Premenopausal women are relatively protected against CVD compared with men(Reference Booth, Kapral and Fung1), but the reasons for this sex difference are not completely understood. An understanding of the underlying mechanisms for this difference may help us to prevent worsening of cardiovascular risk factors in men and women in later life. There is evidence for some sex differences in the plasma metabolic profile in young adults. Short-term fasting reveals sex differences in people who are young and lean; after fasting, women have lower plasma glucose and higher plasma NEFA concentrations than men, as summarised in Soeters et al. (Reference Soeters, Sauerwein and Groener2). The ketone body 3-hydroxybutyrate (3-OHB) is an important energy source for the brain and other tissues during prolonged fasting. Under starving conditions, it is considered to act as a central signal and energy-providing substrate involved in the regulation of food intake(Reference Laeger, Metges and Kuhla3). Also, it seems that 3-OHB has an anti-lipolytic effect and is a ligand for the nicotinic acid receptor. In this way, its effect is similar to nicotinic acid(Reference Taggart, Kero and Gan4). However, to the best of our knowledge, sex comparisons of plasma 3-OHB concentrations have rarely been reported in healthy, lean young men and women. The difference in blood 3-OHB concentrations has been reported to be greater in response to fasting with significantly higher concentrations in women than in men after 30 h fasting, but plasma insulin concentrations were similar(Reference Haymond, Karl and Clarke5). After an overnight fast, plasma TAG concentrations in women were lower than those in men, but this difference did not reach statistical significance and did not change during a further 10 h of fasting(Reference Magkos, Patterson and Mohammed6). However, plasma VLDL-TAG concentrations were lower in women(Reference Magkos, Patterson and Mohammed6) in response to the extended fasting. The results from that study suggested that the liver in women secretes fewer but TAG-richer VLDL particles than the liver in men, and that clearance was faster in women. There are two major classes of VLDL secreted by the liver: VLDL1 is larger and more TAG-rich than VLDL2. The latter can either be secreted directly from the liver or be formed by the peripheral hydrolysis of VLDL1. These two classes of lipoproteins have different properties; in subjects with type 2 diabetes, the secretion of VLDL1 is associated with liver fat content, hypertriacylglycerolaemia and atherogenic risk(Reference Adiels, Taskinen and Packard7). VLDL-TAG production represents the export of fatty acids partitioned towards esterification and secretion rather than storage or oxidation in the liver. Therefore, the balance between the pathways of esterification and oxidation in men and women may be important for the development of CVD risk factors.
The aim of the present study was to investigate sex differences in plasma metabolites and hepatic fatty acid metabolic partitioning in the liver in response to an extended overnight fast. This enabled us to stress metabolic pathways in order to investigate sex differences in young people before the onset of traditional cardiovascular risk factors. Since systemic NEFA are the major substrate for VLDL-TAG and 3-OHB production, we aimed to follow the transfer of a stable isotope label from plasma NEFA into the products of hepatic metabolism.
Materials and methods
Subjects
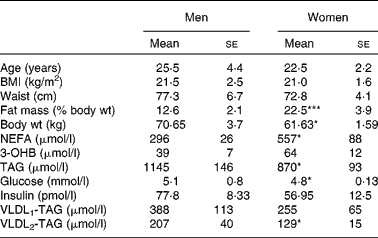
Healthy, lean young men and women (n 12) were studied, and matched for age and BMI with no significant difference in waist circumference, but the women had a greater percentage of body fat (Table 1). Subjects, recruited from the wider Oxford community via advertisement, were free from any disease, weight stable and not taking any lipid-lowering medication or medication that would alter lipid metabolism. The studies were performed during the first week (follicular phase) of the menstrual cycle and conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by the Oxfordshire Clinical Research Ethics Committee. Written informed consent was obtained from all subjects.
Table 1 Volunteer characteristics and baseline overnight fasting (12 h) plasma metabolite concentrations
(Mean values with their standard errors, n 6)
3-OHB, 3-hydroxybutyrate.
Mean values were significantly different from those of men: *P < 0·05, ***P < 0·001.
Study protocol
Before the study day, subjects were asked to avoid foodstuffs naturally enriched in 13C for 48 h and refrain from strenuous exercise and alcohol for 24 h. On the evening before the study, subjects were required to eat a low-fat meal. On the day of the study, subjects arrived at the clinical research unit after an overnight fast. Fat mass was measured by bioelectrical impedance analysis, and fat-free mass was calculated as the difference between total body weight and fat mass. A cannula was inserted into an antecubital vein, and a baseline blood sample was taken for background isotopic enrichment. At time 0, a continuous intravenous infusion (0·04 μmol/kg per min) of potassium [U-13C]palmitate (isotope purity 98 %; Cambridge Isotope Laboratories, Inc., Andover, MA, USA) complexed to human albumin was started, to label the plasma NEFA pool. This was continued until the end of the experiment (at 7 h).
Analyses
Whole blood was collected into heparinised syringes (Sarstedt, Leicester, UK), plasma was rapidly separated by centrifugation at 4°C, and plasma NEFA, VLDL-TAG and 3-OHB concentrations were determined enzymatically, as described previously(Reference Humphreys and Frayn8, Reference Bickerton, Roberts and Fielding9). Plasma insulin concentrations were measured by RIA(Reference Bickerton, Roberts and Fielding9).
In ten subjects, VLDL1 (Svedberg flotation rate (S f) 60–400) and VLDL2 (S f 20–60) were isolated by sequential flotation using density gradient ultracentrifugation in an SW40Ti swinging bucket rotor (Beckman Instruments, Palo Alto, CA, USA) at 40 000 rpm at 15°C for 4 h for S f 60–400 lipoproteins and for a further 16 h to float S f 20–60 lipoproteins.
Fatty acid analysis and isotopic enrichment
Fatty acid methyl esters were prepared from NEFA, VLDL1- and VLDL2-TAG fractions and isotopic enrichment was measured by GC and GC–MS, respectively, as described previously(Reference Bickerton, Roberts and Fielding9). Specific fatty acid concentrations were determined by multiplying the proportion of the specific fatty acid by the corresponding plasma concentration as determined enzymatically for plasma NEFA, VLDL1- and VLDL2-TAG.
Isotopic enrichment from [U-13C]fatty acids appearing in 3-OHB in deproteinised plasma was measured using a modified method of Beylot et al. (Reference Beylot, Beaufrere and Normand10). Solutions of [2,4-13C2]3-OHB (Cambridge Isotope Laboratories, Inc.) and unenriched 3-OHB were prepared in 4 % perchloric acid and diluted in deionised water to make an enrichment standard curve (100 μmol/l). Plasma samples (0·5 ml) were deproteinised with 1 ml perchloric acid (70 g/l). Duplicate 1 ml aliquots of the supernatant were neutralised with 500 μl neutralising reagent (0·5 m-KHCO3 and K2CO3) on ice. After centrifugation, 500 μl aliquots of the deproteinised plasma were pipetted into 10 ml glass tubes and acidified to pH 1 with 1 m-HCl. After prior addition of 50 μl neutralising reagent, 500 μl 3-OHB standards were acidified in the same manner. The 3-OHB from samples and standards was extracted into 4·5 ml ethyl acetate–diethyl ether (1:1, v/v) in 10 ml glass tubes by mixing by hand for 2 min and then by rotary mixing for 1 h. The aqueous and organic phases were separated by centrifugation, and the upper organic phase was evaporated to dryness under N2 at room temperature. t-Butyl dimethylsilyl derivatives of 3-OHB were prepared by the addition of 20 μl pyridine and 20 μl N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide+1 % t-butyl dimethylchlorosilane. After incubation for at least 45 min at room temperature, the contents of the tubes were transferred to GC vials and analysed by GC–MS. The GC was equipped with a 30 m capillary column with a 5 % diphenyl–95 % dimethyl polysiloxane stationary phase (inner diameter 0·32 mm, film thickness 0·25 μm; Thames Restek, Saunderton, UK). The 5890 GC was coupled to a 5973N MSD (Agilent Technologies, Stockport, UK). Ions with mass-to-charge ratios (m/z) of 275 (M+0), 277 (M+2) were determined by selected ion monitoring. We assume that the latter corresponds with the formation of 3-OHB from one labelled [13C2]acetyl-CoA molecule, derived from [U-13C]palmitic acid via β-oxidation. Initially, we selected ions of m/z 279 (M+4) but the GC–MS was not sufficiently sensitive to consistently detect a peak.
The tracer:tracee ratio (TTR) of a baseline measurement (before the administration of the stable isotope tracer) was subtracted from each sample's TTR to account for natural abundance. The TTR for [U-13C]palmitate were multiplied by the corresponding palmitate concentration in plasma NEFA and VLDL1- and VLDL2-TAG to give tracer concentrations. Likewise, the TTR for [13C]3-OHB (M+2):(M+0) were multiplied by the corresponding plasma 3-OHB concentrations to give tracer concentrations. Mole percent excess (MPE) was calculated from the TTR using the formula MPE = 1/(1+1/TTR).
Calculations
The proportion of systemic plasma NEFA contributing to VLDL-TAG at 7 h was calculated as described previously(Reference Hodson, Bickerton and McQuaid11). The remainder are assumed to be fatty acids from splanchnic sources, including those from visceral fat lipolysis, liver fat and de novo lipogenesis.
Whole-body rate of appearance (R a) of NEFA was calculated using the isotopic enrichment in the plasma after an intravenous infusion of the [U-13C]palmitate(Reference Wolfe and Chinkes12) during the last 5 h of the infusion. The rate of disappearance of NEFA was calculated as R a NEFA − dQ/dt, where dQ is the change in the amount of tracee with time(Reference Wolfe and Chinkes12).
The classic mathematical modelling of 3-OHB production could not be performed because the level of enrichment in 3-OHB did not allow for calculation of the precursor pool enrichment. However, we wished to examine the extent to which the enrichment of [13C] in plasma 3-OHB reflected the synthesis from systemic NEFA. Therefore, we estimated the maximal enrichment (MPE) in 3-OHB for (M+2) at 7 h, assuming that the precursor for 3-OHB production was only from the plasma NEFA pool. Since we infused a palmitic acid tracer, we assumed that the enrichment in the mitochondrial acetyl-CoA pool would be diluted by (non-selective) contributions from other specific fatty acids. Therefore, we accounted for the number of carbon atoms in each fatty acid species and their relative proportions in the NEFA pool for each participant. The plasma enrichment of [U-13C]palmitate multiplied by the dilution factor gave an estimation of the [13C]acetyl-CoA enrichment in the mitochondria. The estimated maximal MPE was calculated from the formula for the probability of picking exactly one labelled and one unlabelled element, 2P(1 − P), where P is the probability of an element to be labelled.
The M + 4 isotopomer for 3-OHB in women at plateau enrichment was estimated from the calculated precursor enrichment (MPE) and binomial expansion(Reference Hellerstein, Christiansen and Kaempfer13).
In order to determine an approximation of the relative partitioning of systemic plasma NEFA into 3-OHB and VLDL-TAG, we calculated the ratio of [13C]3-OHB:[13C]VLDL-TAG in the plasma, where [13C] is expressed in μmol/l.
Statistical analysis
Data were analysed using SPSS for Windows version 16 (SPSS, Chertsey, UK). Statistical significance was set at P < 0·05. All data are presented as means with their standard errors. Repeated-measures ANOVA, with time and group as factors, was used to investigate the change between men and women over time while fasting. Comparisons between groups after the extended overnight fast (7 h) were made using a Mann–Whitney test.
Terminology
Conventional terminology for stable isotope tracer techniques is used in the present paper(Reference Wolfe and Chinkes12). The passage of a molecule through the GC–MS results in the production of a major ion of interest with mass M. An isotopomer is a molecule that has the same chemical composition but a different mass because it has an isotopic tracer incorporated somewhere in the molecule. Thus, the M isotopomer for 3-OHB refers to an ion that has no 13C tracer incorporated, and M+2 represents an ion that has a mass that is 2 atomic mass units greater than the M isotopomer; this is assumed to be due to the substitution of two 12C atoms by two 13C atoms.
Results
Differences in plasma variables between men and women after an overnight fast are given in Table 1. After 12 h fasting, the men had a less advantageous metabolic profile in terms of higher plasma glucose (P < 0·05) and TAG (P < 0·05) concentrations but lower plasma NEFA (P < 0·05) concentrations. In order to verify that the twelve subjects whom we chose were representative of the wider community in this respect, we retrieved data from 266 men and 405 women (aged 30–50 years) from the Oxford Biobank(Reference Tan, Neville and Liverani14). All individuals were selected to have a BMI of less than 25 kg/m2. In exact accordance with the six men and six women in the present study, the larger group of women had higher plasma NEFA concentrations (553 (se 13) v. 475 (se 16) μmol/l; P < 0·05); the same plasma 3-OHB concentrations, lower plasma TAG concentrations (870 (se 20) v. 1090 (se 40) μmol/l; P < 0·05); lower plasma glucose concentrations (4·8 (se 0·0) v. 5·2 (se 0·0) mmol/l; P < 0·05) and similar plasma insulin concentrations. After extension of the postabsorptive period during the 7 h of the present experiment, expected changes in plasma variables occurred (Fig. 1). Significant differences between men and women for glucose, TAG and NEFA were maintained, and the marginally higher values for 3-OHB found in women after 12 h fasting (P = 0·078) became significant (P = 0·035). Plasma insulin concentrations decreased significantly with time (P = 0·02) but remained similar between men and women after prolonged fasting.
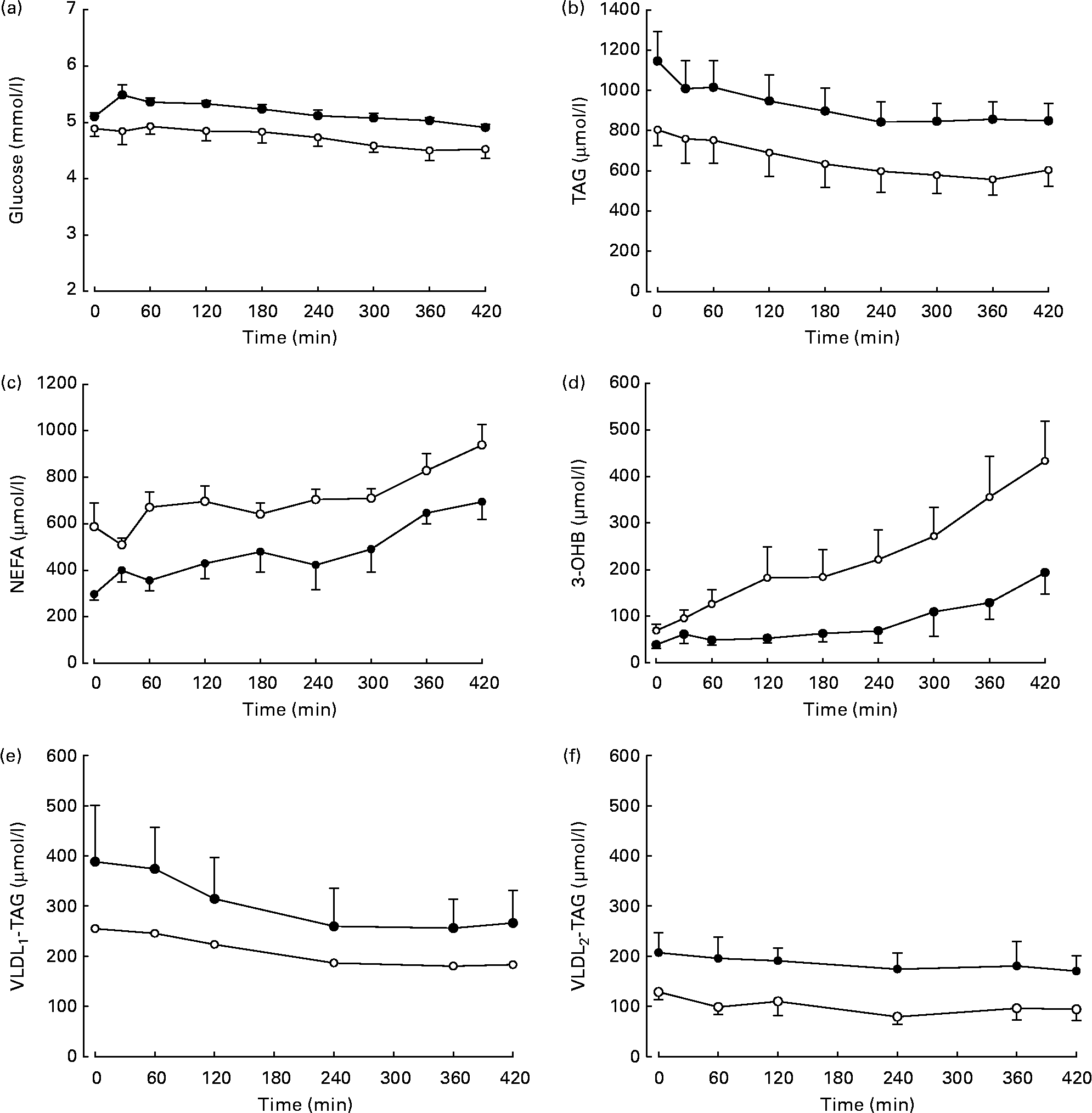
Fig. 1 Plasma concentrations of metabolites in response to continued overnight fasting and analysed by repeated-measures ANOVA. ●, Men (n 6); ○, women (n 6). Values are means, with standard errors represented by vertical bars. (a) Glucose, there was a significant effect of time (P < 0·05) and sex (P < 0·05). (b) TAG, there was a significant effect of time (P < 0·001) and a tendency for an effect of sex (P = 0·087). (c) NEFA, there were significant effects of time (P < 0·01) and sex (P < 0·01). (d) 3-Hydroxybutyrate (3-OHB), there were significant effects of time (P < 0·01) and sex (P < 0·05). (e) Plasma VLDL1 concentrations (men (n 5), women (n 5)), in response to continued overnight fasting. There was a significant effect of time (P < 0·05). (f) Plasma VLDL2 concentrations, there was a tendency for an effect of sex (P = 0·066).
Concentrations of VLDL2-TAG but not VLDL1-TAG were significantly higher in men than in women (Table 1). We observed that VLDL1-TAG concentrations were higher than VLDL2-TAG in both sexes combined (P = 0·028). During prolonged fasting, the concentrations of VLDL1-TAG decreased significantly (Fig. 1), but no further sex differences were revealed. There was a small decrease in VLDL2-TAG concentrations during the study, but this was not significant (females P = 0·275, males P = 0·663).
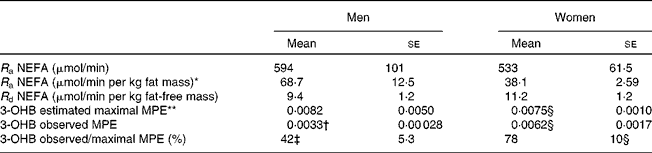
The TTR of [U-13C]palmitate from the intravenous infusion reached a plateau in the plasma NEFA pool by 1 h in men and women (Fig. 2). R a NEFA (μmol/min) was similar in men and women but lower in women when expressed as per kg fat mass (Table 2). When expressed according to fat-free mass, there was no difference between men and women (Table 2).
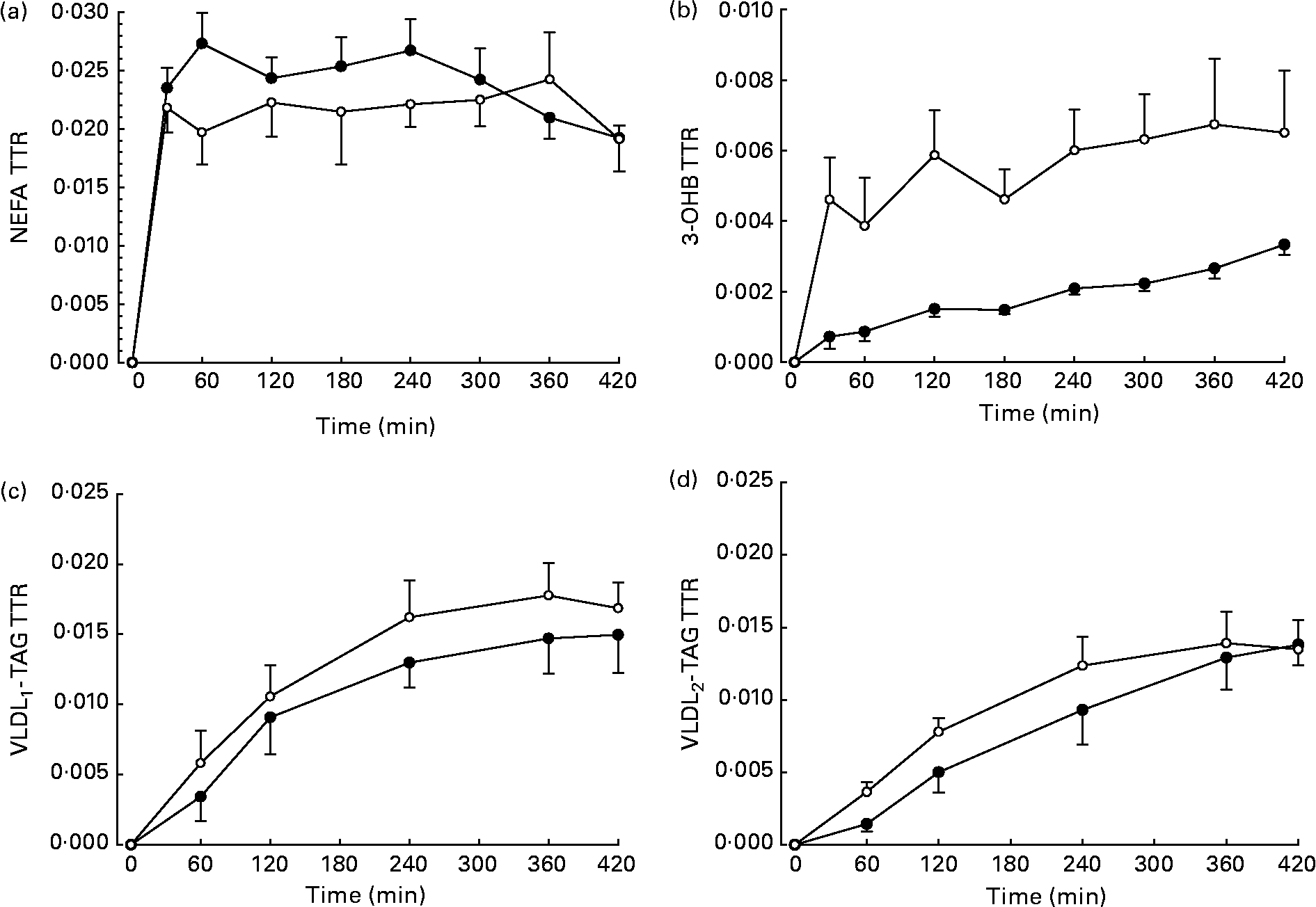
Fig. 2 Plasma tracer:tracee ratio (TTR) resulting from an intravenous infusion of [U-13C]palmitate, starting at time 0. ●, Men (n 6); ○, women (n 6). (a) NEFA (n 6), there were significant effects of time (P < 0·05), time × sex interaction (P < 0·05) and a tendency for an effect of sex (P = 0·086). (b) 3-Hydroxybutyrate (3-OHB, n 5), there was a significant effect of time (P < 0·001) and sex (P < 0·05). (c) VLDL1, there was a significant effect of time (P < 0·001). (d) VLDL2, there was a significant effect of time (P < 0·001). Values are means, with standard errors represented by vertical bars.
Table 2 NEFA kinetics and 3-hydroxybutyrate (3-OHB) 13C enrichment arising from the systemic NEFA pool at 420 min
(Mean values with their standard errors, n 6)
R a, rate of appearance; R d, rate of disappearance; MPE, mole percent excess.
There was a significant time-by-sex effect: ** P < 0·005.
Mean values were significantly different from those of women: † P < 0·05, ‡ P < 0·01. Effect of sex over a period of extended fasting (14–20 h).
§ n 5.
The fatty acid tracer was rapidly incorporated into plasma 3-OHB and VLDL-TAG. Whereas enrichment reached a plateau in plasma 3-OHB at 6–7 h after the start of the infusion in women, it was still increasing at 6 h in men (Fig. 2). Plasma concentrations of [13C]3-OHB were correspondingly higher in women than in men during the course of the experiment (P = 0·007 for the effect of sex, P = 0·004 for the time × sex interaction). In women, the observed isotopic enrichment of the fatty acid tracer in 3-OHB was close to that predicted, if systemic plasma fatty acids were the sole source of 3-OHB production (Table 2). In fact, the observed MPE reached 78 % of the estimated maximal MPE, and thus only 22 % of the 3-OHB could not be accounted for by the systemic NEFA sources. These values are in close agreement with the levels reached for VLDL1- and VLDL2-TAG (Table 3) in these lean women. However, in men, the observed enrichment was less than half expected, implying either an alternative source of fatty acids or a slower rate of production. The incorporation of the [13C] tracer into VLDL1- and VLDL2-TAG was not significantly different between men and women, in terms of either the TTR (Fig. 2) or the [13C]VLDL-TAG concentration (data not shown).
Table 3 Contribution of splanchnic and systemic fatty acids to VLDL-TAG at 7 h in men and women
(Mean values with their standard errors)
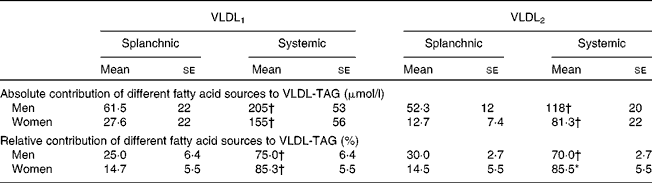
* Mean values were significantly different from those of men (P < 0·05).
† Mean values were significantly different from those of splanchnic sources (P < 0·05).
The ratio of [13C]3-OHB:[13C]VLDL-TAG in the plasma was significantly higher in women than in men (2·73 (se 1·7) v. 0·66 (se 0·30); P = 0·027 for VLDL1 and 3·24 (se 1·6) v. 0·97 (se 0·43); P = 0·014 for VLDL2).
As we have reported previously in the postprandial period for healthy people for total VLDL-TAG(Reference Hodson, Bickerton and McQuaid11), fatty acids from systemic plasma NEFA contributed a greater proportion of fatty acids to VLDL1-TAG and VLDL2-TAG than those from splanchnic sources, in men and women (Table 3). However, the proportion of systemic fatty acids was lower in men than in women for VLDL2-TAG, with a corresponding greater proportion from splanchnic sources.
Discussion
In the present study, we found significant differences in hepatic metabolism in healthy, lean young men and women. Sex comparisons of plasma 3-OHB in very young men and women have rarely been reported, but we are in agreement with previous findings of higher concentrations in young women after a 30 h fast(Reference Haymond, Karl and Clarke5), and we found that a shorter period of fasting (12–19 h) revealed higher concentrations in women. We found no difference in plasma 3-OHB concentrations between men and women without the provocation of a prolonged fast, in either a young age group (n 12) or middle-aged individuals (n 671). Since ketone bodies are only produced in the liver, systemic concentrations, usually of 3-OHB, can be taken to reflect hepatic ketone body production. Lower 3-OHB concentrations have been reported in subjects with hyperlipidaemia(Reference Havel, Kane and Balasse15, Reference Evans, Burdge and Wootton16), obesity(Reference Vice, Privette and Hickner17) and insulin resistance(Reference Hodson, Bickerton and McQuaid11). Therefore, lower 3-OHB concentrations seem to be associated with a less advantageous metabolic profile. These differences could be due to differences in production or clearance. The present findings suggest that in young lean women, a higher plasma concentration of 3-OHB, compared with men, is due to a greater production, although we did not measure production directly.
There were sex differences in the incorporation of carbon units from [U-13C]palmitate, representing systemic fatty acids, into 3-OHB. The higher 3-OHB TTR and attainment of plateau enrichment in women suggests that new 3-OHB substantially contributes to total 3-OHB. Thus, it could be hypothesised that women rapidly switch on 3-OHB production from systemic plasma NEFA, allowing plasma concentrations of 3-OHB to increase rapidly and the TTR to flatten out. Men have a slower 3-OHB switch, and at 7 h, the contribution of plasma NEFA is still increasing as a source of 3-OHB. However, it should be noted that the present findings are not necessarily representative of n-3 PUFA, which may partition away from β-oxidation in women(Reference Burdge and Wootton18).
The reason for a greater ability of women to turn on 3-OHB production is not clear. The production of ketone bodies is dependent, to a large extent, on the supply of plasma NEFA(Reference Grey, Karl and Kipnis19, Reference Johnston and Alberti20). Plasma NEFA concentrations that increased experimentally lead to increased plasma concentrations of 3-OHB(Reference Grey, Karl and Kipnis19, Reference Johnston and Alberti20). Beylot et al. (Reference Beylot, Picard and Chambrier21) found that the percentage conversion of plasma NEFA to 3-OHB increased in a linear fashion as the precursor concentration increased. This is thought to be due to reduced malonyl-CoA and reduced inhibition of carnitine palmitoyl transferase I, and could possibly account for the sex differences that we observed. A greater percentage conversion of plasma NEFA to 3-OHB may have occurred in the women of the present study, whose mean plasma NEFA concentration was almost twice that of men. Glucagon is also an important stimulator of ketone body production, but it is not clear if there is a sex difference. Glucagon levels have been reported as similar in men and women(Reference Haymond, Karl and Clarke5), but in an older study by Merimee & Fineberg(Reference Merimee and Fineberg22), plasma glucagon concentrations were significantly higher in premenopausal women than in men after a prolonged fast (72 h). Insulin reduces 3-OHB production indirectly, via a direct effect on adipose tissue lipolysis, affecting the supply of NEFA. A direct effect on ketone body production has been described in some(Reference Keller, Gerber and Stauffacher23) but not in all studies(Reference Beylot, Picard and Chambrier21, Reference Miles, Haymond and Nissen24).
Although plasma NEFA concentrations were higher in women, they had a similar delivery of fatty acids into the systemic circulation than in men as determined by R a NEFA. However, even though the women were lean, they had a significantly higher fat mass than the men, and when R a NEFA was expressed in relation to fat mass, the R a NEFA was significantly lower. This is in agreement with the recent publication of Mittendorfer et al. (Reference Mittendorfer, Magkos and Fabbrini25) who reported a down-regulation of R a NEFA per unit of fat mass in obesity. So, even within lean individuals, we were able to observe an effect of fat mass on R a NEFA. In contrast to the present findings (no difference between men and women), the study of Mittendorfer et al. found a higher R a NEFA (μmol/min) in women, and reported similar plasma NEFA concentrations. A possible explanation for the observed difference between the two studies may be because we enrolled leaner, younger volunteers.
After 12 h fasting, the men in the present study had a more disadvantageous metabolic profile in terms of higher plasma glucose and TAG concentrations but lower plasma NEFA concentrations. Since the latter are generally thought of as an adverse CVD risk factor, especially in terms of obesity and diabetes(Reference Wilding26), this was unexpected. However, we have shown that a metabolic disadvantage of a low plasma NEFA concentration is the reduced ability of the liver to switch to ketone body production during short-term fasting, and in the long term, this could possibly lead to the development of fatty liver. Plasma NEFA is also a substrate for VLDL-TAG synthesis. In the present study, more than three quarters of VLDL-TAG were delivered from systemic plasma NEFA, in men and women. However, particularly in VLDL2, the contribution of systemic plasma NEFA to VLDL-TAG was lower in men than in women. This may have been due to a lower flux from systemic fatty acids and/or a greater contribution from splanchnic sources. The latter would include de novo lipogenesis, fatty acid from the lipolysis of visceral fat or from cytosolic storage pools.
We calculated an ‘estimated’ maximum 13C enrichment in 3-OHB that would be achieved if systemic fatty acids were the sole precursor pool for 3-OHB production. This was similar for men and women. For women, the achieved value was 78 % of the value expected, implying that systemic fatty acids were the major precursor pool for 3-OHB production. This is in line with the hypothesis that cytosolic TAG fatty acids may not provide substrate for ketone body production, suggesting compartmentalisation of the precursor pool of ketone bodies(Reference Gibbons, Islam and Pease27). It also suggests that fatty acids from visceral fat did not substantially contribute to ketone body production in these lean young women. However, in men, the enrichment of 13C in 3-OHB was less than half the value expected. Consistent with this would be dilution of the precursor pool by non-systemic fatty acids, such as those from visceral fat. Alternatively, a lower 3-OHB production rate in men would mean that the maximum isotopic enrichment had not been reached during the course of the experiment. Parallel to these findings for 3-OHB isotopic enrichment, the VLDL isotopic enrichment in women was close to that of the plasma NEFA pool; thus over 85 % of VLDL were derived from this pool. This suggests that in women, the precursor fatty acids for both metabolic pathways over the duration of the present study were approximately 80 % from plasma NEFA, and implies that plasma NEFA were an immediate and major source of fatty acids for hepatic fatty acid metabolism, contributing equally to 3-OHB and VLDL-TAG synthesis during a prolonged overnight fast. However, since there were no sex differences in VLDL isotopic enrichment, the implication is that women rapidly partition a greater proportion of systemic fatty acids into ketone bodies than men. In men, it cannot be ruled out that the same sources are used for 3-OHB and VLDL-TAG, but since the 3-OHB enrichment is still rising at the end of the experiment, and the total 3-OHB pool is much lower than for women, it is clear either that the time course is considerably slower or that other sources of fatty acids are involved.
Ketone bodies may be infused intravenously to give kinetic information on ketone body production(Reference Beylot, Picard and Chambrier21, Reference Keller, Gerber and Stauffacher23, Reference Avogaro, Nosadini and Bier28), but the use of a fatty acid stable isotope tracer has not been utilised previously to investigate ketone body metabolism in humans. The disadvantage of the latter approach is that we were not able to do classic kinetic modelling, because the lack of detectable M+4 tracer labelling in 3-OHB did not allow for calculation of the precursor pool enrichment. Quantitatively, the M+4 isotopomer would not contribute significantly to the total appearance of the palmitate tracer in 3-OHB. We calculated that in women, the enrichment (MPE) of the 3-OHB M+4 isotopomer was less than 0·5 % of the M+2 isotopomer. However, the results were informative. In particular, we were able to show that in women, systemic plasma NEFA was the main source of fatty acids for 3-OHB production, and that hepatic production of 3-OHB followed different kinetics in men and women. We calculated the [13C]3-OHB:[13C]VLDL-TAG ratio in the plasma, and found that mean values were greater than 2·5 in women. This illustrates the quantitative importance of ketone body production for NEFA turnover in women, in the early fasting period. Beylot et al. (Reference Beylot, Picard and Chambrier21) calculated that for a plasma 3-OHB concentration of 464 μmol/l, 13 % of plasma NEFA are converted to 3-OHB in healthy young men. The present results would suggest an even higher rate of conversion in women.
The hepatic partitioning of fatty acids into ketone bodies or towards esterification is difficult to study in humans but may be an important metabolic regulatory point that warrants further study. We have shown that in young women, plasma fatty acids tend to be readily converted to ketone bodies after an extended overnight fast, possibly partly because of a higher systemic pool than in men. Moreover, there is evidence of preferential partitioning to ketone bodies rather than VLDL-TAG, at least in early starvation. From an evolutionary point of view, women may therefore have been better adapted to cope with periods without food. In modern times, the greater ability of women to oxidise plasma NEFA into ketone bodies may partly explain the fact that premenopausal women have a better metabolic profile than men and may help protect women against the accumulation of liver fat. Fatty acid partitioning could potentially be manipulated by lifestyle changes or pharmacological intervention.
Acknowledgements
This work was funded in part by the project ‘Hepatic and adipose tissue and functions in the metabolic syndrome’ (http://www.hepadip.org/), which is supported by the European Commission as an Integrated Project under the Sixth Framework Programme (Contract LSHM-CT-2005-018734). Funding is also gratefully acknowledged from the European Atherosclerosis Society, Novo Nordisk and the Sahlgrenska Center for Cardiovascular and Metabolic Research funded by the Foundation for Strategic Research. We thank Jane Cheeseman, Louise Dennis and Siobhán McQuaid for expert help during the studies. We confirm no conflict of interest for the work reported here. K. M. carried out the studies, and performed laboratory and data analyses. M. A. analysed the data and helped with the manuscript preparation. L. H. performed laboratory analysis, data analysis and helped with the manuscript preparation. B. A. F. developed stable isotope methods, supervised laboratory work and took the lead on the manuscript preparation. F. K. supervised the studies and helped with the manuscript preparation. K. N. F., L. H., B. A. F. and F. K. designed the study.







