Introduction
Nitridation of sapphire is an important step for many approaches to growing Group III-nitrides. As such, there have been many studies of the nitridation of sapphire by molecular beam epitaxy (MBE) using ammonia, [Reference Grandjean, Massies and Leroux1,Reference Yang, Li and Wang2] an electron cyclotron resonance (ECR) plasma source, [Reference Moustakas, Molnar, Lei, Menon and Eddy3] or an rf-plasma source. [Reference Heinlein, Grepstad, Berge and Riechert4,Reference Ptak, Ziemer, Millecchia, Stinespring and Myers5,Reference Widmann, Feuillet, Daudin and Rouviere6] The chemical evolution of the nitridation layer has been studied by x-ray photoelectron spectroscopy (XPS) [Reference Heinlein, Grepstad, Berge and Riechert4], indicating an initially increasing nitrogen signal which ultimately saturates. A wide range of times have been reported necessary in order to obtain a completely nitrided surface, from ten to twenty minutes for an ECR source to several hours with an RF source. This range of nitridation times can be adequately explained by the different reactivity of the various active nitrogen species. [Reference Ptak, Ziemer, Millecchia, Stinespring and Myers5] After nitridation, some authors find a completely relaxed surface with a final lattice constant equal to that of AlN, [Reference Grandjean, Massies and Leroux1,Reference Moustakas, Molnar, Lei, Menon and Eddy3] while others find a lattice constant consistently smaller than that of AlN. [Reference Widmann, Feuillet, Daudin and Rouviere6] The formation of an AlO1-xNx alloy has been invoked to explain the smaller lattice constant. However, AlO1-xNx formation is unlikely based on thermodynamic considerations, and is contradicted by an in-situ transmission electron microscopy study of nitridation. [Reference Yeadon, Marshall, Hamdani, Pekin, Morkoç and Gibson7] Another consideration is the common observation of the nucleation of cubic grains at the epilayer-sapphire interface. Widmann et al. [Reference Widmann, Feuillet, Daudin and Rouviere6] have related this nucleation of cubic grains to nitridation conditions. In this paper, we present results which indicate that B contamination from an rf-plasma source may explain the latter two phenomena.
Experimental Details
Two rf-plasma sources are used in our lab used to produce various active species of nitrogen for the nitridation of sapphire and growth of GaN. These sources are an Oxford Applied Research (Oxfordshire, England) CARS-25 source and an EPI Vacuum Products (St. Paul, MN) Unibulb source. The Oxford source features a removable pyrolytic boron nitride (PBN) liner and aperture plate. The EPI source contains the standard PBN Unibulb configuration with a 400-hole aperture
Nitridation experiments were performed in two separate systems. For chemical analysis of the evolution of the nitridation layer, nitridation was performed at 400 °C in a chamber connected to an Auger electron spectroscopy (AES) analysis system consisting of a Phi 545 Scanning AES Microprobe with Model 110A Cylindrical Electron Optics. Scans were performed with a 3 keV incident electron beam, a beam current of 2 μA and a nominal spot size of 3 μm. Reflection high energy electron diffraction (RHEED) measurements of the evolution of lattice constant with nitridation were performed in our MBE system, which has been described elsewhere. [Reference Myers, Millecchia, Ptak, Ziemer and Stinespring9]
Evidence for Boron During Growth
We have published several studies detailing the characterization of the active nitrogen flux produced by our two rf plasma sources for various operating conditions. [Reference Ptak, Millecchia, Myers, Ziemer and Stinespring8,Reference Myers, Millecchia, Ptak, Ziemer and Stinespring9,Reference Moldovan, Hirsch, Ptak, Stinespring, Myers and Giles10] During the characterization involving mass spectroscopy, we saw a small but persistent signal indicating that the sources were also producing boron. Subsequent secondary ion mass spectrometry (SIMS) analysis of GaN grown using these sources verified B production. Figure 1 shows typical SIMS measurements from GaN layers grown under standard conditions using both our Oxford (a) and EPI (b) source. A significant background of boron was detected in each case. The flux of B atoms listed was determined from the growth rate of the GaN and assuming 100% incorporation of B. These results are also consistent with levels of B detected in nitrogen-doping studies of ZnSe and CdTe. [Reference Moldovan, Hirsch, Ptak, Stinespring, Myers and Giles10]
The PBN liner is the likely candidate as the source of the boron from the plasma sources. Significant amounts of atomic nitrogen are produced inside the liner. Atomic nitrogen is highly reactive, and may be promoting decomposition of the PBN. We replaced the liner in the Oxford source after verifying the large B background and found similar levels of B in GaN grown with the new liner, indicating that it is an endemic problem. Source configurations can influence this effect as a significantly lower B flux was indicated for our EPI source. Operation almost continually over approximately three years has not changed the amount of B produced by the EPI source. Private discussions with other groups have indicated that B is potentially a universal issue for plasma sources.

Figure 1. Comparison of Boron concentration from two rf-plasma sources.
Rheed Studies
RHEED was used to measure the lattice constant variation occurring during nitridation of sapphire using our EPI source, similar to studies reported previously. [Reference Grandjean, Massies and Leroux1,Reference Widmann, Feuillet, Daudin and Rouviere6] The RHEED pattern was monitored continuously for sapphire exposure to the active nitrogen flux at temperatures of 200, 400 and 700 °C. In contrast to previous results, [Reference Widmann, Feuillet, Daudin and Rouviere6] we do not observe any evidence of nitridation at 200 °C. This result can possibly be explained to be due to differences in the fraction of active species found in different rf plasma sources. [Reference Ptak, Ziemer, Millecchia, Stinespring and Myers5] Evidence of nitridation was readily observed at 400 and 700 °C. Immediately after active nitrogen exposure, streaks began to appear that are generally attributed to the replacement of oxygen by nitrogen in the sapphire lattice. An additional 10 to 15 minutes of exposure to active nitrogen resulted in the disappearance of the underlying sapphire pattern.
Figure 2 illustrates the evolution of the lattice parameter of the nitridation layer measured using RHEED. The measurements were calibrated using the bulk lattice parameters of sapphire. The variation in lattice constant appeared to reach an initial plateau, followed by a second increase to a final value that remained stable for longer times. In general, this final measured lattice constant was several percent lower than the 3.11Å lattice constant of AlN, in agreement with Widmann et al. [Reference Widmann, Feuillet, Daudin and Rouviere6] The lattice constant tended to be significantly smaller for the higher temperature nitridation. Faint and very diffuse rings appear superimposed over the streaks in the RHEED pattern at about the time of the second increase in lattice constant.

Figure 2. Variation of the in-plane lattice constant with nitridation time as observed using RHEED. The x-values represent the composition of Al1-xBxN that would result in this lattice constant.
Auger Analysis During Nitridation
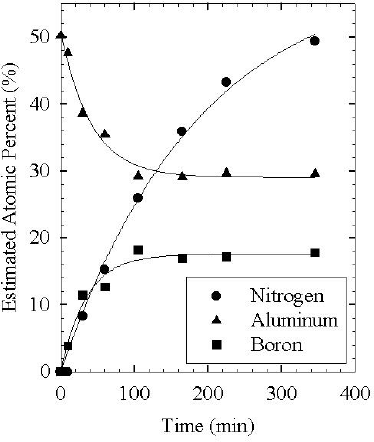
AES was used to perform a detailed examination of the chemical evolution of the nitridation layer as a function of active nitrogen exposure time at 400ºC. The substrate heater in this system did not allow investigation of significantly higher temperatures, and nitridation was not observed for 200ºC or below with the EPI source. As reported previously, [Reference Ptak, Ziemer, Millecchia, Stinespring and Myers5] we observed significant differences in nitridation rates based on whether the active flux was predominantly atomic, ionic or neutral metastable nitrogen. Figures 3 and 4 show the evolution of the Al, N, and B signals measured as a function of nitridation time for two distinct cases. The data in Figure 3 were collected for nitridation under conditions which enhanced ion production with the Oxford source. After about 60 minutes, the Al, N and B signals had reached a plateau, with the B signal small. By assuming that this represented primarily an AlN layer, sensitivity factors relative to nitrogen for Al (0.72) and B (0.9) were obtained for our system to use in determining composition. Of particular significance to this paper is the large amount of B present in the nitridation layer shown in Figure 4, obtained using the EPI source. A previous study of the chemical evolution of a nitridation layer relied on XPS, [Reference Heinlein, Grepstad, Berge and Riechert4] which is not as sensitive to B. The study used an Oxford source similar to ours, which produces primarily atomic and ionic active nitrogen and is not as efficient at capturing boron as illustrated by Figure 3. Focusing on Figure 4, it is readily apparent that both the B and N signal increase rapidly while the Al signal decreases. After about 100 minutes, the Al and B appear to reach equilibrium. We believe that this trend is indicative of the initial formation of a BN layer, which then evolves into an alloy of AlBN during extended nitridation. The continuing increase of the N signal is likely related to the longer mean free path for electrons at this energy, ∼11Å, vs. those of Al (6Å) or B (7Å). Thus, the Al and B signals would saturate for a relatively thin layer tickness while the increasing N signal implies a layer that is still growing with time. Note that the lines in both Figure 3 and Figure 4 are only meant as guides to the eye.

Figure 3. AES study of the change in surface composition upon nitridation with the Oxford source configured for a high ion flux.

Figure 4. Nitridation with the EPI source.
Discussion
It is obvious that B can play a significant role during the long periods required for nitridation using an rf plasma source. AES analysis indicates a final layer that contains about 10 to 20% BN. This is consistent with RHEED lattice constant measurements which also imply significant amounts of BN. If this is due to alloy formation, the AES shown in Figures 3 and 4 would imply approximate x-values of 0.04 and 0.4 respectively. The RHEED studies indicated similar compositions are possible assuming that Al1-xBxN is being formed. RHEED analysis consistently indicates a lattice constant closer to that of pure AlN for lower temperature nitridation implying that the higher temperatures are more effective for B capture in the evolving nitridation layer.
Polyakov et al. [Reference Polyakov, Shin, Qian, Skowronski, Greve and Wilson11] studied the growth of AlBN solid solutions by MOCVD. They found that for compostions larger than about x=0.01 phase segregation occurred into BN and low x-value AlBN. The implication is that high x-value AlBN is thermodynamically unstable. In Polyakov’s study, the second B-rich phase appeared to be wurtzite BN. We cannot identify if this is occurring in our nitridation layers from the measurements made. It is possible that high x-value AlBN can be formed for the thin nitridation layers.However, it is also quite likely that phase separation is indeed occurring, particularly for the more B-rich layers found at higher temperatures. The onset of segregation may be indicated by the relatively abrupt second rise in lattice constant observed by RHEED, accompanied by the appearance of faint, diffuse rings. Indeed, the presence of both the faint ring pattern and the strong, streaky pattern is indicative of an inhomogeneous surface. Wideman et al. [Reference Widmann, Feuillet, Daudin and Rouviere6] found that lower nitridation temperatures led to reduced nucleation of cubic grains in subsequent AlN buffer layer growth. Based on our results, a possible mechanism explaining this result is the formation of larger x-value AlBN at higher temperatures, followed by segregation and nucleation of cubic BN inclusions once a critical thickness is reached. Polyakov et al.’s study suggests that the majority of the B-rich phase may maintain the wurtzite structure, thus higher temperatures and larger B concentrations may be necessary to nucleate the cubic phase.
Conclusions
Boron can be a significant contaminant originating in plasma sources utilizing PBN liners. While it is not clear what role a small background of B has on the properties of GaN, B may have a significant influence on the nitridation of sapphire using rf plasma sources. In particular, the formation of Al1-xBxN alloys can explain observed lattice constants smaller than expected for AlN. Segregation of BN in large x-value alloys coupled with nucleation of the more thermodynamically stable cubic BN phase may explain one possible mode for the subsequent nucleation of cubic GaN or AlN on nitridated sapphire.
Acknowledgements
This work was supported by ONR Grant N00014-96-1-1008 and monitored by Colin E. C. Wood.