victoria, 3. Coihues, 4. Jones, 5. Dina Huapi, 6. Cóndor II, 7. Cóndor I, 8. Chacabuco I, 9. Buitrera, 10. Fg. Chica, 11. Fg. Grande, 12. Condorerita, 13. Pipilcurá, 14. Pichileufú, 15. Chaqueñita, 16. Huechaue, 17. Remolinos, 18. Guanaco, 19. Covunco, 20 Chacabuco II, 21. Chocón.
Study species
We studied three species of obligate scavengers. The Andean Condor (female: 8–11 kg; male: 11–15 kg) feeds in Patagonia, southern South America, mainly on domestic ungulates (Lambertucci et al. Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b) Roosting and nesting occur in mountains (on cliffs, and rock shelters) and foraging occurs in open areas - steppes, grasslands, and beaches along the coast (Del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1994, Lambertucci et al. Reference Lambertucci, Alarcón, Hiraldo, Sanchez-Zapata, Blanco and Donázar2014). It is a species of large dimensions, which displaces smaller species from access to carrion. Due to its large and efficient bill it can open the abdominal cavity of large ungulates, providing access to the viscera for other birds of lower rank (Wallace and Temple Reference Wallace and Temple1987, Del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1994). The study area has one of the best condor populations known (more than 250 individuals; Lambertucci Reference Lambertucci2010). The Turkey Vulture (0.85–2.0 kg) roosts socially on cliffs, rock shelters, and trees. They feed on carcasses of different sizes, soaring at low altitudes in small groups and mainly using their sense of smell, allowing them to locate small carcasses, which are consumed quickly, before other members of the guild arrive (Houston Reference Houston1988, Thomaides et al. Reference Thomaides, Valdez, Reid and Raitt1989, Hiraldo et al. Reference Hiraldo, Delibes, Bustamante and Estrella1991b, Buckley Reference Buckley1997). There are no current estimations of the population size or trends of this species in the area. The Black Vulture (1.1–1.9 kg) inhabits open areas and roosts on cliffs, rock shelters, or trees. It forages and feeds socially in large groups, and searches for food using the sense of sight (Houston Reference Houston1988, Stolen Reference Stolen1996). It feeds on carcasses of different sizes (Coleman and Fraser Reference Coleman and Fraser1989) and is favoured by human activities that gather organic matter in places like garbage dumps and slaughterhouses (Iñigo Elías 1987, Ballejo and De Santis Reference Ballejo and De Santis2013). Black Vulture arrived in the area relatively recently and has expanded its distribution following human activities. Houston (Reference Houston, Buckley, Foster, Morton, Ridgley and Buckley1985, Reference Houston1988) notes that Black Vulture is more abundant in open habitats near human settlements of at least 3,000 people. He also suggests that before the human settlement of South America, Black Vultures were probably restricted to open savannas, swamplands and large river banks, feeding on large mammals, stranded fish, and smaller food items. This range expansion is evidenced in the literature (Darwin Reference Darwin1839, Houston Reference Houston, Buckley, Foster, Morton, Ridgley and Buckley1985, Reference Houston1988, Tonni and Noriega Reference Tonni and Noriega1988, Buckley Reference Buckley1997). Current studies show the greatest abundance of Black Vultures in urban and semi-urban areas (Bellati Reference Bellati2000, Campbell Reference Campbell2014, Barbar et al. Reference Barbar, Werenkraut, Morales and Lambertucci2015). There is no study analysing the expansion of the population in the study area, however, these populations have probably increased in parallel with urbanisation processes as in other regions (Del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1994, Campbell Reference Campbell2014).
Diet survey
During the summer of 2012, we collected vulture pellets from communal roosts to analyse the composition of the diet. The analysis of pellets is one of the most used methods to study bird of prey diet; however, this method overestimates the bone remains of smaller prey. In contrast, skeletal remains found in the nest overestimate the larger prey. Vulture pellets mainly have hair. Therefore this method is reliable with an adequate knowledge of the fauna in the area, good comparison collections, and the application of microscopy techniques (Real Reference Real1996).
Pellets were collected from: a) Eight Black Vulture roosts; four of them characterised by being on trees in Patagonian steppe, near livestock farms in an area that has been used for extensive livestock of sheep, and away from urban centres (Chacabuco I, Condor I, Condor II and Jones); and the last four characterised by being near or within urban areas: the Estacas roost is less than 2 km from a garbage dump; the Victoria roost is on abandoned buildings; and the Coihues and Dina Huapi roosts are on trees within villages with these names; b) two Turkey Vulture roosts, one of them is on trees in rural context near a trout farm (Chacabuco II), and the other is on trees inside a town (Chocón); and c) 11 Andean Condor roosts; which are on cliffs in a natural environment in the Patagonian steppe. Location of roosts is shown in Figure 1. Roosts were classified as rural context (rural and natural environments located more than 15 km from an urban area) and urban context (located within or less than 2 km from an urban area).
Bone and teeth were separated and studied under a stereoscopic microscope (10–40 x). Bone remains were identified using reference materials from the collection at the Museum of Natural Science of La Plata (Buenos Aires Province). Moreover, hair remains were identified using cuticular and medullary patterns with optical microscopy, and using reference materials from the collection at the Museum of La Plata, and the collection of “Patagonia Vertebrate inventory” from the National Parks Administration (Administración de Parques Nacionales, Argentina), as well as with bibliographic sources (Chehébar and Martín Reference Chehébar and Martín1989, De Marinis and Asprea Reference De Marinis and Asprea2006). Feathers and arthropod heads were identified by comparison with a reference collection from the Museum of La Plata, Buenos Aires (Argentina).
In the case of the Andean Condor roosts, samples collected in 2007 and in this study in 2009 were pooled. The 2007 data was published in a previous work, corresponding to the same study area (Lambertucci et al. Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b). To confirm that there has been no change in diet over the years regarding the pellets collected for this work, we applied the Pianka index of trophic niche overlap (O j, k = Σpij applied. Pik / √ (Σpij2. Σpik2) (Pianka Reference Pianka1973), where pij and pik are the proportion of the prey type “i” in the diet of the “j” and “k” species respectively. This index yields values from 0 (no overlap) to 1 (complete overlap) and we did not find any differences (O j, k = 0.985).
We used the minimum number of individuals (MNI) based on the bone elements found in pellets (Grayson Reference Grayson1978). In the cases in which they were not found, hair, feathers and / or scales were counted, considering one individual per pellet; arthropods were quantified based on their heads. In all cases species found were identified at the lowest possible taxonomic level.
Data analysis
We expressed our results on diet composition as a percentage of the total prey (representing the number of times each item was encountered in relation to the overall number of items in all pellets), and percentage occurrence of each item in relation to the overall number of pellets. Diet diversity was calculated using the Shannon index, H ’= -Σpilog2pi, and we used Pianka’s index (Pianka Reference Pianka1973) to compare dietary overlap. From the results of this last index, we created a dendrogram using the statistical software PAST (Paleontological Statistics) version 3.02. The graphic display of this analysis shows clusters in relation to the similarities in the taxa ingested by individuals of each roost. Finally, we carried out a correspondence analysis to examine the relationship between the environment where the roosts were located and the taxa consumed. This type of analysis aims to represent each of the possible values for each variable, where the relative position of the points reflects the degree of association between each of the concepts represented. To perform this analysis a contingency table was drawn up where taxa ingested were placed in rows, and scavenger species in columns, and separated in relation to the environment where their roosts are located (rural or urban context). We used RStudio program (pgirmess package) for this statistical analysis (Giraudoux and Giraudoux Reference Giraudoux and Giraudoux2015).
Results
The three species studied consume carrion of large ungulates (mainly sheep) and hares. In turn, vultures also eat other mammals such as canines, where the genus Lycalopex was the most represented in the pellets in addition to weasels (Mustelidae), felines and cricetid rodents (Table 1). However, vultures also prey on arthropods. The most representative arthropods in the samples were Coleoptera (Tenebrionidae), Hymenoptera and the Orthoptera (Formicidae). But among Coleoptera, the fmailies Scarabaeidae, Carabidae, Elateridae and Curculionidae were also found, in addition to representatives of the order Blattodea and the Chelicerata (family Bothriuridae). In turn, synthetic materials such as polyethylene bags, rubber bands and rubber were also found in vulture pellets. Turkey Vulture has the highest diet diversity, as their sample includes both large ungulates and reptiles as well as teleost fishes and a large number of birds and carnivores. The lowest diversity value is found in the samples of Andean condor, which feeds mainly on domestic ungulates and hares (Table 2).
Table 1. Diet composition of three scavenger species in northwestern Patagonian. We present the number of prey items (n) Frequency percentage (F%) corresponds to the percentage of the total number of taxa. Percentage of occurrence (O%) is the percentage of the total number of pellets.
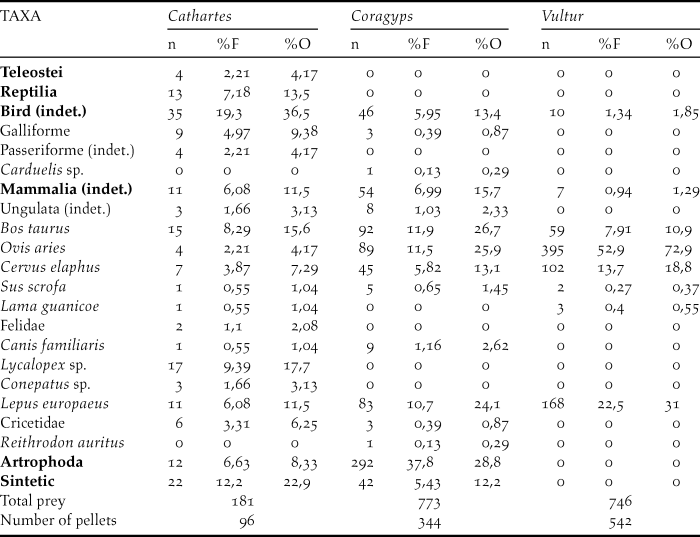
Table 2. Shannon diversity index calculated on the basis of the diet in different communal roosts for three species of scavengers (Cathartidae family)
The dendrogram shows the similarity between prey type consumed among each roost (Figure 2). Three main groups were found, one of which contains only the Andean Condor roosts; the second contains Black Vulture roosts, characterised by being located away from urban centres and near livestock farms; the last group contains the remaining roosts belonging to both species of vultures, most of which are near or within urban centres. However, in this last group there is a clear segregation between both species of vultures. Moreover, the first two groups share a higher degree of similarity to each other (0.4–0.5) than with the third (0.2–0.3).
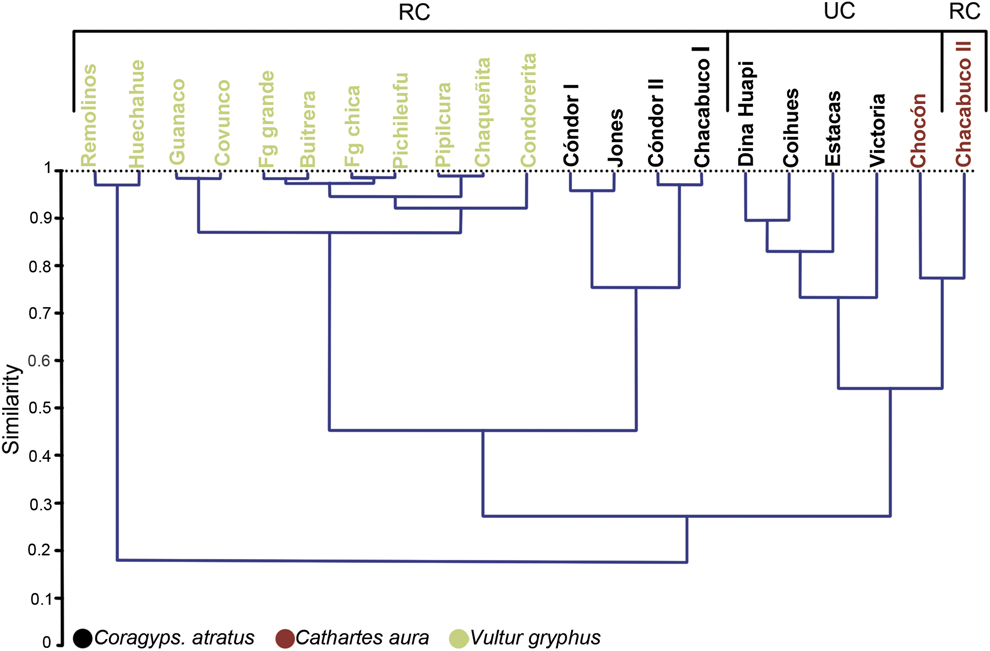
Figure 2. Dendrogram based on the Pianka index of trophic niche overlap of the diet of three species of scavengers surveyed in their communal roosts. Roosts were located in Rural context (RC) or Urban context (UC).
Associations between variables considering the closeness that each of the points in the correspondence analysis (Figure 3) were in agreement with the dendrogram (Figure 2). Black Vultures in a rural context have a similar diet to the Andean Condor, with Lepus europaeus, Ovis aries and Cervus elaphus the taxa with the highest incidences. Black Vultures in urban areas differ from the previous group, with Bos taurus and Sus scrofa being the most frequent prey items. On the other hand, Turkey Vulture roosts are found in both kinds of habitat (rural and urban) and they showed the highest diversity. This is mainly because they are the only species in which reptiles and fish occur in their pellets, and they feed on a higher proportion of felines, canines and cricetid rodents than the other two species (see Table 1).

Figure 3. Correspondence analysis showing the ingested taxa (points) and scavenger species (geometric figures) separated by the environment where their roosts are located. The “other” category includes felids, canids and cricetid rodents.
Discussion
To maintain a demographic balance between populations in a community, species occupy certain trophic niches and have specific foraging strategies. When they move away from this balance, an increase in the trophic niche overlap might take place, increasing competition for resources (Pianka Reference Pianka1985). Our results indicate that such overlaps exists to a significant degree between the Black Vulture and the Andean Condor in rural areas, but not with the Turkey Vulture. The Andean Condor and the Black Vulture feed primarily on domestic livestock carrion (Lambertucci et al. Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b, Ballejo and De Santis Reference Ballejo and De Santis2013, this study). Moreover, they showed higher trophic niche overlap when roosting in a similar context (i.e. they feed on similar food sources when they are far from cities, in a rural context). The Turkey Vulture feeds on the widest range of carrion, regardless of where the roosts are sited, incorporating fish, reptiles and a larger number of birds, carnivores and mice in their diet. As a result, the trophic niche overlap with the other two species is reduced. The difference in selection of carrion might be due to the developed sense of smell which is characteristic of this bird, and which allows them to find carcases of a size that would go unnoticed by the other two species, that mainly use the sense of sight for this purpose (Houston Reference Houston1988). Moreover, their ability to fly at low altitudes allows them to seek alternative sources of food (Coleman and Fraser Reference Coleman and Fraser1989, Stolen Reference Stolen2000).
Black Vulture roosts located in rural areas showed a greater diversity than those in urban areas. An exception is the Estacas roost, which is located near a rubbish dump. The use of garbage as food is well documented in scavengers (Plaza and Lambertucci Reference Plaza and Lambertucci2017). In those places the diversity of food sources and its availability have a positive effect on the development of their populations (Plaza and Lambertucci Reference Plaza and Lambertucci2017, Steigerwald et al. Reference Steigerwald, Igual, Payo-Payo and Tavecchia2015). Differences in diversity of taxa consumed in rural and urban roosts of Black Vulture have been previously documented, with cows and sheep as the main prey consumed, whereas in rural roosts, hares and a large number of arthropods replace that domestic food source (Ballejo and De Santis, Reference Ballejo and De Santis2013). Furthermore, we can assert that the Turkey Vulture has a higher prey diversity in both roosts studied, as found by other authors (Hiraldo et al. Reference Hiraldo, Delibes, Bustamante and Estrella1991b). The Andean Condor tends to occur far from human-occupied areas (Speziale et al. Reference Speziale, Lambertucci and Olsson2008). It has the lowest value in food diversity since they feed almost exclusively on domestic and wild exotic species in rural environments (Lambertucci et al. Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b).
The Old World scavengers are temporally and spatially segregated in the use of carrion (Kruuk Reference Kruuk1967, Blázquez et al. Reference Blázquez, Sánchez-Zapata, Botella, Carrete and Eguía2009, Cortés-Avizanda Reference Cortés-Avizanda2010, Kendall et al. Reference Kendall, Virani, Kirui, Thomsett and Githiru2012, Moreno-Opo et al. Reference Moreno-Opo, Trujillano and Margalida2016). Once they find a carcase, they can dominate birds of lower rank. However, smaller birds can sometimes benefit from their presence as they dismember large corpses (Kruuk Reference Kruuk1967, Moreno-Opo et al. Reference Moreno-Opo, Trujillano, Arredondo, González and Margalida2015). The random distribution of carcases plays a key role, because where carcases are concentrated in small areas, the diversity of scavengers is affected, leaving the resource to be monopolised by the largest and most abundant species (Cortés-Avizanda et al. Reference Cortés-Avizanda, Jovani, Carrete and Donázar2012). On the other hand, they show differences in bill morphology and pecking activity (number of pecks through time), allowing them to specialise in the exploitation of different body parts (Kruuk Reference Kruuk1967, Moreno-Opo et al. Reference Moreno-Opo, Trujillano and Margalida2016). This facilitates coexistence between species. Similarly, New World vultures (Cathartidae) have a clear size hierarchy, in which the larger birds typically displace the smaller (Wallace and Temple Reference Wallace and Temple1987, Houston Reference Houston1988, Hertel Reference Hertel1994). However, when the number of individuals is unbalanced, these hierarchies can be lost (Buckley Reference Buckley1996, Carrete et al. Reference Carrete, Lambertucci, Speziale, Ceballos, Travaini, Delibes, Hiraldo and Donázar2010). In this sense, Turkey Vultures and Andean Condors could be displaced from carcasses when Black Vultures appear in large numbers. Despite this, Turkey Vultures can diversify their diet to incorporate bodies of smaller animals (Hiraldo et al. Reference Hiraldo, Delibes and Donazar1991c). This does not happen with the Andean Condor, probably because small animals hardly cover their nutritional needs (Donázar et al. Reference Donázar, Cortés-Avizanda and Carrete2010). This would be less likely to affect Andean Condor populations if both species consumed different taxa or had extensive dietary diversity. However, our results indicate that both species consume similar taxa in areas of sympatry, which supports the idea that Andean Condor populations could be adversely affected if the number of Black Vultures increased (Carrete et al. Reference Carrete, Lambertucci, Speziale, Ceballos, Travaini, Delibes, Hiraldo and Donázar2010).
The ability to feed on anthropogenic waste is evidenced by the presence of synthetic materials found in the pellets of Black Vultures in this study, as well as in previous works (Iñigo Elías 1987, Sazima Reference Sazima2007, Ballejo and De Santis Reference Ballejo and De Santis2013). Therefore, a scenario of asymmetric competition may arise, with Black Vultures benefiting from the presence of alternative and predictable food resources generated by human activities (Novaes and Cintra Reference Novaes and Cintra2013). This could be the reason why this species has become increasingly common in urban environments (Novaes and Cintra Reference Novaes and Cintra2013, Campbell Reference Campbell2014), extending its distribution and abundance to areas occupied by the Andean Condor (Bellati Reference Bellati2000, Barbar et al. Reference Barbar, Werenkraut, Morales and Lambertucci2015). This type of anthropogenic resource is not used by the Andean Condor in the area, as it is very sensitive to urbanisation (Speziale et al. Reference Speziale, Lambertucci and Olsson2008, Lambertucci et al. Reference Lambertucci, Speziale, Rogers and Morales2009a), nor in most of its distribution range (except in one place in Central Chile). Condor populations have been affected to a different extent in different countries, becoming in danger of extinction in Colombia, Ecuador and Venezuela (Lambertucci Reference Lambertucci2007). In Argentina their populations are more numerous (Lambertucci Reference Lambertucci2010), but face a series of threats, including persecution, ingestion of toxic baits and pesticides, poisoning by ingestion of lead ammunition, and collision with power lines (Lambertucci Reference Lambertucci2007, Lambertucci et al. Reference Lambertucci, Donazar, Huertas, Jiménez, Sáez, Sanchez-Zapata and Hiraldo2011). This situation places the Andean Condor in a vulnerable position which could worsen in the presence of numerous populations of Black Vultures (Carrete et al. Reference Carrete, Lambertucci, Speziale, Ceballos, Travaini, Delibes, Hiraldo and Donázar2010).
The presence of resources resulting from human activities, which are predictable in space and time (e.g. landfill), has direct effects on individuals, but also generates cascade effects on populations and communities worldwide (Oro et al. Reference Oro, Genovart, Taveccia, Fowler and Martínez Abraín2013, Plaza and Lambertucci Reference Plaza and Lambertucci2017). The effects on birds are diverse, for example by generating changes in their home-ranges, since they are limited to areas where food is located (Monsarrat et al. Reference Monsarrat, Benhamou, Sarrazin, Bessa-Gomes, Bouten and Duriez2013); and altering nesting and roosting areas, since these are selected for proximity to sources of food (Kristan and Boarman Reference Kristan and Boarman2007, Selva and Fortuna Reference Selva and Fortuna2007). They can generate a dependency on the resource, so a lack of can trigger sharp declines in the populations that profit from it (Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008) and they can diminish the diversity of communities, since the resource can be monopolised by more aggressive species (Cortés-Avizanda et al. Reference Cortés-Avizanda, Jovani, Carrete and Donázar2012, Plaza and Lambertucci Reference Plaza and Lambertucci2017). But one of the main consequences is that these resources can facilitate the emergence of native invader species, since the availability of readily available food can increase the population of species that can best take advantage of this resource in comparison to others (Carey et al. Reference Carey, Sanderson, Barnas and Olden2012; Oro et al. Reference Oro, Genovart, Taveccia, Fowler and Martínez Abraín2013, Plaza and Lambertucci Reference Plaza and Lambertucci2017). These species might have a similar impact to that caused by alien invader species, which through various mechanisms such as competition, can alter the structure of a community and in severe cases reduce or eliminate populations of native species (Carey et al. Reference Carey, Sanderson, Barnas and Olden2012). Black Vultures show the typical characteristics of a native invader species, with environmental changes generated by human activities facilitating population growth, increasing their survival and reproduction. Therefore, they can take advantage of the vacancies left by other native species that suffer a loss or decline of their populations because of these activities (Carey et al. Reference Carey, Sanderson, Barnas and Olden2012). This kind of competition favoured by human activities should to be incorporated in conservation strategies, particularly those aiming to conserve the most vulnerable and less flexible species to anthropogenic advance.
Acknowledgements
We thank M. Contaldi who helped in the field and F Doyharzabal for revising the English. Managers and owners of La Buitrera, San Ramón, El Cóndor, Chacabuco farms, as well as E Bendstrup and the Administración de Parques Nacionales gave permission to study the roosts. This work has been funded by Project 11/N769 of the Facultad de Ciencias Naturales y Museo, UNLP. We also thank the support of CONICET, and PICT-BID (2014-0725). We thank K. Bildstein, A. Margalida, and an anonymous reviewer for the comments that helped to improve a previous version of this manuscript.







