Prader–Willi syndrome (PWS) is a rare genetic disorder, with a UK population prevalence of ~1 in 52 000.Reference Whittington, Holland, Webb, Butler, Clarke and Boer1 PWS is caused by a lack of a paternal contribution to the critical chromosome region at 15q11-13, because of deletion on the paternally inherited chromosome (del PWS, 70%) or maternal uniparental disomy (mUPD PWS, 25%). Imprinted genes are monoallelically expressed in a parent-of-origin dependent manner. PWS is associated with a distinct physical phenotype including short stature, small hands and feet, hypogonadism and a characteristic facial appearance; and a behavioural phenotype characterised by increased appetite, mood swings, stubbornness, temper tantrums, aggression and repetitive speech.Reference Soni, Whittington, Holland, Webb, Maina and Boer2 PWS is also associated with high rates of psychopathology, including affective disorders. Those with mUPD are considered at greater risk of developing an ‘affective psychosis’ compared with del PWS. The neurological pathophysiology underlying this phenomenon is far from clear.Reference Boer, Holland, Whittington, Butler, Webb and Clarke3, Reference Vogels, De Hert, Descheemaeker, Govers, Devriendt and Legius4
The serotonergic system is a prime candidate for explaining the high rates of affective disorders, and particularly affective psychosis in mUPD PWS. It is well established that serotonin plays a crucial role in brain development and emotional regulation and has been implicated in the development of affective disorders such as major depressive disorder (MDD).Reference Massart, Mongeau and Lanfumey5 Through examining the role of imprinted genes in brain serotonin neurochemistry it may be possible to shed light on the neurobiological basis of higher rates of affective psychosis in those with mUPD PWS. Previous research has shown greater serotonergic turnover, including increased monoamine-oxidase activity in platelets and increased 5-hydroxyindolacetic acid (breakdown product of serotonin) in the cerebrospinal fluid of patients with PWS.Reference Akefeldt, Ekman, Gillberg and Månsson6, Reference Akefeldt and Månsson7 The aim of this study was to explore if serotonin transporter (5-HTT) availability differed significantly between the PWS variants. In keeping with the extant literature on serotonin turnover in PWS and 5-HTT availability in MDD, we hypothesised that people with mUPD PWS will have greater depressive symptoms and lower 5-HTT availability than those with del PWS.Reference Kambeitz and Howes8
Method
This was a UK-wide, cross-sectional study, comparing 5-HTT availability in eight adults with mUPD PWS and ten with del PWS, approved by the West of Scotland Research Ethics Committee and the Administration of Radioactive Substances Advisory Committee. None of the participants were being prescribed medications that interact with 5-HTT uptake. Depressive symptomatology was assessed using the Glasgow Depression Scale for people with Learning Disability (GDS-LD).Reference Cuthill, Espie and Cooper9 Past psychotic episodes were recorded. All participants underwent [123I]-beta-CIT single photon emission tomography (SPECT) imaging using a previously validated protocol.Reference Krishnadas, Nicol, Sassarini, Puri, Burden and Leman10
Brain SPECT imaging was performed with a dedicated, 12-headed Neurofocus 900 scanner (spatial resolution 7 mm full-width at half maximum), which acquires sequential single transaxial brain sections. Participants pretreated with 120 mg of potassium iodide were scanned 3–4 h after intravenous administration of [123I]-beta-CIT in order to establish uptake in 5-HTT rich areas.Reference Manning and Holland11 Region-of-interest (ROI) analysis was carried out by an investigator (A.N.) masked to the participant's clinical and demographic history.Reference Krishnadas, Nicol, Sassarini, Puri, Burden and Leman10 The ROI template consisted of manually drawn regions representing the brain-stem (specific binding) and a reference ROI consisting of the occipital lobe (non-specific/non-displaceable binding) bilaterally. The 5-HTT BPnd (ratio at equilibrium of specifically bound radioligand to that of non-displaceable radioligand in tissue) was compared between the two groups using a general linear model, with brain-stem ROI [123I]-beta-CIT uptake as dependent variable, and group (mUPD PWS v. del PWS) as the categorical predictor variable, and occipital [123I]-beta-CIT uptake, age and gender as covariates.
Results
We scanned eight participants with mUPD PWS and ten participants with del PWS. The SPECT scan from one of the participants with del PWS did not pass quality control and was discarded. Demographic and clinical details are shown in Table 1. The mUPD group had greater GDS-LD scores (non-significant) and had more past episodes of psychosis (non-significant) compared with the del group. On the general linear model, PWS variant was a significant predictor of 5HT BPnd (F(1,12) = 8.15; P = 0.014). The mUPD group had significantly lower 5HT BPnd compared with the del group (mean difference = −0.93, t = −2.85, P = 0.014, d = 2.35, 95% CI 1.12–3.59) (see supplementary Fig. 1, available at https://doi.org/10.1192/bjp.2017.7).
Table 1 Demographic and clinical variables in the two patient groups with Prader–Willi syndrome (PWS)
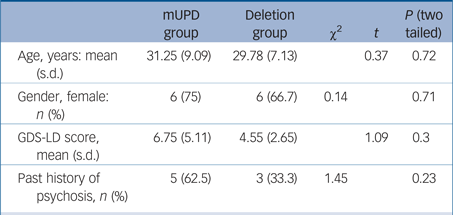
mUPD, maternal uniparental disomy PWS; GDS-LD, Glasgow Depression Scale for people with Learning Disability.
Discussion
This is the first study to explore brain-stem 5-HTT availability in PWS.Reference Manning and Holland11 Our findings reveal an association between PWS genotype and brain-stem 5-HTT availability. The mUPD group had lower brain-stem 5-HTT availability compared with the del group. MDD has previously been associated with low 5-HTT availability as a result of the compensatory downregulation of 5-HTT secondary to low synaptic serotonin concentrations.Reference Kambeitz and Howes8 In keeping with this, we postulate that those with mUPD have a lower synaptic serotonin concentration compared with those with del PWS, and that this may indeed account for the greater prevalence of affective psychotic illness in this population.Reference Kambeitz and Howes8 Greater serotonin turnover (greater plasma monoamine oxidase B and cerebrospinal fluid 5-hydroxyindolacetic acid) has been previously demonstrated in patients with PWS, compared with healthy controls.Reference Akefeldt, Ekman, Gillberg and Månsson6, Reference Akefeldt and Månsson7 In addition, treatment with selective serotonin reuptake inhibitors have been found to be effective in tackling affective and behavioural problems in individuals with PWS. Together with the above findings, our results, suggest that those with mUPD PWS have lower synaptic serotonin concentrations compared with del PWS – a finding that has not been previously demonstrated. Although Akefeldt et al Reference Akefeldt, Ekman, Gillberg and Månsson6 found evidence for lower synaptic serotonin in PWS as a group, compared with healthy controls, they did not test if this finding was driven primarily by the mUPD variants within the group. The lack of a control group in our study prevented us from exploring if 5-HTT availability was indeed lower in the del PWS group compared with healthy controls. Future studies will be required to explore the relationship between serotonin availability and turnover in PWS variants in relation to a healthy population.
Children with mUPD PWS have been found to have smaller cortical and subcortical grey matter volumes compared with neurotypical children.Reference Lukoshe, Hokken-Koelega, van der Lugt and White12 In this context, lower 5-HTT availability may also be the result of a smaller volume of serotonergic neurons in the brain-stem. However, Honea et al found no difference in brain-stem volumes between the two genotypes, suggesting that the lower 5-HTT availability is unlikely to be the result of smaller volume of brain-stem serotonergic neurons.Reference Honea, Holsen, Lepping, Perea, Butler and Brooks13 Nevertheless, serotonin influences neurogenesis, cell migration, synaptic plasticity, dendritic growth and normal spine formation and our findings could therefore be the result of a combination of the above.Reference Sodhi and Sanders-Bush14
Our study has strengths and limitations. It was a UK-wide study with support from the UK Prader–Willi Association. Despite the small sample sizes, at the end of study recruitment, the pool of eligible adults with mUPD PWS who fulfilled the inclusion criteria was exhausted. The 12-detector dedicated head SPECT unit ensured high spatial resolution; and, although more selective ligands for 5-HTT are available, the uptake of [123I]-beta-CIT in the brain-stem at 4 h is a validated measure of 5-HTT availability. Lack of a neurotypical comparison group prevented us from comparing our findings with the healthy population.
In summary, we have shown that a group of adults with mUPD PWS have lower 5-HTT availability compared with the del PWS group. Our findings provide preliminary evidence for the pathophysiology underlying greater affective psychopathology associated with the mUPD group of patients, and the potential reasons why drugs such as selective serotonin reuptake inhibitors are effective in treating this cohort of patients.
Acknowledgements
We thank the Prader-Willi Association, the participants and their carers.
Funding
The Baily Thomas Charitable Trust funded the study.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2017.7




eLetters
No eLetters have been published for this article.