The concept of insight as it applies to psychiatry is a complex phenomenon. David (Reference David1990) proposed that insight consists of three overlapping dimensions: the recognition that one has a mental illness, adherence with treatment and the ability to re-label unusual mental events (delusions and hallucinations) as pathological.
Lack of insight is a common symptom of the acute phase of schizophrenia, being described in 97% of acute cases in the World Health Organization International Pilot Study of Schizophrenia (World Health Organization, 1973). Lack of insight often responds to treatment, but it also persists in a substantial proportion of people with schizophrenia (Reference Cuesta, Peralta and ZarzuelaCuesta et al, 2000), and tends to be associated with non-adherence (Reference McEvoy, Applebaum and AppersonMcevoy et al, 1989a ; Reference Sanz, Constable and LopeziborSanz et al, 1998).
Non-adherence itself is associated with relapse, re-hospitalisation (Reference Haywood, Kravitz and GrossmanHaywood et al, 1995; Reference Fenton, Blyler and HeinssenFenton et al, 1997) and social breakdown, which result in substantial hardships and cost to patients, their families, and society as a whole. Depot antipsychotic medication is commonly prescribed in cases of poor adherence, particularly when covert non-adherence is suspected (Reference Valenstein, Copeland and OwenValenstein et al, 2001). Administration of a depot ensures either that a patient receives adequate levels of medication or that their failure to receive medication is detected early (through refusal or failure to attend for depot administration). However, long-term treatment with depot antipsychotics has disadvantages. In particular, it is associated with an increased risk of extrapyramidal side-effects, tardive dyskinesia, weight gain and depression (Reference CooksonCookson, 1991). Depot medication involves painful injections, may require attendance at depot or primary care clinics, could make patients feel less in control of their illness, and dose adjustments to reach optimal dose can be time consuming.
Over the past decade, the introduction of atypical antipsychotics has been accompanied by a reduction in the use of depot antipsychotics (Reference Patel and DavidPatel & David, 2005). A substantial number of patients have switched from depot antipsychotics to oral atypicals with apparently beneficial results. For example, an observational study by Desai et al (Reference Desai, Huq and Martin1999) reported that switching from depot antipsychotics to risperidone tablets resulted in significant improvement of positive and negative symptoms, level of functioning, parkinsonism and dyskinesia.
However, a substantial number of patients remain on depot antipsychotic medication long term. This raises the question of whether this is due to inertia, or reflects a rational clinical decision based on the benefits of reducing covert non-adherence in a subgroup of patients with poor insight. To answer this question, we tested the hypothesis that patients with schizophrenia on depot medication would have lower levels of insight than similar patients receiving oral antipsychotics. We also aimed to control for potential confounding variables such as symptom severity, side-effects and duration of illness.
Method
A cross-sectional assessment was undertaken of stable community patients with schizophrenia at two sites in the north west of England (Preston and Blackburn), both served by Lancashire Care NHS Trust. The local research ethics committee approved the study.
The inclusion criteria were that participants were aged 18–65 years, had an ICD–10 (World Health Organization, 1992) diagnosis of schizophrenia, had been stable in the community for the previous 6 months, and were currently prescribed either depot or oral antipsychotic medication. Patients who were prescribed clozapine were excluded, as they were considered to represent a subgroup that would be pre-selected for treatment resistance, as were patients who were taking both oral and depot medication. Written informed consent was obtained from all participants. A pre-study power calculation specified that 26 participants per group were required for an 80% power to show a statistically significant difference between the two groups at the 5% significance level, using a two-sided t-test.
Basic demographic, clinical and social characteristics were collected using a structured questionnaire. Insight and attitude to treatment was assessed using the Insight and Treatment Attitude Questionnaire (ITAQ) (Reference McEvoy, Apperson and ApplebaumMcevoy et al, 1989b ). The ITAQ is a semi-structured interview of 11 items that measures awareness of illness (first 5 items) and attitude to medication/hospitalisation and follow-up evaluation (6 items). Scores range from 0 (no insight) to 22 (full insight).
The Brief Psychiatric Rating Scale (BPRS; Reference Overall and GorhamOverall & Gorham, 1962) was used to assess psychopathology. The BPRS contains 16 items that measure negative symptoms (2 items), positive symptoms (5 items) and general psychopathology (9 items).
Akathisia was assessed using the Barnes Akathisia rating scale (BARS; Reference BarnesBarnes, 1989). This scale is observer rated and includes items for the objective and subjective aspects of akathisia as well as a global rating. The Extra Pyramidal Side Effects Scale (ESES; Reference McEvoy, Hogart and SteingardMcevoy et al, 1991) was used to assess bradykinesia, rigidity and tremor. Tardive dyskinesia was assessed using the Abnormal Involuntary Movements Scale (AIMS; Reference Guy and BanGuy & Ban, 1979).
The dosage of each antipsychotic was converted to its chlorpromazine equivalents (Reference Lehman and SteinwachsLehman & Steinwachs, 1998; Reference WoodsWoods, 2003).
The Statistical Package for Social Science (SPSS) for Windows, Version 10 was used for the analysis. Univariate tests were used to assess differences between the depot antipsychotic group and the oral antipsychotic group. For all tests, the level of significance (P) was set at the conventional level of 0.05 (two-sided).
Multiple regression was used to analyse the relative contribution of symptom severity (BPRS score), side-effects (BARS, ESES and AIMS scores), duration of illness and group membership (depot or oral) to insight (ITAQ scores).
Results
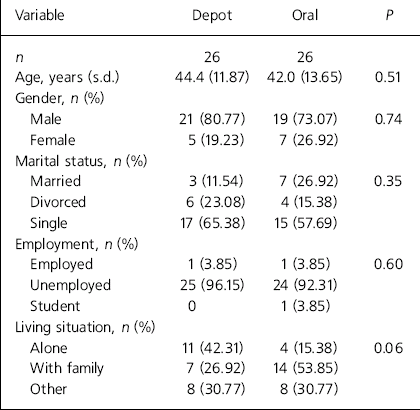
We recruited 52 participants, of which 26 were receiving depot medication and 26 were on oral medication. The demographic characteristics of the participants are summarised in Table 1. The mean age of participants was 44.4 years (s.d.=11.9) in the depot group and 42.0 years (s.d.=13.7) in the oral group. In both groups the majority of the participants were male (depot 80.7%; oral 73.1%) and single (depot 65.4%; oral 57.7%). More participants from the depot group were living alone (depot 42.3%; oral 15.4%) and the majority of participants were unemployed (depot 95.2%; oral 92.3%). No differences reached statistical significance.
Table 1. Characteristics of the depot and oral antipsychotic groups
| Variable | Depot | Oral | P |
|---|---|---|---|
| n | 26 | 26 | |
| Age, years (s.d.) | 44.4 (11.87) | 42.0 (13.65) | 0.51 |
| Gender, n (%) | |||
| Male | 21 (80.77) | 19 (73.07) | 0.74 |
| Female | 5 (19.23) | 7 (26.92) | |
| Marital status, n (%) | |||
| Married | 3 (11.54) | 7 (26.92) | 0.35 |
| Divorced | 6 (23.08) | 4 (15.38) | |
| Single | 17 (65.38) | 15 (57.69) | |
| Employment, n (%) | |||
| Employed | 1 (3.85) | 1 (3.85) | 0.60 |
| Unemployed | 25 (96.15) | 24 (92.31) | |
| Student | 0 | 1 (3.85) | |
| Living situation, n (%) | |||
| Alone | 11 (42.31) | 4 (15.38) | 0.06 |
| With family | 7 (26.92) | 14 (53.85) | |
| Other | 8 (30.77) | 8 (30.77) |
In the oral antipsychotic group all the participants were on atypical antipsychotics. In the depot group 23 participants were on typical depot medication and 3 were on risperidone long-acting injection. The mean chlorpromazine equivalent dose of antipsychotic was much higher in the depot group than the oral antipsychotic group (1388.7, s.d.=1870.7 v. 391.0, s.d.=205.5; z=–3.16, P=0.002).
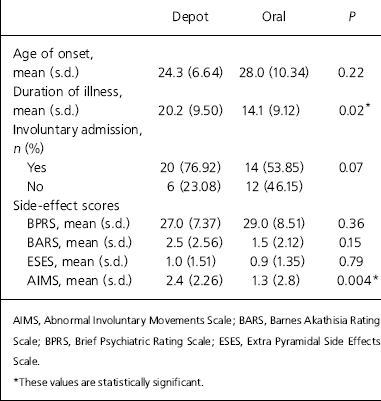
Illness variables for the two groups are summarised in Table 2. The mean age at onset of psychotic symptoms for the whole sample was 26.1 years (s.d.=8.8) and the mean duration of illness was 17.2 years (s.d.=9.7). The mean duration of illness was significantly longer for the depot group than the oral group (20.2, s.d.=9.5 v. 14.1, s.d.=9.1; P=0.02, 95% CI 0.9 to 11.3). More patients from the depot group had a history of compulsory admission under the Mental Health Act (76.9% v. 53.9%, P=0.07) compared with the oral group.
Table 2. Illness variables and side-effects scores between the depot (n=26) and oral (n=26) antipsychotic groups
| Depot | Oral | P | |
|---|---|---|---|
| Age of onset, mean (s.d.) | 24.3 (6.64) | 28.0 (10.34) | 0.22 |
| Duration of illness, mean (s.d.) | 20.2 (9.50) | 14.1 (9.12) | 0.02* |
| Involuntary admission, n (%) | |||
| Yes | 20 (76.92) | 14 (53.85) | 0.07 |
| No | 6 (23.08) | 12 (46.15) | |
| Side-effect scores | |||
| BPRS, mean (s.d.) | 27.0 (7.37) | 29.0 (8.51) | 0.36 |
| BARS, mean (s.d.) | 2.5 (2.56) | 1.5 (2.12) | 0.15 |
| ESES, mean (s.d.) | 1.0 (1.51) | 0.9 (1.35) | 0.79 |
| AIMS, mean (s.d.) | 2.4 (2.26) | 1.3 (2.8) | 0.004* |
The mean BPRS score of the total sample was 28.0 (s.d.=7.9). The depot group had a slightly better mean BPRS score than the oral group but this difference was not statistically significant (27.0, s.d.=7.4 v. 29.0, s.d.=8.5; P=0.36, 95% CI –6.5 to 2.4).
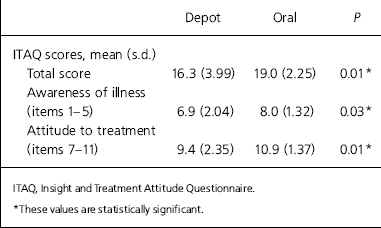
Overall the level of insight was fairly high, with the total sample having a mean ITAQ score of 17.6 (range 10–22, s.d.=3.5). However, the patients on oral medication had a statistically significantly higher mean score than those on depot (16.3, s.d.=4.0 v. 19.0, s.d.=2.3; z=–2.4, P=0.01). A sub-score analysis of the ITAQ showed that the oral medication group scored significantly higher than the depot group on both awareness of illness and attitude to treatment (Table 3).
Table 3. ITAQ scores in the depot (n=26) and oral (n=26) antipsychotic group
| Depot | Oral | P | |
|---|---|---|---|
| ITAQ scores, mean (s.d.) | |||
| Total score | 16.3 (3.99) | 19.0 (2.25) | 0.01* |
| Awareness of illness | |||
| (items 1-5) | 6.9 (2.04) | 8.0 (1.32) | 0.03* |
| Attitude to treatment | |||
| (items 7-11) | 9.4 (2.35) | 10.9 (1.37) | 0.01* |
Overall the sample had relatively low levels of side-effects (Table 2). There was no significant difference between the two groups other than for the tardive dyskinesia (AIMS score).
Total scores for BPRS, AIMS, ESES and BARS, duration of illness and group were entered as independent variables in a stepwise multiple regression analysis, with ITAQ score (as the dependent variable). Only group (depot or oral) contributed significantly to the regression model (adjusted R 2=0.135, F=8.99, P=0.004).
Discussion
The participants in this study had relatively high levels of insight into their illness. The study sample consisted of stable out-patients. This finding is consistent with previous reports of higher insight among out-patients with schizophrenia (Reference Garavan, Browne and GervinGaravan et al, 1998; Reference Williams and CollinsWilliams & Collins, 2002).
The findings of the study confirmed our hypothesis that participants receiving depot antipsychotics would have significantly less insight than those receiving oral antipsychotics. This result was supported by our additional finding that participants on depots were also more likely to have been admitted to hospital under the Mental Health Act, suggesting that they were less adherent with medication than the group receiving oral antipsychotics. This suggests that psychiatrists were taking adherence and/or insight into account when deciding to start and maintain patients on depot medication.
It can be hypothesised that the majority of the participants on oral antipsychotics were started and maintained on oral antipsychotics because of their better insight and adherence. However, because of the cross-sectional nature of the study design, it is not possible to ascertain the insight of the participants when they were started on their medication.
The finding of poorer insight among participants taking depot medication is open to an alternative explanation that the depot medication was less effective than the oral medication at improving insight. However, against this explanation is the fact that the two groups had similar levels of symptomatology. This finding, together with the similar level of side-effects between the two groups, reflects the broader evidence base that depots are as effective and safe as oral antipsychotic medication for the treatment of schizophrenia (Reference Adams, Fenton and QuaraishiAdams et al, 2001).
Insight was shown to be a clinical modulator of long- and short-term adherence with treatment and is a good indicator of prognosis (Reference BuchananBuchanan, 1992). The clinical relevance of the findings from this study is that appropriate interventions should be offered to patients on depot medication to improve their insight. This will have a beneficial effect on adherence and long-term prognosis.
We found that the participants on depot antipsychotics were on significantly higher doses of antipsychotic medication compared with those on atypical medication, to the ratio of 1:35. The participants on depot medication were on a mean chlorpromazine equivalent dose of 1388.7 mg; well above the recommended range of 300–1000 mg chlorpromazine equivalent. Clinicians should review the patients on depot antipsychotics at regular intervals and review the dose of their depot medication.
It is possible that the findings of the study are only locally applicable to the sites in east Lancashire, as the study is relatively small and restricted to patients from this area. None the less, as far as we are aware, this study provides the best evidence so far that the decision to put patients on depot rather than oral medication is being made on a rational basis. Future studies should address this question in larger cohorts of patients, where changes in adherence are observed over time from the inception of the antipsychotic medication.
Declaration of interest
None.
Acknowledgements
We thank Dr Saleem and Maureen Eka Harrison (Queens Park Hospital, Blackburn) for their help in recruiting participants. These findings were presented as a poster at the Royal College of Psychiatrists Annual General Meeting of the Royal College of Psychiatrists, Edinburgh, June 2005.






eLetters
No eLetters have been published for this article.