Chronic obstructive pulmonary disease (COPD) is a progressive life-threatening chronic respiratory disease that commonly causes breathlessness with recurrent exacerbations and serious illness(Reference Labaki and Rosenberg1,Reference Barnes2) . According to the Global Burden of Disease Study, COPD affected about 251 million people worldwide as of 2016(Reference Mathers and Loncar3). An estimated 3·17 million deaths were caused by COPD in 2015 (accounting for 5 % of all deaths globally in that year). The primary risk factor of COPD is exposure to tobacco smoke, which causes oxidative stress of lung parenchyma and peripheral airways and triggers chronic inflammatory responses(Reference Barnes2,Reference Celli, Decramer and Wedzicha4–Reference MacNee9) . Thus, drugs or supplements with anti-inflammatory properties may be of potential benefit for reducing risk of COPD.
Glucosamine is a very popular non-vitamin, non-mineral dietary supplement in many countries(Reference Qato, Wilder and Schumm10,Reference Sibbritt, Adams and Lui11) and commonly taken for osteoarthritis and joint pain(Reference Jordan, Arden and Doherty12–Reference Runhaar, Rozendaal and van Middelkoop15). A number of laboratory(Reference Sibbritt, Adams and Lui11,Reference Largo, Alvarez-Soria and Díez-Ortego16–Reference Rajapakse, Kim and Mendis18) , animal(Reference Campo, Avenoso and Campo19–Reference Wen, Tang and Chang21) and human studies(Reference Kantor, Lampe and Navarro22–Reference Kantor, O’Connell and Du24) have shown that glucosamine may have anti-inflammatory properties. Notably, different from other drugs with anti-inflammatory properties, glucosamine is considered relatively safe because it has no known serious adverse effects, such as intracerebral or gastrointestinal haemorrhage(Reference Thorat and Cuzick25–Reference Solomon, Wittes and Finn27). Thus, there is a substantial interest in assessing whether regular use of glucosamine is inversely associated with the risk of COPD. Moreover, if an association exists between glucosamine use and COPD risk, it is clinically important to determine whether smoking is a potential confounding factor or an effect modifier in the association.
We therefore evaluated the association of regular glucosamine use with the risk of incident COPD using data from the UK Biobank, a large-scale cohort of more than half a million participants. Furthermore, we explored whether the association between glucosamine use and incident COPD risk varied by different smoking subgroups.
Methods
Study setting and participants
The UK Biobank is a valuable research resource with the aim of widely exploring the prevention, diagnosis and treatment of the most common and life-threatening illnesses(Reference Sudlow, Gallacher and Allen28). As detailed elsewhere(Reference Fry, Littlejohns and Sudlow29), this prospective cohort recruited approximately half a million community-based participants aged 40–69 years from across the UK between 2006 and 2010. At baseline, each participant completed a touchscreen self-reported questionnaire and a face-to-face oral interview at one of twenty-two assessment centres after signing an informed consent. Then, they had standardised anthropometric measurements taken and provided biological samples. Follow-up information was collected through linking to the national routine health-related data resources. We excluded participants who dropped out during the study (n 1329), those with missing information on glucosamine use (n 6156), those with history of COPD at baseline (n 9476), as well as those with missing values on smoking status before analyses (n 1860). Therefore, our analyses included 483 703 participants. Furthermore, participants with missing information on smoking pack-years (n 72 134) were also excluded (Fig. 1). The research activities were approved by the North West Multi-Center Research Ethics Committee (London, UK). Additionally, ethics approvals were obtained from the National Information Governance Board for Health & Social Care in England and Wales and the Community Health Index Advisory Group in Scotland.
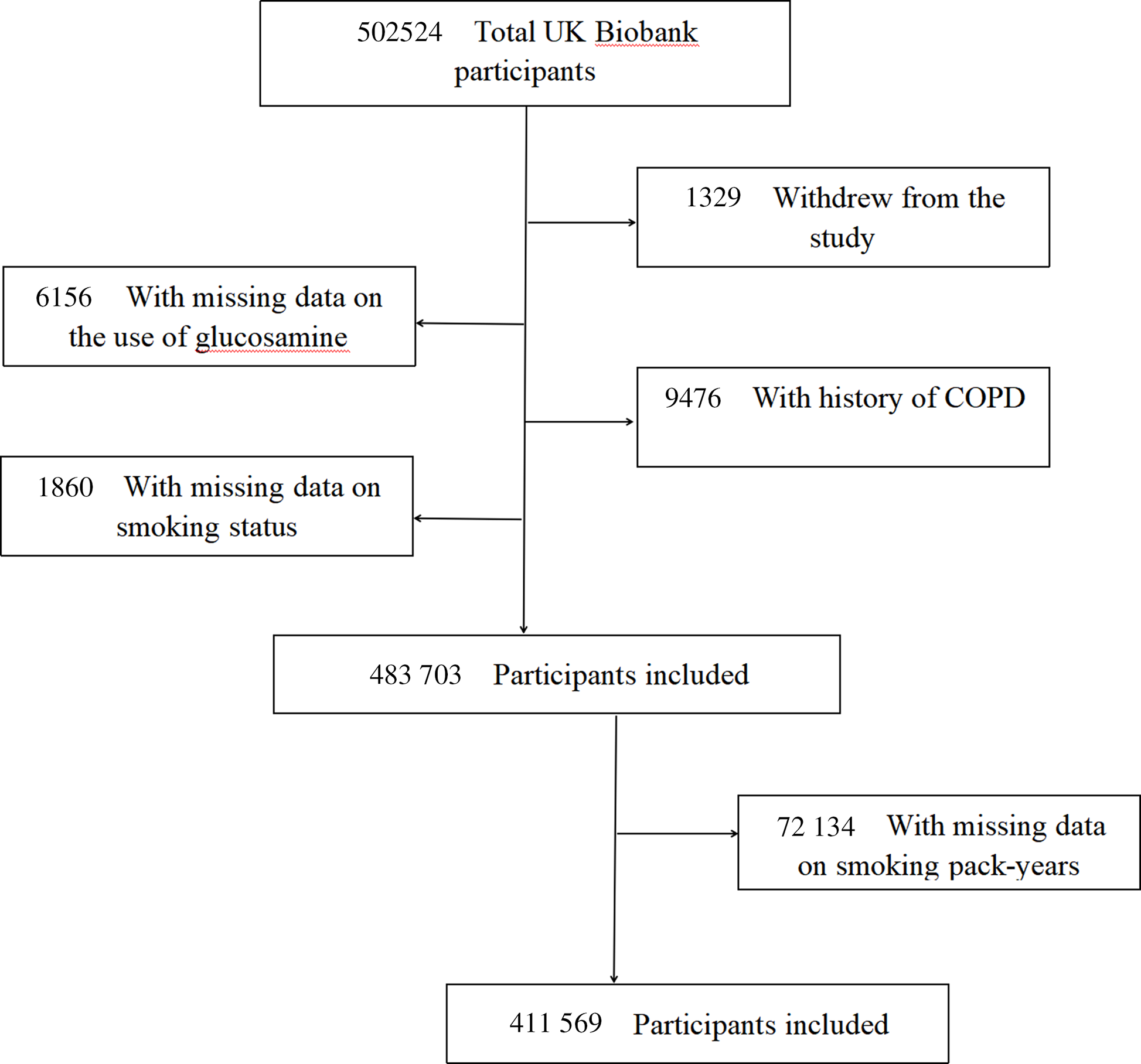
Fig. 1. Flow chart of participant enrolment.
Assessment of regular glucosamine use
One of the questions in the baseline electronic questionnaire was ‘Do you regularly take any of the following?’. Each participant could select answers from a list of supplements, including glucosamine, fish oil, Se, Fe, Zn and Ca, or select a final option of ‘none of the above’ indicating that they took none of listed supplements. According to this information, we scored regular glucosamine supplement use as ‘1 = yes’ or ‘0 = no’.
Assessment of smoking
Information on smoking was collected by touchscreen electronic questionnaire at baseline. All eligible participants were classified as the following groups: never smokers, former smokers or current smokers based on their smoking status; or none smokers (0 pack-years), hardly ever smokers (0·1–10·0 pack-years), light smokers (10·1–20·0 pack-years), moderate smokers (20·1–30·0 pack-years), heavy smokers (> 30 pack-years) or the group of no available data according to their smoking pack-years. Smoking pack-years is a composite index of smoking based on number of cigarettes per day, age stopped smoking and age start smoking. Detailed definitions of smoking status and smoking pack-years were provided in Supplementary Table S1 in the Supplement.
Outcome ascertainment
Incident COPD in this cohort was determined based on having a diagnosis in hospital admission electronic records or in death register databases. Death information was obtained via linking to national death registries. Causes of death and diseases diagnoses in the UK Biobank cohort were coded using the International Classification of Diseases, 9th Revision and 10th Revision. COPD was defined as International Classification of Diseases, 9th Revision codes 492, 492·0, 492·8, 496.X or 10th Revision codes J43, J43·0, J43·1, J43·2, J43·8, J43·9, J44, J44·0, J44·1, J44·8 and J44·9. We calculated follow-up person-years of included participants from the date of conducting the baseline survey until the date of the first COPD diagnosis, date of death or the date of the end of follow-up (February 28, 2017, for Scotland and February 25, 2018, for England and Wales), whichever was earliest.
Ascertainment of covariates
We collected information on risk factors of COPD and correlates of glucosamine use at baseline to assess several potential confounders: socio-demographic characteristics (age, sex, ethnicity, education and household income), lifestyle and health-related behavioural factors (BMI, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking and occupational exposure), drug use (cholesterol-lowering medication, anti-hypertensive drug, insulin, aspirin, non-aspirin non-steroidal anti-inflammatory drugs (NSAID), chondroitin and cortisone), vitamin supplementation (vitamin A, vitamin B, vitamin C, vitamin D, vitamin E, folic acid and multivitamin), mineral or other dietary supplementation (fish oil, Se, Zn, Fe and Ca) and disease history (CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, arthritis and asthma). BMI was calculated as the weight divided by the square of the height (kg/m2). According to the validated International Physical Activity Questionnaire embedded in the touchscreen electronic questionnaire, the intensity and duration of physical activity were ascertained(Reference Bassett30). Passive smoking was defined as being exposed to other people’s tobacco smoking for more than one hour per week in the home or other relatively closed space. Occupational exposure was classified based on the self-reported frequency of exposure to diesel exhaust, paints, thinners, glues, pesticides, asbestos or other chemical smog in daily work. Further details on covariates are available on the UK Biobank website (www.ukbiobank.ac.UK).
Statistical analysis
The distribution of participants’ baseline characteristics was summarised by habitual glucosamine use as mean (standard deviation (sd)) for normally distributed continuous variables, median (interquartile range) for skewed distributed continuous variables or as number (percentage (%)) for categorical variables. Correspondingly, t test, Wilcoxon rank sum test or χ 2 were used to examine the difference of participant characteristics. We conducted multiple imputation with chained equations to assigned missing values (all missing values <3 %), thus minimising the possibility for inferential bias(Reference Buuren and Groothuis-Oudshoorn31).
Cox proportional hazards models with progressive adjustment for potential confounders were performed to calculate hazard ratios (HR) along with 95 % CI for associations of habitual glucosamine use or smoking with incident COPD risk, respectively. Model 1 was adjusted for age (numerical variable), sex (male or female), ethnicity (white or others), education (lower qualification or higher qualification), household income (<£18 000, £18 000–£30 999, £31 000–£51 999, £52 000–£100 000 or >£100 000), BMI (numerical variable), alcohol consumption (never, 1–2, 3–4 or ≥ 5 times/week), physical activity (regular physical activity, some physical activity or no regular physical activity), fruit consumption (<1, 1–3, 3–4 or ≥ 4 servings/d), vegetable consumption (<3, 3–4, 4–6 or ≥ 6 servings/d), passive smoking (yes or no), occupational exposure (rarely/never, sometimes or often), CVD (yes or no), hypertension (yes or no), diabetes (yes or no), cancer (yes or no), chronic pulmonary infections (yes or no), rheumatoid arthritis (yes or no), osteoarthritis (yes or no), joint pain (yes or no), arthritis (yes or no), asthma (yes or no), aspirin use (yes or no) and non-aspirin NSAID use (yes or no). In model 2, we adjusted for not only the same confounding factors as model 1 but also for the following variables: smoking status (former, current or never), cholesterol-lowering medication use (yes or no), anti-hypertensive drug use (yes or no), insulin use (yes or no), vitamin supplementation (yes or no), mineral or other dietary supplementation (yes or no), glucosamine use (yes or no), chondroitin use (yes or no) and cortisone use (yes or no). In model 3, smoking status was replaced by smoking pack-years (numerical variable), which represented participants’ total active smoking exposure. It is worth noting that glucosamine use and smoking status/pack-years were adjusted for each other. We used a Schoenfeld residuals plot to evaluate the proportional hazards assumption; no violation of this assumption was observed in our study. The linear trend test was performed by treating each smoking category as a continuous variable. Additionally, incidence rates of COPD per 1000 person-years were calculated.
Multivariable adjusted stratified analyses were conducted by smoking status (never, former or current) or smoking pack-years (none, hardly ever, light, moderate or heavy) to explore the association between glucosamine use and incident COPD. Additionally, we also conducted stratified analysis by sex (male or female), age (<60 or ≥ 60 years), obesity (BMI < 30 or ≥ 30 kg/m2), CVD (yes or no), hypertension (yes or no), diabetes (yes or no), cancer (yes or no), osteoarthritis (yes or no), joint pain (yes or no), asthma (yes or no), vitamin use (yes or no), minerals and other dietary supplements use (yes or no), aspirin use (yes or no) and non-aspirin NSAID use (yes or no) to assess the potential modification effect. The statistical interaction was evaluated by adding the cross-product term of the stratifying variable with glucosamine use to fully adjusted Cox regression models.
We performed several sensitivity analyses to examine the robustness of the results. First, we categorised all eligible participants as glucosamine/chondroitin users (who taken glucosamine alone, or chondroitin alone or taken both of them) or glucosamine/chondroitin nonusers (who taken neither glucosamine nor chondroitin) to explore the association between glucosamine/chondroitin use and incident COPD. Second, we excluded participants who used chondroitin. Third, participants who developed COPD within the first two years of follow-up were removed in order to minimise the possibility of reverse causation. Final, given the poor health of NSAID users, we excluded participants who taken aspirin or non-aspirin NSAID at baseline.
The population-attributable fraction, an estimated fraction of all COPD cases that would not have occurred if all individuals would have been in the less smoking category and/or have taken glucosamine(Reference Mansournia and Altman32), was calculated according to Miettinen’s formula(Reference Miettinen33). We used R software version 3.6.1 (R Development Core Team) to conduct all statistical analyses; all tests in our study were 2-sided, and P < 0·05 was considered statistically significant.
Results
Baseline characteristics of participants
Table 1 shows the baseline features of the eligible participants stratified by glucosamine use status (users v. nonusers). Of the 483 703 participants (mean (sd) age, 56·5 (8·1) years), 263 992 (54·6 %) were male. At baseline, a total of 92 593 (19·1 %) participants self-reported habitual glucosamine use. Compared with nonusers, glucosamine users were older, more likely to be female, white, current non-smokers and passive smokers. They were also likely to have a lower education qualification, lower household income, more physically activity, more alcohol consumption and more frequent occupational exposure. They also had a higher prevalence of cancer, osteoarthritis, joint pain and arthritis, but a lower prevalence of diabetes, hypertension and CVD. Additionally, glucosamine users more frequently took NSAID, vitamins, chondroitin and minerals and other dietary supplements than nonusers. Of note, glucosamine users have a lower C-reactive protein concentration than nonusers.
Table 1. Baseline characteristics of study participants by glucosamine use
(Numbers and percentages; mean values and standard deviations)
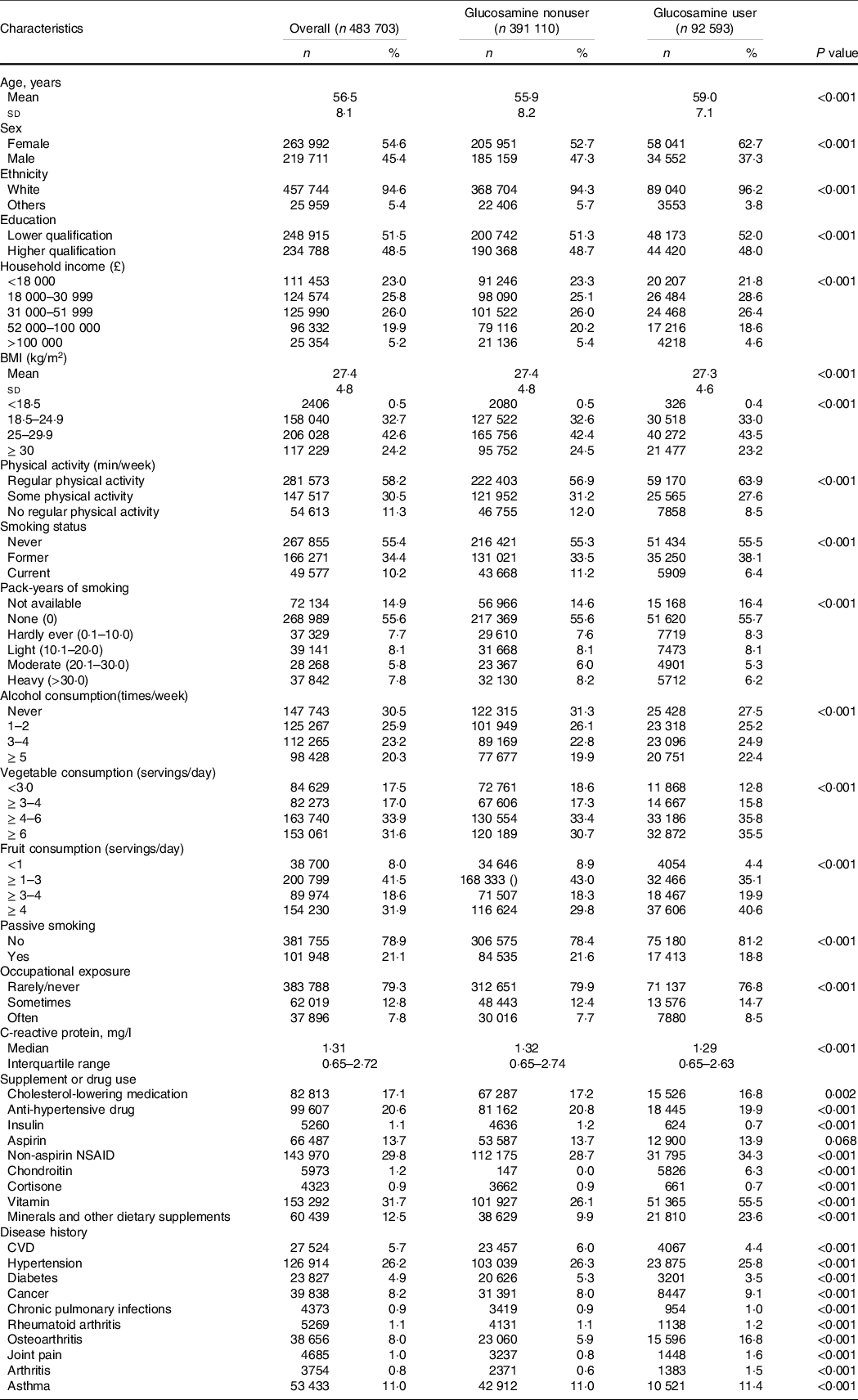
Values are numbers (%) unless stated otherwise.
NSAID, nonsteroidal anti-inflammatory drug.
Associations between smoking and incident chronic obstructive pulmonary disease risk
Table 2 shows the associations between smoking and incident COPD. During a median follow-up of 8·96 years (interquartile range 8·29–9·53 years), a total of 9016 participants developed incident COPD. Incidence rates and HR (P for trend < 0·001) of incident COPD were increased in association with smoking status and increases of smoking pack-years. Compared with never smokers, the multivariable adjusted HR of former smokers and current smokers was 3·09 (95 % CI, 2·91, 3·28) and 10·61 (95 % CI, 9·96, 11·29)); similarly, the multivariable adjusted HR of hardly ever smokers, light smokers, moderate smokers and heavy smokers was 1·92 (95 % CI, 1·72, 2·14), 3·24 (95 % CI, 2·99, 3·53), 5·58 (95 % CI, 5·17, 6·01) and 10·42 (95 % CI, 9·79, 11·09), respectively (Table 2).
Table 2. Risk of chronic obstructive pulmonary disease (COPD) according to smoking categories
(Numbers and percentages; Hazard ratios and 95 % confidence intervals)
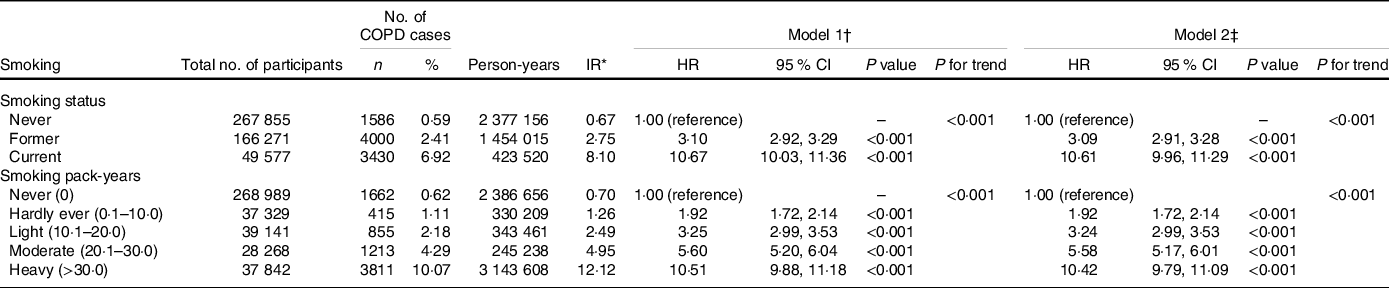
COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IR, incidence rate.
* Incidence rates are provided per 1000 person-years.
† Model 1: Cox proportional hazards regression adjusted for age and sex, ethnicity, education, household income, BMI, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, aspirin use and non-aspirin NSAID use.
‡ Model 2: Cox proportional hazards regression adjusted for model 1 and cholesterol-lowering medication use, anti-hypertensive drug use, insulin use, vitamin use, minerals and other dietary supplements use, chondroitin use, glucosamine use and cortisone use.
Inverse associations between regular glucosamine use and incident chronic obstructive pulmonary disease risk
Table 3 shows the associations between habitual glucosamine use and incident COPD. In model 1, we found a significant inverse association between regular use of glucosamine and risk of incident COPD (HR = 0·73, 95 % CI, 0·69, 0·78; P < 0·001). Regular glucosamine use was significantly associated with a reduced risk of incident COPD with the multivariable adjusted HR of 0·80 (95 % CI, 0·75, 0·85; P < 0·001) and 0·78 (95 % CI, 0·73, 0·84; P < 0·001) in model 2 and model 3, respectively.
Table 3. Risk of incident chronic obstructive pulmonary disease (COPD) according to glucosamine use
(Numbers and percentages; Hazard ratios and 95 % confidence intervals)

COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IR, incidence rate.
* Incidence rates are provided per 1000 person-years.
† Model 1: Cox proportional hazards regression adjusted for age and sex, ethnicity, education, household income, BMI, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, aspirin use and non-aspirin NSAID use.
‡ Model 2: Cox proportional hazards regression adjusted for model 1 and smoking status, cholesterol-lowering medication use, anti-hypertensive drug use, insulin use, vitamin use, minerals and other dietary supplements use, chondroitin use and cortisone use.
§ Model 3: Cox proportional hazards regression adjusted for model 1 and smoking pack-years (numerical variable), cholesterol-lowering medication use, anti-hypertensive drug use, insulin use, vitamin use, minerals and other dietary supplements use, chondroitin use and cortisone use.
|| Model 4: Cox proportional hazards regression adjusted for model 2 and smoking pack-years.
Joint associations of glucosamine use and smoking with incident chronic obstructive pulmonary disease
We conducted multivariable adjusted stratified analyses by smoking to explore whether smoking status or smoking pack-years could modify the association between habitual glucosamine use and incident COPD risk (Table 4). We found that glucosamine use was associated with a lower risk on incident COPD with the adjusted HR of 0·84 (95 % CI, 0·73, 0·96; P = 0·009), 0·84 (95 % CI, 0·77, 0·92; P < 0·001) and 0·71 (95 % CI, 0·63, 0·80; P < 0·001) among never, former and current smokers. The hazard ratio of incident COPD associated with glucosamine use was 0·82 (95 % CI 0·72, 0·94; P = 0·003) among none smokers, 0·78 (95 % CI 0·68, 1·02; P = 0·065) among hardly ever smokers, 0·70 (95 % CI 0·57, 0·85; P < 0·001) among light smokers, 0·66 (95 % CI 0·55, 0·79; P < 0·001) among moderate smokers and 0·85 (95 % CI 0·77, 0·94; P = 0·002) among heavy smokers. We observed a significant interaction between glucosamine use and smoking pack-years on the risk of incident COPD (P for interaction = 0·019). A similar interaction pattern was not found in the analyses stratified by smoking status (P for interaction = 0·078).
Table 4. Risk of chronic obstructive pulmonary disease (COPD) according to glucosamine use within each smoking category
(Numbers and percentages; Hazard ratios and 95 % confidence intervals)
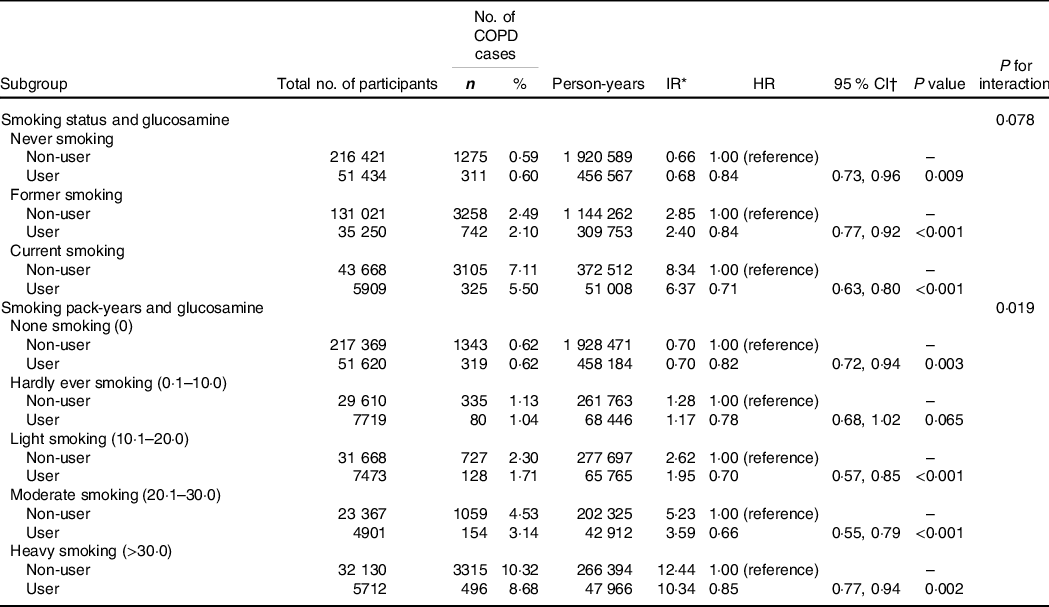
HR, hazard ratio; IR, incidence rate.
* Incidence rates are provided per 1000 person-years.
† Cox proportional hazards regression adjusted for age, sex, ethnicity, education, household income, BMI, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, cholesterol-lowering medication use, anti-hypertensive drug use, insulin use, aspirin use, non-aspirin NSAID use, vitamin use, minerals and other dietary supplements use, chondroitin use and cortisone use.
Other subgroup analyses
We conducted stratified analyses for the association of glucosamine use with incident COPD risk according to other potential risk factors using the fully adjusted model (Fig. 2). The association between the use of glucosamine use and the risk of incident COPD was seemed not to be significantly modified by sex, age, obesity, CVD, hypertension, diabetes, cancer, osteoarthritis, joint pain, asthma, vitamin use, minerals and other dietary supplements use, aspirin use or Non-aspirin NSAID (all P for interaction > 0·05).
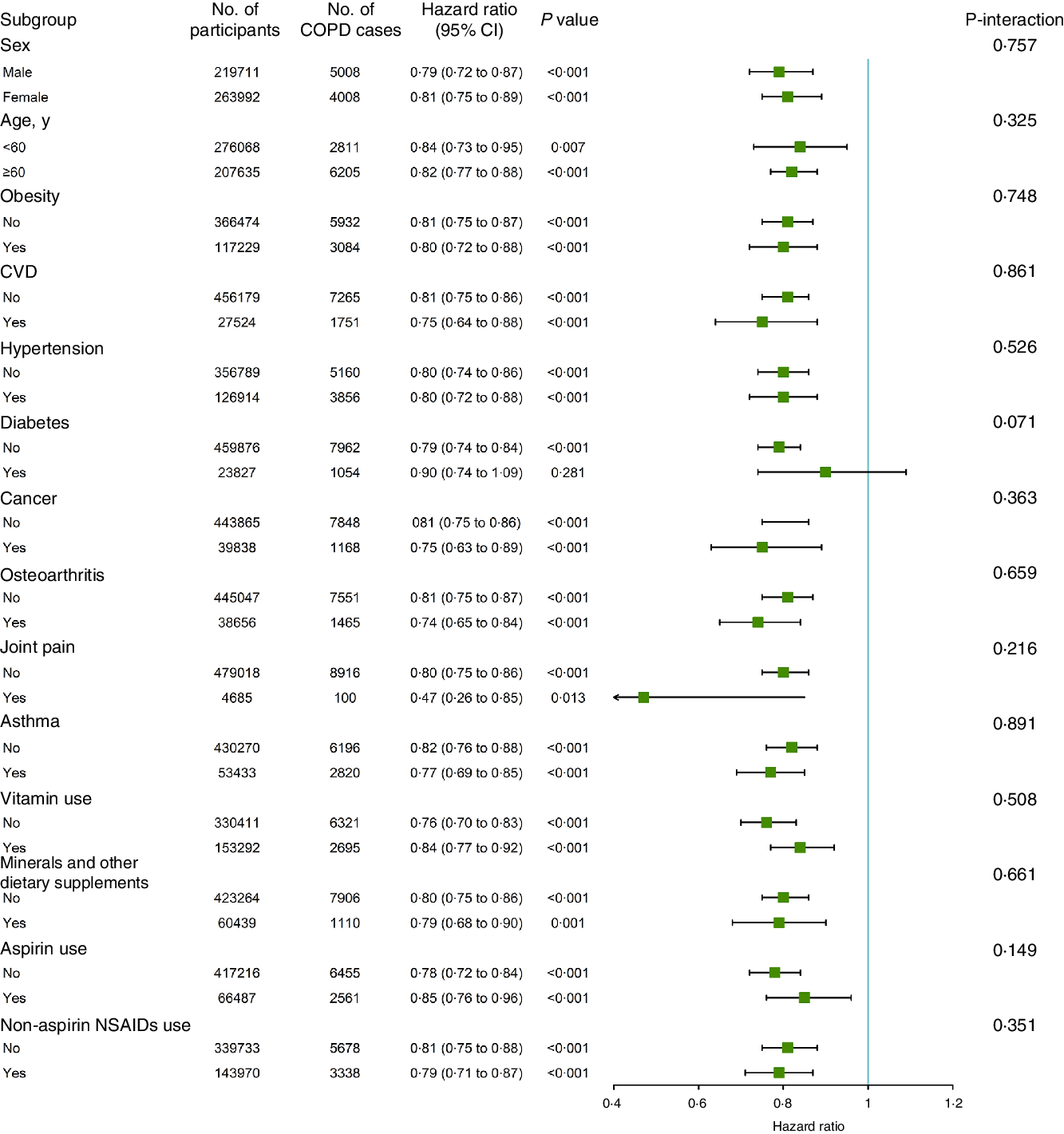
Fig. 2. Association between glucosamine use and incident chronic obstructive pulmonary disease (COPD) risk stratified by other potential risk factors.
Sensitivity analyses
When all eligible participants were categorised as glucosamine/chondroitin users or nonusers, no substantial changes of results were observed, whether or not stratified by smoking (online Supplementary Tables S2 and S3). Likewise, when we removed participants who regularly took chondroitin (online Supplementary Table S4), or who reported COPD events within the first two years of follow-up (online Supplementary Table S5), there was no significant change on the association between glucosamine use and incident COPD. When the analyses were restricted NSAID nonusers, the result still shown significant inverse associations between the glucosamine use and the new-onset COPD events risk (online Supplementary Table S6).
Population attributable fractions
We calculated the population-attributable fraction. If current smokers who were glucosamine nonusers regularly took glucosamine supplements before the baseline evaluation, new-onset COPD cases could be reduced by 25·53 % (95 % CI, 17·49, 32·70). If all individuals who currently smoke actively and did not regularly take glucosamine quit smoking during follow-up and regularly took glucosamine before baseline, 61·74 % (95 % CI, 59·98, 63·30) of incident COPD could be prevented. If they had never smoked and regularly took glucosamine before baseline, the new-onset COPD events could be reduced by 83·72 % (95 % CI, 82·79, 84·52).
When the heavy smokers and glucosamine nonusers were defined as the reference group, COPD events could be reduced by 13·83 % (95 % CI, 6·35, 20·62), 61·45 % (95 % CI, 455·37, 66·63), 75·29 % (95 % CI, 71·14, 78·75), 83·19 % (95 % CI, 79·52, 86·08) and 83·83 % (95 % CI, 82·91, 84·72) for groups who (i) regularly took glucosamine, (ii) were moderate smokers as well as regularly took glucosamine, (iii) were light smokers as well as regularly took glucosamine, (iv) were hardly ever smokers as well as regularly took glucosamine and (v) were none smokers as well as regularly took glucosamine before baseline, respectively (Table 5).
Table 5. Population aetiologic fraction according to smoking category and glucosamine use
(Hazard ratios and 95 % confidence intervals)
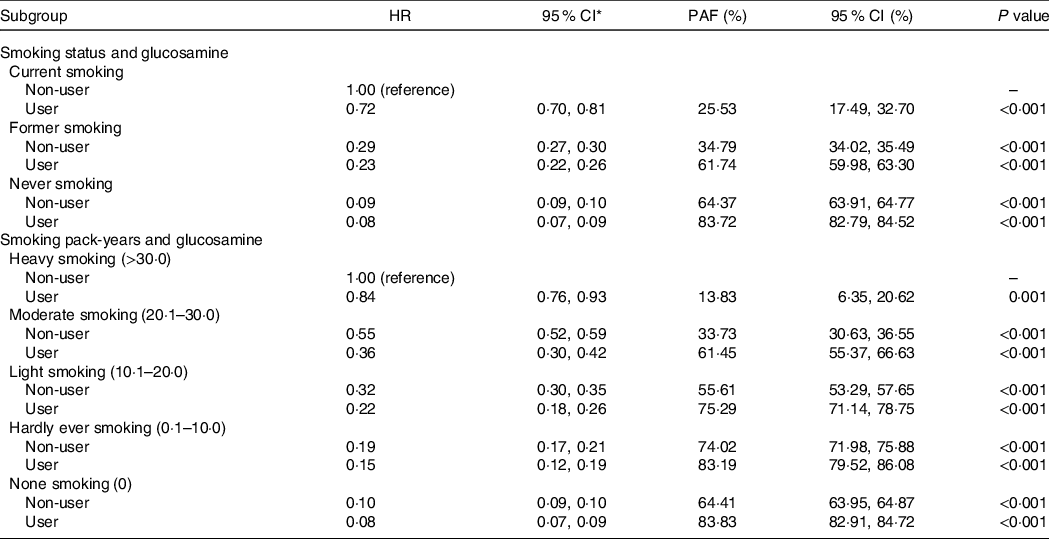
HR, hazard ratio; PAF, population aetiologic fraction; NSAID, non-steroidal anti-inflammatory drugs.
†Cox proportional hazards regression adjusted for age, sex, ethnicity, education, household income, BMI, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, cholesterol-lowering medication use, anti-hypertensive drug use, insulin use and aspirin use, non-aspirin NSAID use, vitamin use, minerals and other dietary supplements use, chondroitin use and cortisone use.
Discussion
In this large-scale prospective cohort study involving 483 703 individuals, we found a significant inverse association of regular glucosamine use with incident COPD risk. This association was independent of potential confounders, including socio-economics characteristics, lifestyle and health-related behavioural factors, other dietary supplementation consumption, health conditions and drugs use. Furthermore, we observed a prominent interaction between glucosamine use and smoking pack-years on the risk of incident COPD.
In our study, 19·1 % of participants reported regular glucosamine use. Similarly, regular glucosamine use has been reported by 22·0 % of Australians aged 45+ years(Reference Sibbritt, Adams and Lui11) and 16·7 % of Americans aged 50+ years(Reference Qato, Wilder and Schumm10). To our knowledge, this is first study exploring the relationship between regular glucosamine use and incident COPD risk in human populations; thus, it is challenging to contextualise our finding with respect to the current knowledge base. Of note, several previous epidemiological studies have demonstrated glucosamine supplementation use was associated with a lower risk of incident diseases(Reference Satia, Littman and Slatore34–Reference Ma, Li and Sun38) and mortality(Reference Ma, Li and Sun38–Reference Li, Gao and Chung40). For instance, the VITamins And Lifestyle cohort study suggested negative associations of glucosamine use with incident lung cancer and colorectal cancer(Reference Satia, Littman and Slatore34,Reference Brasky, Lampe and Slatore35) . A recent study including 43 163 individuals from the Health Professionals Follow-up Study, Nurses’ Health Study, and Nurses’ Health Study II indicated that glucosamine use may have a protective effect on new-onset colorectal carcinogenesis events in older adults(Reference Lee, Cao and Zong36). The results from a surveillance, epidemiology and end results cancer registry suggested that glucosamine use was associated with a lower total mortality and with reductions of some broad causes of death in adults aged 50–76 years(Reference Bell, Kantor and Lampe39). Based on the UK Biobank cohort, Ma and colleagues found that habitual use of glucosamine was associated with lower risk of multiple conditions including, 17 % for incident type 2 diabetes(Reference Ma, Li and Zhou37), 18 % for incident CHD, 18 % for incident stroke and 22 % for CVD death(Reference Ma, Li and Sun38); Li and colleagues also reported that regular use of glucosamine was associated lower risk of multiple conditions including 15 % for all-cause mortality, 27 % for respiratory mortality, 26 % for digestive mortality, 18 % for CVD mortality and 6 % for cancer mortality(Reference Li, Gao and Chung40).
Although the precise biological mechanisms underlying the inverse association between regular use of glucosamine and risk of COPD remain to be determined, a wealth of emerging evidence provides various plausible explanations for the inverse association. Given the detrimental roles of inflammation in the development of COPD, we assumed that glucosamine supplementation might reduce the incident COPD risk at least partly through the anti-inflammatory effect. First, glucosamine may achieve its beneficial effect by reducing the translocation of nuclear factor kappa B and inhibiting the activation of nuclear factor kappa B, a well-characterised transcription factor involved in inflammatory response, and thus suppress the subsequent cascade of related events(Reference du Souich41,Reference Zahedipour, Dalirfardouei and Karimi42) . An animal study in which mice received an injection of lipopolysaccharide to induce endotoxic shock and systemic inflammation has demonstrated that glucosamine decreased the production of inflammatory cytokines related to nuclear factor kappa B activation(Reference Silva, Olivon and Mestriner43). A number of vitro and vivo studies suggested glucosamine use decreases levels of various proinflammatory cytokines(Reference Largo, Alvarez-Soria and Díez-Ortego16–Reference Chou, Vergnolle and McDougall20,Reference Hong, Park and Choi44–Reference Zhang, Chen and Dou48) , such as IL-1β, PGE2, COX-2 and TNF-α, which lies downstream stream of nuclear factor kappa B (Reference Romano, Mallardo and Romano49,Reference Li, Withoff and Verma50) . Second, some evidence, even if limited, indicated a potential mechanism by which glucosamine exerts an anti-inflammatory effect by regulating the metabolic, composition, or immunological activities of gut microbiota(Reference Hao, Shang and Liu51,Reference Coulson, Butt and Vecchio52) . Additionally, glucosamine, a significant component of intestinal mucin, could potentially affect intestinal immune responses and gut permeability(Reference Lee, Han and Ryu53,Reference Sicard, Vogeleer and Le Bihan54) .
Several previous human studies(Reference Kantor, Lampe and Navarro22–Reference Kantor, O’Connell and Du24,Reference Kantor, Lampe and Vaughan55) also have shown that circulating levels of C-reactive protein, a marker of low-grade systemic inflammation, were significantly lower in glucosamine users compared with nonusers; a small randomised controlled cross-over clinical trial also suggested that glucosamine use may significantly reduce C-reactive protein concentrations compared with the placebo group(Reference Navarro, White and Kantor23). Interestingly, we found that the C-reactive protein level at baseline was significantly lower in glucosamine users than in nonusers.
Although some previous studies suggested the positive effect of aspirin use among patients with COPD(Reference Goto, Faridi and Camargo56,Reference Fawzy, Putcha and Aaron57) , results of our study (online Supplementary Table S7) and other studies showed that the use of either aspirin or ibuprofen was not associated with COPD or lung function(Reference McKeever, Lewis and Smit58,Reference Aaron, Schwartz and Hoffman59) . The potential benefit of glucosamine supplementation for incident COPD was significant and was independent of a series of potential confounding factors. Additionally, glucosamine is considered relatively safe because no known serious adverse effects related to it have been reported(Reference Wen, Tang and Chang21). Furthermore, even though we observed a significantly positive association between cigarette smoking and new-onset COPD events, regular glucosamine use could reverse this relationship to a certain extent. Glucosamine seems promising as a recommended protective agent for the prevention of COPD. It should be noted that, given the limitations in this study, including potential residual confounding, and a sparse dose and duration information, PAR may provide an overestimation and misrepresentation of the potential preventive effect glucosamine has on COPD.
This study has several notable strengths, including the large sample size, the prospective cohort study design and detailed information on socio-economic characteristics, lifestyle and health-related behavioural factors, supplementation use, drugs use, health status and other covariates.
Nevertheless, there are several limitations of our study. First, information on regular dietary supplements intake was obtained via self-reported baseline questionnaire, and detailed information on the formulation of supplements was not collected. Some participants who take compound preparation containing glucosamine and chondroitin might only reported the glucosamine use. Second, dosage, duration and frequency of glucosamine use were not collected; further studies are needed to explore those associations. Third, glucosamine users were likely to have a healthier lifestyle. However, it is difficult to distinguish the effects of a healthy lifestyle from habitual glucosamine use in this observational study. Although we carefully adjusted for a great many potential confounding factors, the observed inverse associations might have been driven by some unmeasured health-related lifestyles. Additionally, the possibility of residual confounders due to other unknown factors or imprecise measurements cannot be eliminated in our study.
Conclusions
In summary, this large-scale prospective cohort study showed that a considerable proportion (19·1 %) of the UK population reported regular glucosamine use. Our study suggests that regular glucosamine use is inversely associated with incident COPD. The inverse association was modified by smoking pack-years. Functional studies and clinical trials are needed to enhance our understanding of potential benefits of glucosamine supplementation for incident COPD.
Acknowledgements
The authors would like to thank the UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 43795.
This work was supported by the National Natural Science Foundation of China (81973109, 82173588), the Project Supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), the Guangdong Basic and Applied Basic Research Foundation (2021A1515011629), the National Key Research and Development Program of China (2018YFC2000404), the Construction of High-level University of Guangdong (G621331128, G621339832), the Guangzhou Science and Technology Project (202002030255) and the National Institutes of Health/National Institute on Aging of USA (P30-AG028716). The funders played no role in the study design or implementation; data collection, management, analysis or interpretation; manuscript preparation, review or approval or the decision to submit the manuscript for publication.
X. R. Z. and P. D. Z. are joint first authors, contributed to the concept and design, statistical analyses and had primary responsibility for drafting the manuscript. They contributed equally to this article. Z. H. L., P. Y., X. M. W. and H. M. L. contributed to the data cleaning. F. L., J. D. W., Y. S., D. S., P. L. C., W. F. Z., Q. M. H., D. L., Z. H. W. and V. B. K. contributed to the analysis or interpretation of the data and the editing of this manuscript. C. M. ([email protected]) acquired the data and directed the study and is considered the corresponding author. All authors critically reviewed the manuscript for important intellectual content. The corresponding author (C. M.) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. C. M. is the study guarantor and takes responsibility for the integrity of the data and the accuracy of the data analyses.
The authors declare that they have no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452100372X










