The capacity of rainbow trout (Oncorhynchus mykiss) to use dietary carbohydrates is controversial(Reference Wilson1–Reference Stone3), although several authors have claimed that diets with a high content of digestible starch (20–30 %) can stimulate growth(Reference Kim and Kaushik4, Reference Capilla, Médale and Navarro5). However, while a protein-sparing effect from lipids has been reported in this species(Reference Beamish and Medland6–Reference Rasmussen, Ostenfeld and McLean8), there is no clear evidence that carbohydrates also have a protein-sparing effect. The replacement of fishmeal by plant ingredients is a common practice in aquaculture, even in carnivorous fish. Since plant sources contain large amounts of carbohydrates, the use of this alternative energy source is of interest. The physical state of the animal, the molecular complexity and the amount of starch in the diet influence carbohydrate digestibility and tolerance, and also the efficiency of fish growth. If the protein content of the diet is adequate, low levels of gelatinised starches promote growth in carnivorous fish, such as European eel (Anguilla anguilla)(Reference Degani, Viola and Levanon9), cod (Gadus morhua)(Reference Hemre, Lie and Lied10), sturgeon (Acipenser transmontanus)(Reference Hung, Fynn-Aikins and Lutes11), Atlantic salmon (Salmo salar)(Reference Hemre, Sandnes and Lie12, Reference Hemre and Hansen13) and turbot (Scophthalmus maximus)(Reference Stephan, Dreanno and Guillaume14). As we have shown in brown trout, uptake and use of glucose by tissues depends on plasma glucose concentration(Reference Blasco, Fernández and Marimón15) and after an aortic glucose overload, almost all tissues increase glucose uptake. This is particularly true for skeletal muscle, which is the main target of the glucose load(Reference Blasco, Marimón and Viaplana16). This effect may also be exerted by high levels of dietary carbohydrates, although, to our knowledge, this has not been measured experimentally.
Fish generally swim aerobically at submaximal velocities(Reference Weihs17–Reference Quinn19). Several fish species, when made to swim at about 1·3 body lengths/s (BL/s), show improved growth rate and food conversion efficiency(Reference Davison20) through the increase of aerobic potential of red muscle (RM) and white muscle (WM). There is no general agreement on how metabolic fuels support aerobic swimming in fish(Reference Lauff and Wood21). Protein and lipids were traditionally believed to be the main source of energy during sustained swimming in teleost fish, and carbohydrate utilisation was considered to be minimal(Reference Driedzic, Hochachka, Hoar and Randall22–Reference Moyes, West, Hochachka and Mommsen26). However, Alsop & Wood(Reference Alsop and Wood27) found that in satiation-fed rainbow trout, protein did not become more important as a fuel source during exercise. Further, Lauff & Wood(Reference Lauff and Wood21), using respirometric analyses, demonstrated that the most oxidised substrates during moderate swimming (55–85 % U crit; critical swimming speed) were lipids, followed by carbohydrates, and then protein. Kieffer et al. (Reference Kieffer, Alsop and Wood28) also found in rainbow trout that during swimming at 1 or 3 BL/s, protein use decreased to 15 % while the relative contribution of both lipid and carbohydrates increased. On the other hand, Shanghavi & Weber(Reference Shanghavi and Weber29) noted that sustained swimming for a period of 3 h causes a 33 % decline in hepatic glucose production, but plasma glucose levels are maintained stable by closely matching peripheral glucose utilisation. However, glucose disposal can also be conditioned by the source or type of diet, carbohydrate content, feeding regimen, gelatinisation process, etc. (reviewed by Hemre et al. (Reference Hemre, Mommsen and Krogdahl2)). Traditionally, studies on fish metabolism have used radioactive isotopes, but labelling feed ingredients with radioactive markers can be harmful to users and the aquatic environment. Stable isotopes are now used to study protein metabolism in fish. Thus, 15N has been administered orally to measure protein synthesis in species such as rainbow trout (O. mykiss)(Reference Carter, Owen and He30), flounder (Pleuronectes flesus)(Reference Carter, Houlihan and Owen31) or carp (Cyprinus carpio)(Reference Meyer-Burgdorff and Rosenow32). In the present study, we used stable isotopes as dietary tracers ([13C]starch and [15N]protein), as a preferred method for tracing dietary nutrient allocation in fish(Reference Beltrán, Fernández-Borrás and Médale33), with the following two aims: (1) to measure the effects of gelatinisation of starch on [13C]glucose uptake and use by tissues in rainbow trout fed a carbohydrate-rich diet; (2) to determine the effects of sustained swimming on the efficiency of carbohydrate use in this species. To our knowledge, there are no studies on routing both carbohydrates and protein from [13C]starch and [15N]protein added to the diet. This alternative method has allowed us to show improved assimilation and distribution of dietary carbohydrates, and their protein-sparing effect in rainbow trout under sustained swimming.
Experimental methods
Experimental design and sampling
Expt 1: effects of raw and gelatinised starch on glucose uptake by tissues of rainbow trout fed with carbohydrate-rich diets
Rainbow trout from a local fish farm (Truchas del Segre, Lleida, Spain) were held in the facilities of the Faculty of Biology (University of Barcelona, Barcelona, Spain) in 1000 litre tanks with fresh water within a semi-closed system (10 % of water renovation daily) with physical and biological filters, ozone skimmers and continuous aeration at 15°C and a 12 h light–12 h dark photoperiod. Fish with an average body weight of 180 g were randomly distributed into two experimental groups (twenty-five fish/tank), which were fed with two experimental diets with a high level of raw starch (RS) or gelatinised starch (GS) (see diet compositions given in Table 1). After 1 month, fourteen fish from each group were lightly anaesthetised and then force-fed a bolus, equivalent to 1 % of body weight, with a gastric cannula. Fish were held in separated tanks for only a few minutes to check the acceptance of the forced meal. Any fish showing some degree of regurgitation was disqualified, and another one was used in its place. Fish were returned to their respective tanks and maintained for 11 or 24 h post-feeding. These two periods were chosen as they represent the post-absorption maximum (11 h) and nutrient use completion (24 h) time points. Diets were labelled with 3 % [13C]starch ([13C]algal starch; Martek Biosciences Corporation, Columbia, MD, USA). Another four animals from each group received the same dietary ration containing non-labelled starch, and they were used to measure the background level of 13C, to establish the natural abundance (i.e. blank value of each sample). At 11 h after the oral administration of the diets, half of the fish (seven fish fed the diets labelled with stable isotope plus two fish as blanks) were anaesthetised, killed by sectioning the spinal cord and sampled. Blood samples from the caudal vessel were centrifuged (12 000 g, 5 min at 4°C) to obtain plasma. Portions of the liver, and WM and RM and viscera (gut plus perivisceral fat) were excised, frozen in liquid N2 and stored at − 80°C until analysis, as were the rest of the fish and plasma samples. The entire sampling procedure took less than 3 min from the death of the fish, and the tissues with high glycogen hydrolytic capacity, such as muscle, were frozen first. After 24 h of forced-feeding, the remaining animals were sampled using the same protocol.
Table 1 Ingredients and chemical composition of the experimental diets
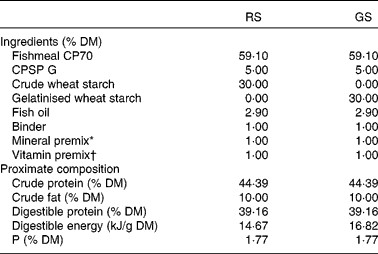
RS, raw starch; GS, gelatinised starch; CPSP G, fish soluble concentrate protein with high-fat level.
* Supplied the following (mg/kg diet, except as noted): calcium carbonate (40 % Ca) 2·15 g, magnesium hydroxide (60 % Mg) 1·24 g, potassium chloride 0·9 g, ferric citrate 0·2 g, potassium iodide 4 mg, NaCl 0·4 g, calcium hydrogen phosphate 50 g, copper sulphate 0·3, zinc sulphate 40, cobalt sulphate 2, manganese sulphate 30, sodium selenite 0·3.
† Supplied the following (mg/kg diet): retinyl acetate 2·58, dl-cholecalciferol 0·037, dl-α-tocopheryl acetate 30, menadione sodium bisulphite 2·5, thiamin 7·5, riboflavin 15, pyridoxine 7·5, nicotinic acid 87·5, folic acid 2·5, calcium pantothenate 2·5, vitamin B12 0·025, ascorbic acid 250, inositol 500, biotin 1·25, choline chloride 500.
Expt 2: effects of sustained swimming on the use of carbohydrates by rainbow trout
Juvenile rainbow trout (with an average weight of 60 g) from the same fish farm were acclimatised indoors as in the previous experiment. For individual monitoring, sixty fish were identified with a passive integrated transponder (PIT) tag (Trovan Electronic Identification Systems, Madrid, Spain) near the dorsal fin, and were randomly distributed into four 200 litre circular tanks (fifteen fish/tank) at a density of 4 kg/m3. Of these four tanks, two were kept on standard rearing conditions, with a water flow of 350 litres/h and vertical water inflow. Fish in these conditions presented only spontaneous movements (voluntary swimming) and were used as the control group. The other two tanks (exercise group) were kept in a circular, uniformly distributed flow of 700 litres/h, induced by the perpendicular water entrance at the surface and a submerged water pump at the bottom of the tank, isolated from the free-living area. The shape of the tank prevented the fish from entering a central area of lower velocity, thus guaranteeing similar swimming velocities throughout the experiment. Consequently, water volume and fish density were the same as in the control group. This design and water flow resulted in a swimming velocity of 1·3 BL/s, measured and adjusted at three different tank depths (surface, mid-tank and near the bottom) using a low-speed mechanical flow meter (General Oceanics, Inc., Miami, FL, USA). All fish were kept in the same semi-closed circuit, guaranteeing that physico-chemical water parameters were the same for both groups, and they were fed twice a day to apparent satiety with the diet rich in digestible carbohydrates (GS) for 1 month (see diet composition given in Table 1). Feed intake was recorded daily for each tank and the specific growth rate (100 × (ln final weight − ln initial weight)/d) and food conversion ratio (feed intake:wet body-weight gain) were also calculated for each tank at the end of the experimental period. After 1 month, eighteen fish from the exercise group and twelve from the control group were lightly anaesthetised and force-fed with a gastric cannula a ration of 1 % of diet labelled with 1 % [15N]Spirulina protein and 3 % [13C]algal starch. From each group, two other fish received the same dietary ration containing similar proportions of non-labelled Spirulina protein and algal starch. These four fish were used to measure natural abundances of 15N and 13C in samples (blank values). After force-feeding, fish were held for a few minutes in separate tanks as indicated for the first experiment. Then, fish were returned to their respective tanks and maintained for 11 or 24 h post-feeding (exercise group swam until the moment they were sampled). At 11 h after feeding, nine fish from the sustained swimming group and six from the control group were anaesthetised and killed by sectioning the spinal cord. Samples of blood were extracted from caudal vessels, and then, samples of liver, WM and RM, viscera (gut plus perivisceral fat) and the rest of the fish were rapidly excised, frozen in liquid N2 and stored at − 80°C until analysis. As in the first experiment, the entire sampling procedure took less than 3 min from the death of the fish, and the tissues with high glycogen hydrolytic capacity, such as muscle, were frozen first. The same procedure was repeated at 24 h post-feeding with the other nine and six fish from the sustained swimming and control groups, respectively. Although the initial body weight of rainbow trout differed between the two experiments, all fish can be considered as juvenile fish in a linear phase of growth. For the exercise trial, we used fish of 60 g constrained by the size and number of the tanks available for implementing sustained swimming.
Before conducting the animal trials, prior approval of the Comitè Ètic d'Experimentació Animal de la Universitat de Barcelona (CEEA-UB, Ethics Committee) was obtained. The specific ethics approval number for the protocol was CEEA-96/09.
Plasma analysis and proximal composition of tissue samples
Plasma was used to determine glucose concentration (Commercial Kit Glucofix, Menarini, Italy) based on the enzymatic method of glucose oxidase described by Werner et al. (Reference Werner, Rey and Wielinger34). Tissue samples (liver, muscles and viscera) were homogenised in liquid N2 using a pestle and mortar to obtain a fine powder. The rest of the fish was homogenised at − 20°C using a food homogeniser (Pacojet AG, Zug, Switzerland). Samples were apportioned for the various analyses: percentage of lipids, proteins, glycogen and water determination, and one part of the sample was used for isotopic analysis. Tissue water content was determined gravimetrically after drying the samples at 100°C for more than 24 h. Lipids were extracted as described by Folch et al. (Reference Folch, Lees and Sloane Stanley35). The washed lipid extracts were dried under a N2 atmosphere and the lipid content was determined gravimetrically. Protein was purified from defatted samples via precipitation with 10 % (v/v) trifluoroacetic acid. Protein extracts were dried by a vacuum system (Speed Vac Plus, AR, Savant Speed Vac Systems, South San Francisco, CA, USA) and protein content was calculated from N obtained by elemental analysis (Elemental Analyser Flash 1112, ThermoFinnigan, Bremen, Germany), assuming that N content is 1 g for every 6·25 g of protein. Glycogen was extracted and purified by alcoholic precipitation after alkaline tissue hydrolysis with 30 % KOH in heat(Reference Good, Cramer and Somogy36). Glycogen content was analysed using the anthrone colorimetric method described by Fraga(Reference Fraga37).
δ15N and δ13C determination in tissues and expression of results
The enrichments in 13C were determined in both experiments, and the enrichments in 15N were measured in the second experiment. Dried samples of diets and tissues, as well as the purified lipid, glycogen and protein fractions of each tissue, were lyophilised and ground in a mortar to a homogeneous powder for isotope-ratio mass spectrometry analysis. Aliquots ranging from 0·3 to 0·6 mg were accurately weighed in small tin capsules (3·3 × 5 mm; Cromlab, Barcelona, Spain). Samples were analysed for C and N isotope composition using a Mat Delta C isotope-ratio mass spectrometry (Finnigan MAT, Bremen, Germany) coupled to an elemental analyser (Flash 1112) at Barcelona University ‘Serveis Cientifico-Tècnics’. Isotope ratios (15N/14N, 13C/12C) are expressed on a relative scale as deviation, referred as δ units with the notation ‰, parts per thousand, relative to the isotope ratio content of international standards: Pee Dee Belemnite (a calcium carbonate) for C and air for N.
δ values were determined as follows:
where R sa = 15N/14N or 13C/12C of samples and R st = 15N/14N or 13C/12C of standards. The same reference material analysed over the analysis period was measured with about 0·2 ‰ precision for natural materials and about 0·4 ‰ precision for enriched materials. The δ values are expressed as atom percentage (at %) as follows:
The net enrichments (atom percentage excess) in 13C and 15N of glycogen, lipid, protein and whole tissue were calculated by the difference between the atom percentage of samples and their corresponding blank values:
Finally, using the values of atom percentage excess, molecular weight and Avogadro's number, the results are expressed as the percentage of marker in relation to the ingested dose (g/100 g 13C or 15N ingested) in each tissue fraction (glycogen, lipid and protein), which was calculated as follows:
where t. fr. is the tissue fraction and b.w. is the body weight. The free pool of each tissue was calculated as the difference between isotope levels in the entire organ or tissue and the sum of the three tissue fractions. So, the measure in an entire organ, or tissue, represents the sum of all fractions (Eq. (1)+free pool) for 13C or 15N. In whole fish, it was calculated as the sum of all tissues (liver, WM and RM, viscera and the rest of the fish) for 13C or 15N.
For liver and viscera, the exact mass of the total tissue sample was measured by weighing the entire individual organs from the experimental fish. However, in order to estimate the total mass of WM and RM, we made accurate dissections of another ten fish under the same conditions as indicated earlier. The muscle-somatic index (g muscle/100 g body weight) obtained presented the values of 40 % for WM and 4 % for RM.
Statistics
Results are presented as means with their standard errors. Unpaired t tests were used to compare the two experimental groups at 11 and 24 h, respectively, and in the two periods of the same condition. All statistical analyses were performed using SPSS version 14 (SPSS, Inc., Chicago, IL, USA).
Results
Expt 1: effects of raw and gelatinised starch on glucose uptake by tissues of rainbow trout fed with carbohydrate-rich diets
Plasma glucose levels and tissue proximal composition
The two forms of starch, RS and GS, in the diets are digested and absorbed at different rates, as reflected in the postprandial plasma glucose levels shown in Fig. 1. At 11 h after the meal, rainbow trout in the GS group presented plasma glucose levels 3-fold higher than the RS group (17 and 5·6 mm, respectively). Although at 24 h after a meal, plasma glucose levels in the GS group decreased by 30 %, the hyperglycaemic situation was maintained (10·3 mm; P < 0·05). Higher amounts of assimilated carbohydrates also entailed 5-fold higher liver glycogen content in the GS group than in the RS group (GS, 10·28 (sem 1·58) % wet weight; RS, 2·02 (sem 0·29) % wet weight; P < 0·05), causing hypertrophy of the organ (hepatosomatic index: GS, 1·82 (sem 0·09) % wet weight; RS, 1·29 (sem 0·03) % wet weight; P < 0·05) and a concomitant reduction of the other tissue components (lipid: GS, 2·7 (sem 0·2) % wet weight; RS, 4·3 (sem 0·12) % wet weight; P < 0·05; protein: GS, 11·2 (sem 0·3) % wet weight; RS, 16·2 (sem 0·4) % wet weight; P < 0·05).

Fig. 1 Plasma glucose concentration (mm) in rainbow trout fed with the raw starch (RS) and gelatinised starch (GS) diets, 11 (■) and 24 h (□) after force-feeding. Values are means, with their standard errors represented by vertical bars (n 9). ** Mean values were significantly different between the RS and GS groups (P < 0·01). †† Mean values were significantly different between 11 and 24 h (P < 0·01).
δ13C taken up by tissues
The total recovery of 13C from whole fish and entire organs is shown in Fig. 2. Changes in this variable paralleled those observed in plasma glucose. Thus, while there were no differences in the RS group in the 13C recovered between 11 and 24 h (18 and 22 %, respectively), higher levels were recovered in the GS group at 11 h (28 %), with a marked decrease at 24 h (18 %). The uptake of 13C in RM and WM increased from 11 to 24 h post-feeding in both groups. Thus, in the RS group, 13C increased by 60 % in RM and 78 % in WM, and in the GS group, it increased by 175 % in RM and 100 % in WM. Taking into account the total muscle mass of rainbow trout, more than 40 % of body weight, the 13C taken up by muscles was the main allocation site of the dietary [13C]starch (43 % of the 13C ingested was recovered in the RS group and 41 % in the GS group).

Fig. 2 Recovery of 13C (as a percentage of ingested isotope) from entire organs or tissues (WM (![]() ), white muscle; RM (■), red muscle; L (
), white muscle; RM (■), red muscle; L (![]() ), liver; V (▧), viscera; R (□), the rest of the fish) of rainbow trout fed with the raw starch (RS) and gelatinised starch (GS) diets, 11 and 24 h after force-feeding. The sum of the stacked bar represents the total recovery from whole fish (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 7). * Mean values were significantly different between the RS and GS groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
), liver; V (▧), viscera; R (□), the rest of the fish) of rainbow trout fed with the raw starch (RS) and gelatinised starch (GS) diets, 11 and 24 h after force-feeding. The sum of the stacked bar represents the total recovery from whole fish (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 7). * Mean values were significantly different between the RS and GS groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
The comparison of the values of 13C recovered in each tissue fraction (protein, lipid and glycogen) in liver and WM is shown in Fig. 3. There were clear differences between the two diets in the fate of nutrients. The highest amount of 13C in the liver of the RS group was found in protein (36 % of total) and in glycogen (32 %), with only 8 % in lipids. In the GS group, the highest labelled fraction in liver was lipids (27 % of total), then glycogen (19 %) and protein (12 %) (Fig. 3(a)). In WM, the levels of 13C recovered in protein and lipid components of the GS group increased significantly between 11 and 24 h post-feeding (Fig. 3(b)). The incorporation of 13C from dietary starch to muscle glycogen is shown in Fig. 4. No differences were found between the diets, although there was a significant correlation between 13C levels in glycogen and the amount of glycogen present in RM (r 0·84, P < 0·01) and WM (r 0·94, P < 0·001), where 13C deposition in glycogen in RM was 9-fold higher than that in WM.

Fig. 3 Recovery of 13C (as a percentage of ingested isotope) from (a) liver and (b) white muscle fractions (protein, lipid and glycogen) of rainbow trout fed with the raw starch (RS) and gelatinised starch (GS) diets, 11 (■) and 24 h (□) after force-feeding (see the Experimental methods section for details of the calculations). Recovery of 13C from the glycogen fraction of white muscle was below the limit of detection (n.d., not detected). Values are means, with their standard errors represented by vertical bars (n 7). * Mean values were significantly different between the RS and GS groups (P < 0·05). †† Mean values were significantly different between 11 and 24 h (P < 0·01).

Fig. 4 Relationship between the glycogen content (g/100 g wet weight (w.w.)) and the percentage of [13C]glycogen recovered from dietary starch in white muscle (■; y = 10·315x − 0·2796, R 2 = 0·8829; P < 0·001) and red muscle (□; y = 32·18x+4·316, R 2 = 0·7049; P < 0·01) of rainbow trout.
Expt 2: effects of sustained swimming on the use of carbohydrates by trout
Food intake, fish growth, plasma glucose levels and tissue proximal composition
Sustained swimming caused a significant increase in food intake (control, 2·54 (sem 0·14) % body weight; exercise, 3·09 (sem 0·15) % body weight; P < 0·05), reflecting higher metabolic costs but without impairing growth (specific growth rate: control, 2·24 (sem 0·34) % body weight/d; exercise, 2·63 (sem 0·03) % body weight). The food conversion ratio did not change significantly (control, 1·32 (sem 0·37); exercise, 1·36 (sem 0·11)). Under exercise, plasma glucose levels increased at 11 h after feeding (P < 0·05), but decreased significantly with respect to the control group at 24 h (Fig. 5).

Fig. 5 Plasma glucose concentration (mm) in rainbow trout subjected to sustained swimming (exercise, E) or to voluntary swimming (control, C), 11 (■) and 24 h (□) after force-feeding. Values are means, with their standard errors represented by vertical bars (n 6, C) and (n 9, E). * Mean values were significantly different between the two experimental groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
Proximate composition of liver, RM and WM, at both 11 and 24 h post-feeding, is shown in Table 2. Exercise modified liver composition due to a transient increase in glycogen content at 11 h and lipid mobilisation at 24 h post-feeding. Fish under exercise increased the glycogen content in RM and the lipid content in WM.
Table 2 Proximal composition of liver and muscle in rainbow trout subjected to sustained swimming
(Mean values with their standard errors, n 6)

HSI, hepatosomatic index; RM, red muscle; WM, white muscle.
* Mean values were significantly different between the two experimental groups (P < 0·05).
† Mean values were significantly different between 11 and 24 h (P < 0·05).
δ13C and δ15N taken up by tissues
The recoveries of 13C in glycogen, lipid, protein and free pool in liver, and WM and RM are shown in Fig. 6. In liver, sustained swimming induced higher total recoveries of 13C (2-fold) with respect to the control group at both 11 and 24 h, also in parallel with plasma glucose changes. The higher recovery in the liver of the exercise group was due, in part, to significant depositions of 13C in protein and lipid fractions at 11 h (4- and 5-fold higher, respectively) and in the free pool at 24 h (7-fold increase). In WM, sustained swimming induced a significantly higher recovery of 13C (P < 0·05), especially due to the recovery in the free pool at 11 h post-feeding. The 13C recovered in the protein fraction increased at 24 h (P < 0·05) in both groups, but the incorporation of 13C in the other tissue stores (lipid and glycogen) differed in the two groups. Thus, the control group significantly increased 13C in glycogen (control, 95 (sem 29) v. exercise, 2·2 (sem 0·3) mg [13C]glycogen/100 g of 13C ingested; P < 0·05), whereas in the exercise group higher deposition of 13C in the lipid fraction was observed (control, 29 (sem 12) v. exercise, 92 (sem 16) mg [13C]lipid/100 g of 13C ingested; P < 0·05). In RM, more than 85 % of 13C labelling was in the free pool, whereas depositions in protein and glycogen reserves were lower and no deposition was observed in lipids. However, the exercise group presented lower deposition of 13C in the glycogen fraction than that of the control group.

Fig. 6 Recovery of 13C (as a percentage of ingested isotope) from (a) liver, (b) white muscle and (c) red muscle fractions (protein (■), lipid (![]() ), glycogen (
), glycogen (![]() ) and free pool (□)) of rainbow trout subjected to sustained swimming (exercise, E) or to voluntary swimming (control, C), 11 and 24 h after force-feeding (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 6, C) and (n 9, E). * Mean values were significantly different between the two experimental groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
) and free pool (□)) of rainbow trout subjected to sustained swimming (exercise, E) or to voluntary swimming (control, C), 11 and 24 h after force-feeding (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 6, C) and (n 9, E). * Mean values were significantly different between the two experimental groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
The recoveries of 15N in protein and the free pool fractions of liver, and WM and RM are shown in Fig. 7. Sustained swimming did not modify the 15N recovery in liver, and the fate of 15N from dietary protein revealed the same pattern in WM and RM. The greatest recovery of total 15N, due to the higher recovery in the protein fraction, occurred at 24 h in both muscles of the exercise group (P < 0·05).

Fig. 7 Recovery of 15N (as a percentage of ingested isotope) from the protein (■) and free pool (□) fractions of the (a) liver, (b) white muscle and (c) red muscle of rainbow trout subjected to sustained swimming (exercise, E) or to voluntary swimming (control, C), 11 and 24 h after force-feeding (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 6, C) and (n 9, E). * Mean values were significantly different between the two experimental groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
As a summary of all results, Fig. 8(a) and (b) presents the total recoveries of 13C and 15N, respectively, after a single forced meal, including all tissues and fractions. The high 13C recovery in whole fish under exercise, although not significantly different than that of the control group at 11 h, decreased significantly between 11 and 24 h. At 24 h, the total 15N recovered in whole fish of the exercise group was significantly higher.

Fig. 8 Recovery of (a) 13C and (b) 15N (as a percentage of ingested isotope) from entire organs or tissues (WM (![]() ), white muscle; RM (■), red muscle; L (
), white muscle; RM (■), red muscle; L (![]() ), liver; V (▧), viscera; R (□), the rest of the fish) of rainbow trout subjected to sustained swimming (exercise, E) or to voluntary swimming (control, C), 11 and 24 h after force-feeding. The sum of the stacked bar represents the total recovery from whole fish (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 6, C) and (n 9, E). * Mean values were significantly different between the E and C groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
), liver; V (▧), viscera; R (□), the rest of the fish) of rainbow trout subjected to sustained swimming (exercise, E) or to voluntary swimming (control, C), 11 and 24 h after force-feeding. The sum of the stacked bar represents the total recovery from whole fish (see the Experimental methods section for details of the calculations). Values are means, with their standard errors represented by vertical bars (n 6, C) and (n 9, E). * Mean values were significantly different between the E and C groups (P < 0·05). † Mean values were significantly different between 11 and 24 h (P < 0·05).
Discussion
For the first time, two stable isotopes ([13C]starch and [15N]protein) have been incorporated into fish diets as labels to study the fate of both nutrients in a species of reference, the rainbow trout. The use of [13C]starch allowed us to analyse the distribution of dietary carbohydrates depending on the degree of gelatinisation. However, we should point out two limitations with this method that reduces its value for the assessment of the use of [13C]starch: the amount of label lost with the undigested food and the small amount of glucose lost via the urine in fish with the highest glycaemia(Reference Blasco, Fernández and Marimón15, Reference Bucking and Wood38). The long-lasting hyperglycaemia observed in the group fed the GS diet indicates higher absorption of carbohydrates in this group than in those fed RS. The digestibility of the two diets was not analysed in the present study, but we assume that the theoretical values of 58 % for RS and 90 % for GS are correct (see Brauge et al. (Reference Brauge, Corraze and Médale39) and Kaushik et al. (Reference Kaushik, Médale and Fauconneau40)). This assumption is consistent with the postprandial glucose levels measured. Long-lasting hyperglycaemia in trout was previously seen as a result of feeding a high-carbohydrate diet(Reference Bergot41) or of a high glucose dose administrated orally(Reference Blasco, Marimón and Viaplana16, Reference Palmer and Ryman42). Many fish species correct hyperglycaemia following a high-carbohydrate meal less rapidly than endotherms(Reference Wilson1, Reference Furuichi and Yone43, Reference Moon44). Apart from the differences in body temperature and metabolic rate, another reason, at least in rainbow trout, is a persistent, high level of endogenous glucose production from the liver(Reference Enes, Panserat and Kaushik45). Key enzymes of gluconeogenesis in this species are always highly expressed, independently of nutritional status(Reference Panserat, Plagnes-Juan and Kaushik46). In the present study, fish fed GS took up much more glucose into tissues, as shown by their higher plasma glucose levels and higher total recovery of 13C in tissues at 11 h. These higher uptake rates could contribute to the marked reduction of plasma glucose levels at 24 h in this group. These results are consistent with our previous study showing that brown trout (Salmo trutta) after an aortic glucose load labelled with 14C presented higher glucose uptake rates into tissues, in proportion to the hyperglycaemia(Reference Blasco, Marimón and Viaplana16). In the present study, the [13C]glucose dilution was different in the RS and GS groups due to the similar amount of tracer delivered and the different glycaemia observed. Since the percentage of total 13C recovered in muscle (WM plus RM) was similar in the two groups (nearly 40 % of the total 13C recovered), the total amount of glucose taken up by the GS group must have been higher than that by the RS group. These results also reinforce the idea that skeletal muscle of rainbow trout is the main peripheral site of glucose disposal, similarly to what was observed in cod(Reference Hemre and Kahrs47), Atlantic salmon(Reference Hemre and Storebakken48) and brown trout(Reference Blasco, Marimón and Viaplana16). The 13C deposition rate into glycogen depots in RM was nine times higher than in WM, in agreement with the different capacity of glycogen repletion of each kind of muscle in exercised rainbow trout(Reference West, Schulte and Hochachka49). So, the importance of WM as the main site of glucose disposal cannot be overlooked, based on the large relative mass of the tissue. Higher carbohydrate uptake by the muscles of the GS group caused a proportional increase in 13C recovered in all muscle reserves, especially in protein and lipid, in agreement with the results observed in brown trout after an aortic glucose load(Reference Blasco, Marimón and Viaplana16). In rainbow trout, high dietary levels of digestible carbohydrates increased hepatic lipid content(Reference Brauge, Medale and Corraze50), suggesting hepatic lipogenesis from carbohydrates(Reference Panserat, Hortopan and Plagnes-Juan51). In accordance with these studies, the present results show that higher glucose uptake in the liver of the GS group produced an increase of the glycogen depots (observed after 1 month of the GS diet) and the de novo synthesis of lipids in the liver. In the present study, nearly 20 % of the total 13C ingested was recovered at 24 h, whereas in Atlantic salmon, the 14C recovered ranged between 13 and 15 %(Reference Hemre and Storebakken48). Differences in species and in methodological conditions (tracer, starch or glucose) might explain these discrepancies. Glucose oxidation can be calculated from the difference between 13C ingested and 13C recovered from the whole fish after 24 h post-feeding, but the values of 82 % of glucose oxidised in the GS group and 77 % in the RS group can be overestimates of the actual values due to the losses indicated before. The main issue, however, is that the measurement of 13C in tissue stores confirms that excess glucose promotes lipogenesis, leading to significantly higher depots of newly formed, saturated fat in liver and muscle, even in trout accustomed to this diet.
Summarising all the results of the first experiment, gelatinisation of starch improved the absorption of dietary carbohydrates, causing a long-lasting hyperglycaemia, and glucose uptake by tissues rose in proportion to the plasma glucose concentrations in the post-absorptive period. This higher rate of glucose uptake by tissues favoured the synthesis of lipid in the liver. A level of 30 % digestible carbohydrates fed daily, however, exceeds the capacities of rainbow trout to use this nutrient, as reflected in the hyperglycaemia maintained after 24 h and the deposition of lipids in WM. Consequently, if these lipids are not consumed as energy fuel, they will be deposited as saturated fat, which in excess can affect the fish fillet quality. This can be prevented by increasing energy expenditure through the induction of exercise. In the second experiment, only the GS diet was used to determine the effects of moderate, sustained swimming on the use of nutrients as energy fuels. The swimming regimen of 1·3 BL/s for 1 month was used as a ‘metabolic promoter’. In this situation of induced activity, rainbow trout increased feed intake to compensate for the higher energy costs due to the exercise. Although Davison & Goldspink(Reference Davison and Goldspink52) found an increase of food conversion ratio in exercised brown trout fed chopped liver, no significant differences were observed between the groups in the present study, perhaps due to the dietary differences. Rainbow trout growth rate showed a tendency to increase in exercise, in agreement with the results reported by Houlihan & Laurent(Reference Houlihan and Laurent53) and Farrell et al. (Reference Farrell, Johansen and Steffensen54).
A carbohydrate-rich diet fed under exercise also induced transitory hyperglycaemia 11 h post-feeding, but glycaemia returned to control values at 24 h. This transitory hyperglycaemia in the swimming group is attributed to higher food ingestion. As indicated before, the maintenance of hepatic gluconeogenesis in rainbow trout feeding carbohydrate-rich diets(Reference Enes, Panserat and Kaushik45, Reference Panserat, Médale and Blin55) can contribute to hyperglycaemia. As observed in the first experiment, hyperglycaemia raised glucose uptake rates in liver and WM. Thus, in liver, the total recovery of 13C was 2-fold higher in the exercise group, indicating greater uptake and deposition into the various tissue reserves. The postprandial variation of glycogen content, related to glycaemia, was evidence of higher carbohydrate uptake and use in exercised fish than in controls. Moreover, hepatic lipogenesis from dietary carbohydrates was also enhanced and, combined with the lower level of liver fat in the exercise group at the end of the experiment, this observation supports the idea of higher mobilisation of hepatic lipids to extra-hepatic tissues. The increase in muscle lipid content in the exercised rainbow trout, in agreement with the increase in muscle lipid content found in several fish species under exercise conditions(Reference Davison and Goldspink52, Reference Totland, Kryvi and Jødestøl56, Reference Yogata and Oku57), should in part be caused by such mobilisation. Fish rely mainly on fatty acids to fuel submaximal exercise(Reference Kieffer, Alsop and Wood28), mediated by an enhancement of lipoprotein lipase activity in RM(Reference Magnoni and Weber58). However, the origin of these lipids has not been traced previously. The addition of [13C]algal starch to the diet enabled us to ensure that lipid synthesised de novo in the liver is mobilised and transported to skeletal muscles and oxidised or used to replenish stores. The present results on the lack of 13C in the lipid fraction of RM support the notion that lipids are highly used as aerobic energy fuel by this tissue, as reported by Magnoni & Weber(Reference Magnoni and Weber58). On the other hand, the balance in WM results in a net lipid deposition. Additional information can be drawn from high levels of 13C recovered in the free pool of RM. This labelling corresponds to several kinds of molecules of the intermediary metabolism and is indicative of the higher metabolic activity of RM. During moderate and sustained swimming, RM burns not only fatty acids provided by the liver, but also glycogen. The present results support that dietary carbohydrates play a key role in muscle metabolism during exercise. Moreover, West et al. (Reference West, Arthur and Suarez59) reported a 30-fold rise in glucose use by RM in rainbow trout under a steady swimming speed at 80 % U crit.
Non-protein energy sources spare dietary protein from oxidation as fuel, thus releasing it for growth. Thus, high protein efficiency ratios in rainbow trout fed diets containing about 30 % of digestible carbohydrates have been reported(Reference Kim and Kaushik4, Reference Kaushik and Oliva Teles60). In the present study, the use of [15N]protein as a dietary tracer allowed us to measure the amount of protein allocated to the main tissues following a single meal. Houlihan & Laurent(Reference Houlihan and Laurent53) reported that both protein synthesis and protein degradation increased in exercised rainbow trout, leading to increased growth rate. In the present study, the greatest recovery of total 15N for exercised fish at 24 h, mainly in the protein fraction of RM and WM, is evidence that exercise improves protein deposition. Exercise may also reduce N wastes. In conclusion, one forced-feeding with labelled nutrients, [13C]starch and [15N]protein, has allowed us to show that sustained swimming in rainbow trout improves the use of digestible carbohydrates and of lipid and glycogen depots, resulting in an enhancement of the protein-sparing effect.
Acknowledgements
We thank Dr F. Medale (INRA, St Pee) for providing the experimental diets. The present study was supported by grants from the Catalan government and the Spanish government (AGL2002-03987 to J. V. P.). O. F. and M. M.-P. received fellowships from FPI-2007 and FI-2007 of the Spanish government and the Catalan government, respectively. The authors' responsibilities were as follows: J. F.-B., A. I., and J. B. planned the project. J. F.-B., A. I., J. B., O. F., M. B. and M. M.-P. carried out the experiment and collected the samples. O. F. carried out the laboratory analyses. O. F., A. I., J. F.-B., J. V. P. and J. B. carried out the data analysis and interpretation and O. F and J. B. wrote the manuscript; all authors read and approved the final manuscript. The authors declare that they have no competing interests to report.












