INTRODUCTION
According to the latest World Health Organization (WHO) report, there were 8·6 million new tuberculosis (TB) cases and 1·3 million deaths globally in 2012 [1]. Most of the new cases were notified from Asia (58%) and the African region (27%) [1]. Despite achieving 100% geographical directly observed therapy, short course (DOTS) strategy coverage and increased financial and human resources for TB control since 2001 [2, Reference Ukwaja3], Nigeria is currently ranked 10th among the 22 high TB burden countries worldwide – with a prevalence rate of 161/100000 population – up among the highest rates in Africa [1]. Moreover, 23% of TB patients were HIV co-infected in Nigeria in 2012 [1].
Several reports from both high- and low-income countries have evaluated gender differences in the incidence of TB [1]. Previous WHO reports showed that globally almost twice as many men as women are being diagnosed with TB annually [1, 4]. In 2012 there were an estimated 2·9 million female cases – about half the number notified for men [1]. Several factors have been proposed to explain gender differences in TB burden [510]. One of these is the possibility of under-notification of TB in women because they face greater difficulties in gaining access to health services and in obtaining timely diagnosis and treatment, particularly in developing countries – primarily because of social, cultural and economic factors [Reference Yamasaki-Nakagawa5–Reference Karim8]. However, a meta-analysis of ~30 reports on notifications and prevalence data concluded that the higher male:female ratio in TB burden observed was not due to differential access to healthcare in most cases [Reference Borgdorff9]. Other factors such as increased social interaction of men compared to women due to gender-specific social roles [Reference Vlassoff and Garcia10], and biological factors like differences in sexual hormones, genetic factors and immunological response have been also been proposed to account for a higher susceptibility of men to TB [Reference Neyrolles and Quintana-Murci11–Reference Fortin12].
It is likely that these biological and socioeconomic factors which were suggested to reduce susceptibility to TB infection or decrease access to TB care in women may also impact on their abilities to complete TB treatment or their final treatment outcome. However, only a few studies have evaluated gender differences in TB treatment outcomes worldwide [Reference Jimenez-Corona13–Reference Feng18], and none was studied in sub-Saharan Africa. Knowledge of the impact of gender on TB treatment outcome is critical for informing health policy solutions needed to improve the outcomes of TB and control the transmission of the disease.
The aim of this study was to describe gender differences in the epidemiological characteristics and treatment outcomes of TB in Nigeria. Specific objectives were to determine in adults (aged ⩾ 15 years) with TB in Nigeria in 2011 and 2012: (i) the number and proportion of TB cases notified according to gender; (ii) gender differences in their demographic and clinical characteristics; (iii) gender differences in their treatment outcomes stratified by; age, TB regimen received, residence, treatment category, human immunodeficiency virus (HIV) status and facility where care was given, and (iv) if gender was a predictor of unsuccessful treatment outcome in TB patients.
METHODS
Study design and setting
This was a retrospective cohort study using routine programme data from the national TB control programme (NTP). TB treatment registers of two large healthcare facilities – one rural (private) secondary-care and one urban (public) tertiary-care facility in Ebonyi, Southeastern Nigeria were reviewed. Ebonyi state, one of Nigeria's 36 states, has a population of over 2.5 million people, and 75% of them reside in the rural areas [19]. The state notifies about 2300 TB cases annually to the NTP [Reference Ukwaja3]. It is divided in to three zones with 130 healthcare facilities providing DOTS services for TB [Reference Ukwaja3]. In 2008, DOTS coverage reached 100% [Reference Ukwaja3], and treatment success rate for smear-positive TB cases was 88·7% [Reference Ukwaja20]; however, the TB case detection rate was about 40% [Reference Ukwaja21]. One rural secondary-care (faith-based) private hospital and the only tertiary (public) hospital in the state were selected as the study sites. The study sites were selected based on high TB notification rates and geographical spread. The two hospitals notified about 50% of all TB cases in Ebonyi state in 2009, and they serve an estimated 1·5 million people in the state and beyond [Reference Ukwaja3].
The participants were adult (⩾15 years) patients who received TB treatment at the study facilities during 1 January 2011 to 31 December 2012. The following information was retrieved from the TB treatment registers: age, gender (female vs. male), treatment category (new vs. retreatment case), residence (rural vs. urban), type of TB (pulmonary vs. extrapulmonary TB), HIV status (HIV positive vs. HIV negative), treatment regimen (6 months vs. 8 months) and treatment outcome.
Diagnosis of TB
TB diagnosis was based on standard WHO/NTP guidelines [2, 22]. Briefly, any individual coughing for ⩾2 weeks with or without symptoms such as: weight loss, night sweats, fever, chest pain, shortness of breath, anorexia and haemoptysis was suspected of having pulmonary TB [2, 22]. Such persons were asked to provide three sputum samples (spot-morning-spot) for microscopy using Ziehl–Neelsen staining. Individuals with at least one sputum sample smear positive for acid-fast bacilli were registered as having smear-positive pulmonary TB [2, 22]. Patients suspected of having TB with smear-negative sputum; with or without radiographic abnormalities consistent with active pulmonary TB, who did not respond to a course of broad-spectrum antibiotics (excluding anti-TB drugs, fluoroquinolones and aminoglycosides), having a repeat sputum-smear microscopy testing negative, and whose clinical presentation warrants a decision to treat for TB by the clinician were registered as having smear-negative TB [2, 22]. Extrapulmonary TB patients were identified as persons with a histological diagnosis of TB [2, 22]. All persons suspected of having TB had HIV counselling and testing at the time of laboratory investigation for TB.
Treatment of TB
Before 2012, all new TB patients were treated with standard anti-TB medications that included isoniazid (H), rifampicin (R), ethambutol (E) and pyrazinamide (Z) in the intensive phase for at least 2 months, followed by a regimen that included isoniazid and ethambutol for 6 months in the continuous phase (2RHZE/6EH) [2]. However, in accordance with the current WHO guidelines, from January 2012 all new TB patients received a 6-month regimen consisting of 2RHZE/4RH [19]. Treatment of re-treatment TB cases during the study period remained the same and consisted of an 8-month rifampicin-containing regimen (2RHZES/1RHZE/5RHE) with 3 months intensive phase – streptomycin (S) is added to RHZE in the first 2 months – and a 5-month continuation phase. The community DOTS strategy was used to deliver the TB treatment where a DOTS supporter (family member/community volunteer) monitored the daily intake of the drugs by the patients.
Treatment outcomes
The TB treatment outcomes reported were based on standard WHO/NTP definitions, i.e. (cured, treatment completed, defaulted, death, treatment failure and transfer out) [1, 2, 19]. Patients who were cured or completed treatment were classified as having a successful treatment outcome while those who died, defaulted, had treatment failure or were transferred out were classified as having an unsuccessful treatment outcome [1]. Further, we evaluated sputum smear conversion after 2 and 5 months of treatment for smear-positive pulmonary TB patients.
Data analysis
The data were checked for errors, double-entered, and analysed using Epi Info v. 3·4·1 (CDC, USA). Data were analysed by frequencies and percentages for demographic, clinical characteristics and treatment outcomes according to gender. We also identified independent determinants of unsuccessful treatment outcomes in the patients studied. The outcome variable was successful vs. unsuccessful outcome and the main predictor variable was gender. Other covariates considered were: age, residence, treatment category, treatment regimen, health facility, and HIV status. Univariate analyses (χ 2 tests) were used to determine significant associations between the outcome variable and the explanatory variables. We performed a stratified analysis to determine the occurrence of interaction and confounding between the outcome variable and the explanatory variables. Multivariable logistic regression analysis was performed including all variables with a univariate P < 0·25 and all variables of clinical importance. P < 0·05 was considered statistically significant
Ethical approval
This study was approved by the Ethics and Research Advisory Committee of the National Tuberculosis Control Programme, Ministry of Health, Ebonyi state, Nigeria.
RESULTS
Socio-demographic characteristics
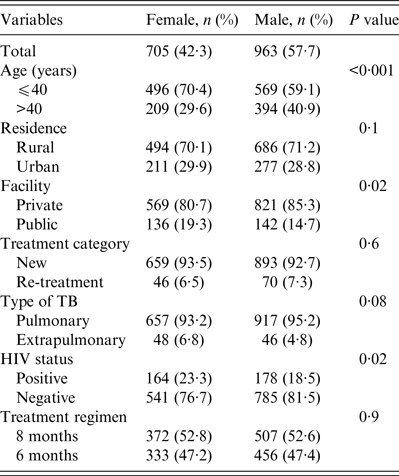
A total 1668 patients were studied, 963 (57·7%) were male and 705 (42·3%) were female with an overall male:female ratio of 1·4:1. The mean ages of males and females were 40·2 (±14·7) and 36·1 (±14·6) years, respectively (t test 6·62, P < 0·001). Overall, 70% of the women were aged < 40 years compared to 59·1% of men (Table 1). Fewer women received treatment at the rural private facility compared to males (80·7% vs. 85·3%, P = 0·02). Compared to men, more women were diagnosed with extrapulmonary TB (6·8% vs. 4·5%, P = 0·04) and HIV co-infection (23·3 vs. 18·5%, P = 0·02). There were no gender differences in the proportion of TB patients in terms of their residence, treatment category or treatment regimen (Table 1).
Table 1. Demographic and clinical profile of tuberculosis patients studied according to gender
Treatment outcomes
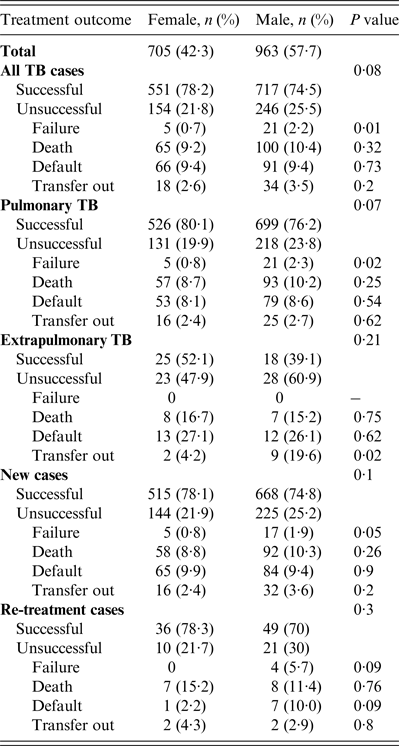
Treatment outcomes by type and category of TB are shown in Table 2. Of all TB cases seen during the study period, the treatment success rate in women was 551 (78·2%) compared to 717 (74·5%) in men (P = 0·08). A higher proportion of unsuccessful outcomes in all TB patients seen in men was mainly due to treatment failure which was 0·7% in women vs. 2·2% in men (P = 0·01, Table 2). Of patients with pulmonary TB (Table 2), treatment success rate in women was 526 (80·1%) vs. 699 (76%) in men (P = 0·05). The higher rate of unsuccessful outcomes in men compared to women was also mainly due to treatment failure (0·8% vs. 2·3%, P = 0·02). In extrapulmonary TB, new TB or re-treatment TB cases, treatment success rates were generally higher in women compared to men although the differences were not statistically significant. Moreover, men outnumbered women in all the unfavourable outcomes including death, treatment failure, and default although none of these differences were statistically significant (P > 0·05).
Table 2. Tuberculosis treatment outcomes stratified by gender in Ebonyi state, Nigeria, 2011–2012
In 985 smear-positive pulmonary TB patients studied, 929 (94·4%) had a sputum smear microscopy done after the intensive phase of treatment, i.e. 563 (60·6%) males and 366 (39·6%) females. Of the women, 64 (17·5%) failed to smear convert after the intensive phase of treatment compared to 123 (21·8%) men (P = 0·06). Further, of the 860 patients who had sputum smear test done after 5 months of treatment, 5/343 (1·5%) of the women still had a positive smear compared to 22/517 (4·3%) men (P = 0·02).
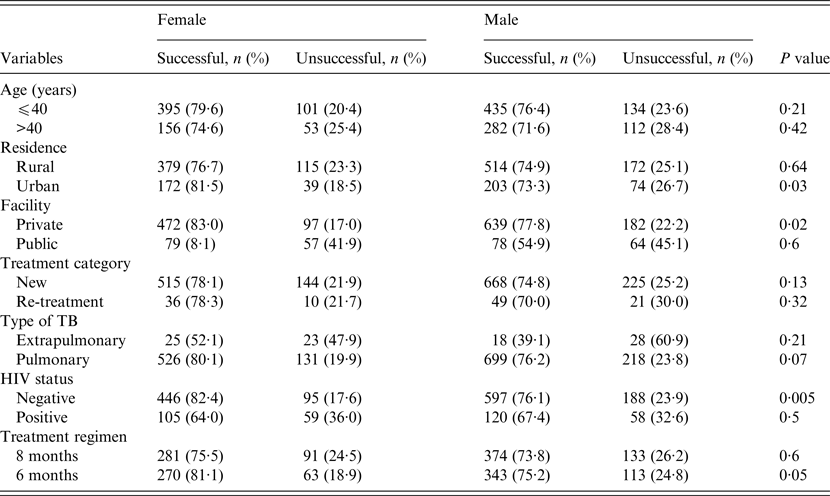
The treatment outcomes of TB patients according to gender, stratified by their demographic and clinical characteristics are given in Table 3. Across patients' residence, health facility where care was provided and HIV status (Table 3), the treatment success rates were significantly higher in women compared to men in urban residence (81·5% vs. 73·3%, P = 0·03), higher in women who received care at the private health facility (83% vs.77·8%, P = 0·02), and higher in HIV-negative women (82·4% vs.76·1%, P = 0·005). In patients who received the longer (8-month) anti-TB regimen, rates of successful outcomes in women were higher than in men but the difference was not statistically significant (75·5% vs. 73·8%, P = 0·6). However, in those who received the 6-month anti-TB regimen, rates of successful outcomes were higher in women compared to men (81·1% vs. 75·2%, P = 0·05) (Table 3). No significant differences exist regarding the outcomes of treatment of men and women according to their age groups, type of TB or treatment categories (Table 3).
Table 3. A comparison of treatment outcomes between female and male tuberculosis patients stratified by their demographic and clinical characteristics, Ebonyi state, Nigeria, 2011–2012
HIV, Human immunodeficiency virus.
Risk factors of unsuccessful outcomes
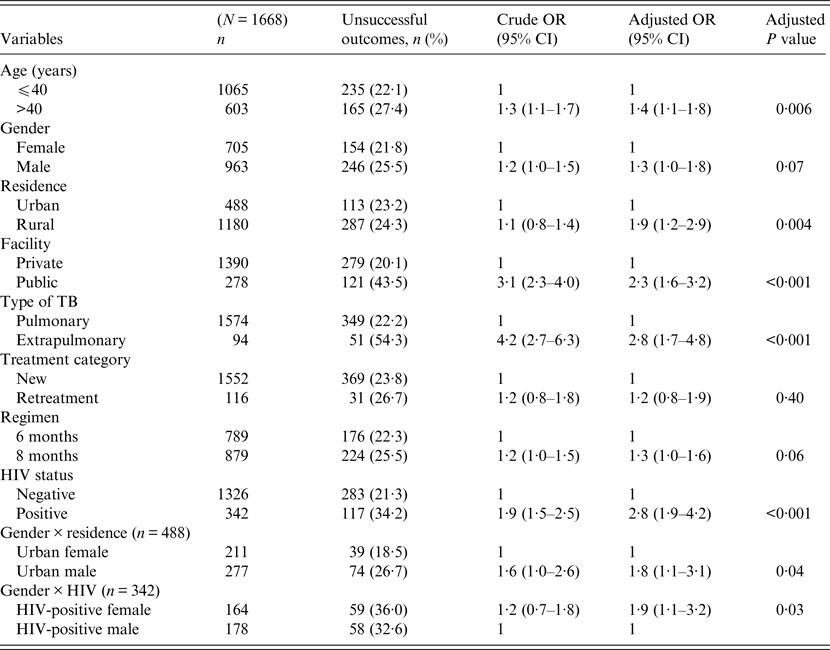
Univariate and multivariable logistic regression analyses were performed to determine socio-demographic and clinical risk factors for unsuccessful outcomes in all (Table 4), female (Table 5) and male (Table 6) TB patients. For all TB cases, the independent predictors for unsuccessful outcomes were: older age [adjusted odds ratio (aOR) 1·4, 95% CI 1·1–1·8], rural residence (aOR 1·9, 95% CI 1·2–1·9) care at the public facility (aOR 2·3, 95% CI 1·7–4·8), extrapulmonary TB case (aOR 2·8, 95% CI 1·7–4·8), HIV co-infection (aOR 2·8, 95% CI 1·9–4·2), being an urban male (aOR 1·8, 95% CI 1·1–3·1), and being a HIV-infected female (aOR 1·9, 95% CI 1·1–3·2). In female TB patients (Table 5), independent predictors for unsuccessful TB outcomes were: rural residence (aOR 2·0, 95% CI 1·2–3·0), treated at the public facility (aOR 2·8, 95% CI 1·7–4·6), extrapulmonary TB (aOR 2·5, 1·2–5·3), receiving the 8-month regimen (aOR 1·5, 95% CI 1·1–2·2), and HIV co-infection (aOR 2·7, 95% CI 1·8–4·2). In male TB patients (Table 6), independent predictors for unsuccessful TB outcomes were: older age (aOR 1·4, 95% CI 1·01–1·8), treated at the public facility (aOR 2·0, 95% CI 1·3–3·1), extrapulmonary TB (aOR 3·2, 95% CI 1·5–6·5), and HIV co-infection (aOR 1·5, 95% CI 1·3–2·2).
Table 4. Multivariable logistic regression analysis of factors associated with unsuccessful outcomes in all adult tuberculosis patients, Ebonyi state, Nigeria, 2011–2012
HIV, Human immunodeficiency virus; OR, odds ratio; CI, confidence interval.
Table 5. Multivariable logistic regression analysis of factors associated adverse outcomes in adult female tuberculosis patients, Ebonyi state, Nigeria, 2011–2012
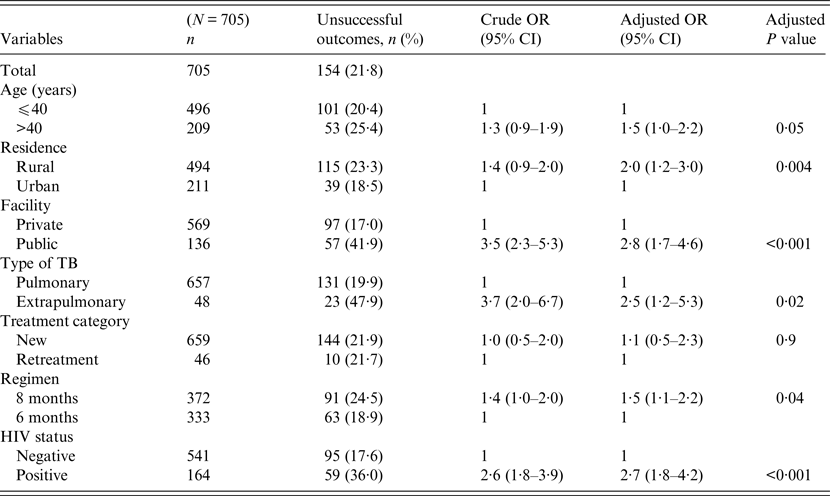
HIV, Human immunodeficiency virus; OR, odds ratio; CI, confidence interval.
Table 6. Multivariable logistic regression analysis of factors associated adverse outcomes in adult male tuberculosis patients, Ebonyi state Nigeria, 2011–2012
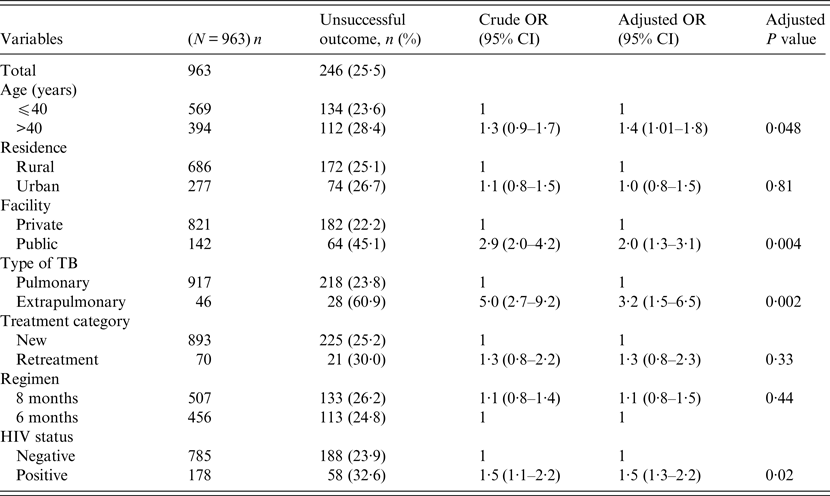
HIV, Human immunodeficiency virus; OR, odds ratio; CI, confidence interval.
DISCUSSION
In this study, we have shown that the male:female ratio was 1·4:1, and men were in general older than women. Moreover, we found that a higher proportion of women were treated at the private facility, had extrapulmonary TB and had higher rates of TB/HIV co-infection. Compared to men, treatment success rates were marginally higher in women and the difference was accounted for mainly by treatment failure. Furthermore, we observed gender differences in sputum conversion rates with a higher proportion of men failing to smear convert after 2 and 5 months of treatment. In addition, treatment success rates of women were superior to that of men for patients who were urban residents, treated at a private facility, or who were HIV-negative. Independent predictors of unsuccessful outcomes in all patients were: older age, being an urban male, being a HIV-infected female, rural residence, care at the public facility, extrapulmonary TB, and HIV co-infection.
In this study, we found that more males than females were diagnosed with TB and men overall were older. This observation has been documented in Nigeria and elsewhere and suggests that gender-specific strategies are needed to improve TB control [Reference Weiss, Somma and Abouihia6–Reference Borgdorff9, Reference Feng18, Reference Lawson23]. More than two-fifths of men were aged >40 years. Consistent with previous studies, this suggests a dominance of males in older TB patients [Reference Borgdorff9, Reference Feng18]. This difference may be because older women face greater socio-cultural and economic barriers in accessing healthcare services. Based on the most recent population census report of Ebonyi state and Nigeria, there were no differences in the proportion of men and women according to their age groups; and women accounted for 52·7% and 50% of the total and adult (⩾15 years) population, respectively [19]. Yet, we found that overall, more men than women were diagnosed with TB. The reasons why a higher proportion of men had TB and the relative under-diagnosis of TB in women is a crucial issue that needs to be explored further in Nigeria and other high-burden settings where the TB prevalence/notification ratio is very high but case detection rates are low [1, Reference Ukwaja21].
Although treatment success rates were marginally higher in women compared to men, the difference did not reach statistical significance. The influence of gender on TB treatment outcomes has shown inconsistent results in previous studies. In smear-positive TB patients in Mexico, treatment success rates were 88% in women and 79·6% in men [Reference Jimenez-Corona13]. Further, across TB types in India, treatment success rates were higher in women than men [Reference Balasubramanian14]. By contrast, no statistically significant differences in treatment success rates existed between men and women in Brazil, Egypt and Syria – although women had a higher proportion of treatment success in these countries [Reference Belo16, Reference Kamel17, Reference Bashour and Mamaree24].
No differences existed in the mortality rates between male and female TB patients in Nigeria. Most of the differences in unsuccessful outcomes between men and women in this study were mainly due to treatment failure. Higher mortality and greater treatment failure have been reported in Mexico, India and Taiwan in male TB patients [Reference Jimenez-Corona13, Reference Balasubramanian14, Reference Feng18]. Although better treatment compliance in women than men has consistently been reported [Reference Jimenez-Corona13–Reference Belo16, Reference Bashour and Mamaree24], in the present study, there was no gender difference in treatment default – which occurred in 8–10% of cases across all categories of TB studied. This suggests the need for interventions to improve treatment compliance and reduce treatment default in male and female TB patients in our setting. Only a few studies have reported on gender differences in sputum conversion rate. Our study agrees with the finding of a previous study which reported significantly lower sputum conversion rates in male TB patients [Reference Feng18].
This study showed that the treatment success rate of women were superior to that of men in patients who were: urban residents, treated at a private facility, or without HIV infection. In order to better understand these differences, we assessed for determinants of unsuccessful outcomes in all patients studied adjusting for their socio-demographic and clinical factors. Being an urban male TB patient was an independent predictor for unsuccessful outcome. Several in vitro studies suggest that the female sex hormone modulates and improves the immune response [Reference Neyrolles and Quintana-Murci11, Reference Calippe25–Reference Flanagan28]. Very broadly, oestrogens are Th2-biasing and pro-inflammatory; thus they can enhance the secretion of interferon-gamma and potentiate macrophage activation; on the other hand, testosterone is Th1-skewing and may inhibit the immune response [Reference Neyrolles and Quintana-Murci11, Reference Calippe25–Reference Flanagan28]. This may have accounted for the marginal higher treatment success rate observed in women. However, following HIV infection, it appears the effect of sex hormone-induced immunomodulation in women is lost. Indeed, our study suggests that with HIV infection, there appears to be a reversal of the immunoprotective effect of female sex – as being a HIV-positive female was a predictor for unsuccessful TB outcome. There have been recent calls for the evaluation of the role of sex on outcomes of infectious diseases in general [Reference Flanagan28], and in TB patients in particular [Reference Neyrolles and Quintana-Murci11, Reference Nhamoyebonde and Leslie27]. Our study supports the need for a detailed evaluation of the impact of sex hormones on TB epidemiology and treatment outcomes as well as its relationship with HIV. The reason why rural male TB patients were not at higher risk than females of having unsuccessful outcomes was not clear. It may be that the impact of the immunosuppressive effect of the male sex hormone on TB outcomes was being countered by economic and social barriers faced by rural women in accessing TB services. There is, however, a need for further studies to confirm/refute this. In previous studies male sex have been found to predict unsuccessful TB treatment outcomes in the UK and Malaysia [Reference Atif29, Reference Ditah30]
Irrespective of the patients' gender; care at the public facility, having extrapulmonary TB, HIV co-infection and older age were independent predictors for unsuccessful outcomes. The reasons why patients who received care at the public facility and those with extrapulmonary TB had poorer treatment outcomes are not clear. It may be that TB patients who visited the tertiary (public) hospital were more ill. There is therefore a need for interventions to reduce unsuccessful treatment outcomes in these patient groups. HIV co-infection was also a predictor of unsuccessful outcome irrespective of patients' gender. This is supported by other studies where similar results were documented [Reference Maruza31, Reference Ukwaja32]. Older age was another independent predictor for unsuccessful outcome. This may be due to a higher reduction in immunity with increasing age, higher delay in seeking care in older patients – which might lead to worsening of the disease [Reference Ukwaja33], or it may be because older patients had a higher rate of loss to follow-up during treatment [Reference Ukwaja32]. Previous studies have documented a higher risk of unsuccessful TB outcomes with increasing age in TB patients [Reference Atif29, Reference Ditah30, Reference Ukwaja32]. Furthermore, the reason why being an older male was a predictor for unsuccessful outcome and being an older female was not in this study was not clear. Treatment regimen was not a predictor for unsuccessful outcome in all patients, although there was a tendency towards a higher rate of successful outcomes in patients who received the shorter regimen.
In women, rural residence and receiving the 8-month regimen were found to be additional predictors for unsuccessful outcome. The reason(s) why female patients who were rural residents had higher rates of unsuccessful treatment outcomes may be because in our setting rural patients are poorer and do face substantial socio-economic barriers in accessing TB services [Reference Ukwaja33–Reference Ukwaja35]; these barriers are more likely to affect women than men. A previous study has suggested that poorer TB patients are less able to complete TB treatment [Reference Wei36]. The reason why women who received the longer regimen had a higher risk of unsuccessful outcome was not clear. It is reassuring that this regimen is no longer administered.
There are, however, a number of strengths and limitations of this study that require elucidation. This was a retrospective study based on routinely collected surveillance data – thus, our findings may reflect operational reality. However, because we could not collect additional data from the study participants, we were unable to assess for relationships between gender disparities in TB incidence/treatment outcomes and other important variables, e.g. comorbidities (diabetes mellitus), duration of symptoms, health-seeking behaviour, educational status, and income levels which may affect TB notifications/outcomes. Moreover, as TB/HIV collaborative activities were only recently expanded in Ebonyi state, other important data like details of antiretroviral therapy use, cotrimoxazole prophylaxis and CD4+ count were not adequately collected in the TB treatment registers; these may affect TB treatment outcomes. Furthermore, our study was conducted in a high TB/HIV under-resourced setting in southeastern Nigeria; it may not be representative of the whole country. However, the enrolled patients in our study were representative of Ebonyi state and Nigeria with respect to rural residence and burden of TB/HIV co-infection, reducing concerns that the studied sample was systematically different than the Nigerian population as a whole. Although our findings may not be generalizable to all settings, it might be applicable to similar high TB/HIV settings where patients are managed by a TB control programme with significant resource limitations. Finally, sputum culture for Mycobacterium sp. and their drug sensitivity testing are not performed routinely by the NTP for TB patients. Indeed, it is recommended (if available) only for patients who had failed the re-treatment anti-TB regimen [2]. Therefore, we are unable to report on the burden of drug resistant-TB in the study population. However, with the ongoing expansion of the Xpert (GeneXpert) MTB/RIF testing kits by the NTP [Reference Gidado and Obasanya37], the burden of rifampicin-resistant TB in Nigeria may soon be adequately documented.
In conclusion, more male than female TB patients were notified and treated during the study period and men were in general older than women. Compared to men, treatment success rates were marginally higher in women mainly due to higher treatment failure rates in men; and women had significantly higher rates of sputum conversion following treatment. These findings demonstrate gender disparities in TB profile and treatment outcomes in Nigeria and have implications that could modify NTP policy. We recommend that: (1) urgent strategies to reduce treatment default and mortality in men and women with TB need to be implemented; (2) TB case finding in older women should be improved; (3) specific measures to reduce unsuccessful outcomes for patients in the high-risk groups should be implemented; (4) socioeconomic interventions to improve treatment success for rural TB patients, especially women, should be implemented; and (5) further studies on the impact of biological factors on TB treatment outcomes in men and women need to be carried out and the influence of HIV co-infection, sex hormones and poverty assessed.
ACKNOWLEDGMENTS
We acknowledge all the staff of the National Tuberculosis Control Programme, Ebonyi state, the Centre for Development and Reproductive Health (CDRH) and all healthcare workers who participated in the meticulous data collection and reporting for their contributions. This research was supported through an operational research writing grant, which is a component of the Wave 3 TBREACH grant from the WHO/Stop TB Partnership with funds from the Canadian International Development Agency (CIDA).
DECLARATION OF INTEREST
None.









