1 Introduction
Schizophrenia (SCH) is a severe, complex, multifactorial disorder that affects approximately 0.7% of the world population Reference van Os and Kapur[1, Reference Wittchen, Jacobi, Rehm, Gustavsson, Svensson and Jonsson2]. In recent years, there have been changes in the approach to SCH, with a focus on the search for biological markers Reference Ding, Guo, Hu, Jiang and Wang[3–Reference Pickard7]. In this sense, there is renewed interest in immune-inflammatory changes and their associated oxidative-nitrosative consequences as key pathophysiological mechanisms of the neuroprogressive pathways of this disorder Reference Kirkpatrick and Miller[8].
Several hypotheses involving inflammatory processes caused both by external and endogenous factors have been implicated in SCH Reference Kirkpatrick and Miller[8–Reference Leza, Garcia-Bueno, Bioque, Arango, Parellada and Do11]. Inflammation is a complex biological protective mechanism, but when excessive in intensity or time, it becomes harmful. Intracellular events, such as cytoplasmic/-nuclear transcription factors, mainly kappaB (NFκB), control the expression of several oxidative and nitrosative mediators through activation of inducible enzymes, as key factors in this regulation. Furthermore, intercellular elements such as cytokines and chemokines are crucial elements of proper inflammatory response. Such a complex defense mechanism is finely regulated by compensatory anti-inflammatory pathways Reference García-Bueno, Caso and Leza[12]. One of these mechanisms involves cyclopentenone prostaglandins (PGs) such as 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) Reference Prasad, Giri, Singh and Singh[13], one of the proposed endogenous ligands for the gamma isoform of peroxisome proliferator-activated nuclear receptors, PPARγ. The PPARγ is a transcription factor that mitigates inflammation by repressing the expression of pro-inflammatory cytokines and the inducible isoforms of COX and NOS: COX-2 and iNOS Reference Bernardo, Levi and Minghetti[14, Reference Subbaramaiah, Lin, Hart and Dannenberg15].
Early studies in SCH described elevations in plasma levels of pro-inflammatory cytokines Reference Garcia-Alvarez, Garcia-Portilla, Gonzalez-Blanco, Saiz Martinez, de la Fuente-Tomas and Menendez-Miranda[16, Reference Müller and Schwarz17] and decreases in anti-inflammatory cytokines Reference Maes, Bosmans, Ranjan, Vandoolaeghe, Meltzer and De Ley[18]. However, recent studies focus on intra- and intercellular biochemical pathways controlling inflammatory response and found a systemic imbalance in some pro-/anti-inflammatory mediators in these patients Reference Leza, Garcia-Bueno, Bioque, Arango, Parellada and Do[11]. Most of the imbalance studies have been carried out in early stages of the disease: subtle alterations in immune-inflammatory mediators and oxidative-nitrosative stress have already been found at disease onset Reference Borovcanin, Jovanovic, Radosavljevic, Djukic Dejanovic, Bankovic and Arsenijevic[19, Reference Martinez-Cengotitabengoa, Mac-Dowell, Leza, Mico, Fernandez and Echevarria20]. In particular, there was an increase in levels of pro-inflammatory NFκB, iNOS and COX-2 in patients with a first episode of psychosis (FEP) compared with healthy controls (HC) Reference García-Bueno, Bioque, Mac-Dowell, Barcones, Martinez-Cengotitabengoa and Pina-Camacho[21] and of PGE2 in patients with established SCH Reference Kaiya, Uematsu, Ofuji, Nishida, Takeuchi and Nozaki[22]. Furthermore, the inhibitory subunit of NFκB, 15d-PGJ2, and PPARγ expression and transcriptional activity were lower in FEP patients Reference García-Bueno, Bioque, Mac-Dowell, Barcones, Martinez-Cengotitabengoa and Pina-Camacho[21] along with anti-inflammatory PGs in peripheral monocytes in patients with established SCH Reference Martínez-Gras, Pérez-Nievas, García-Bueno, Madrigal, Andrés-Esteban and Rodríguez-Jiménez[23]. In addition, the systemic pro-/anti-inflammatory deregulation found in FEP became more severe after a 1-year follow-up Reference García-Bueno, Bioque, MacDowell, Santabarbara, Martinez-Cengotitabengoa and Moreno[24].
C-reactive protein (CRP) is a widely used biomarker of systemic inflammation, and higher CRP levels have been reported in SCH compared with HC patients Reference Fernandes, Steiner, Bernstein, Dodd, Pasco and Dean[25–Reference Singh and Chaudhuri29], even in patients without antipsychotic treatment Reference Fawzi, Fawzi and Said[30]. For homocysteine (Hcy), an intermediate amino acid containing a sulfhydryl radical that can act as an oxidant, the results are controversial. While some studies found higher levels of this oxidative stress biomarker in several subgroups of SCH Reference Dietrich-Muszalska, Olas, Glowacki and Bald[31–Reference Nishi, Numata, Tajima, Kinoshita, Kikuchi and Shimodera33] compared with HC patients Reference Dietrich-Muszalska, Olas, Glowacki and Bald[31, Reference Bouaziz, Ayedi, Sidhom, Kallel, Rafrafi and Jomaa34], others did not Reference Di Lorenzo, Amoretti, Baldini, Soli, Landi and Pollutri[35, Reference Wysokinski and Kloszewska36].
A review of the literature suggests that there could be an overlap in peripheral immune-inflammatory mechanisms across severe mental disorders (SMD), what justify our research Reference Catala-Lopez, Moher and Tabares-Seisdedos[37]. For example, Goldsmith et al. (2016) describe similarities in the pattern of cytokine alterations in SCH, bipolar disorder (BD), and major depressive disorder (MDD) during the acute (significant increases of IL-6, TNF-α, sIL-2R, and IL-1RA) and chronic (significant increases of IL-6, sIL-2R, and IL-1β) phases of illness that may suggest the existence of common underlying pathways for immune dysfunction Reference Goldsmith, Rapaport and Miller[38]. Thus, it is necessary to evaluate markers of inflammation and immune activation across the whole psychosis continuum. In this sense, it was recently found that there is a strong increase in the levels of inflammatory activity in SCH and a relatively lesser increase in schizoaffective and affective disorders respectively Reference Morch, Dieset, Faerden, Hope, Aas and Nerhus[39]. In line with the proposed psychosis continuum model, the aim of our study was to identify the potential differential intra- and intercellular biochemical pathways controlling inflammatory response and their oxidative-nitrosative consequences in SCH, compared with BD and healthy controls (HC). An additional aim was to identify whether there are differential pathways of the psychopathological (positive, negative, and depressive), cognitive, and functional dimensions of SCH.
2 Methods
2.1 Study design
Cross-sectional, naturalistic study of a cohort of SCH patients in outpatient treatment at two mental health centers in Oviedo (Corredoria and Ería) in northern Spain, and their controls (BD patients and HC). The Clinical Research Ethics Committee of Hospital Universitario Central de Asturias in Oviedo approved the study protocol. All participants gave written informed consent prior to their enrollment.
2.2 Participants
Of the 325 participants recruited, a total of 305 individuals were included in the analysis after removing outliers. Of these, 123 were SCH patients (mean age 40.75, 67.5% males), 102 BD patients (mean age 48.37, 37.3% males), and 80 HC (mean age 35.81, 38.8% males). If an alfa error of 5% with a power of 90% is considered for the ANCOVA tests performed in this work, an effect size value of f=0.2634 is obtained. This value, according to Cohen (1988) can be considered as a medium effect size and suitable for our research purposes Reference Cohen[40].
Outpatients attending their regular appointments with their clinicians were offered to participate in the study and healthy controls were recruited by snowball sampling. There were statistically significant differences among the groups in age and gender (F=27.668, P<0.0001; Chi2=25.707, P<0.0001).
Inclusion criteria for SCH and BD were: (1) DSM-IV-TR diagnosis of SCH or BD; (2) age>17 years; and (3) written informed consent. Inclusion criteria for HC: (1) no past or current mental disorder (DSM-IV-TR diagnosis) and (2) written informed consent. Exclusion criteria for patients and controls were: (1) no written informed consent; (2) physical comorbidity that could interfere with immune-inflammatory biomarkers (acute infection, fever, acute allergies, cancer, or autoimmune diseases) was determined by directly asking patients about them; (3) treatment with immunosuppressive drugs or vaccines within the 6 months prior to enrollment in the study, or treatment with anti-inflammatory drugs within the two days prior to blood collection.
2.3 Assessments
2.3.1 Psychometric instruments
Psychopathology in SCH patients was evaluated using the Spanish versions of the Clinical Global Impression (CGI) Reference Guy[41], which assesses the severity in global psychopathology, and the Positive and Negative Syndrome Scale (PANSS) Reference Peralta and Cuesta[42], which measures the severity of positive, negative, and general psychopathology symptoms. In addition, the Negative Symptom Assessment-16 (NSA-16) Reference Garcia-Alvarez, Garcia-Portilla, Saiz, Al-Halabi, Bobes-Bascaran and Bascaran[43] and the Hamilton Depression Rating Scale (HDRS) Reference Bobes, Bulbena, Luque, Dal-Re, Ballesteros and Ibarra[44] were employed to assess the severity of negative and depressive symptoms, respectively. The Screen for Cognitive Impairment in Psychiatry (SCIP) Reference Pino, Guilera, Rojo, Gomez-Benito, Bernardo and Crespo-Facorro[45] was used to assess cognition. Finally, to assess patient functioning, we used the Personal and Social Performance (PSP) Reference Garcia-Portilla, Saiz, Bousono, Bascaran, Guzman-Quilo and Bobes[46].
2.3.2 Specimen collection and preparation
Venous blood samples (10mL) were collected at 8:00 am after fasting overnight.
2.3.3 Biochemical analyses of PBMC samples
To perform all biochemical analyses, PBMC samples were first fractionated into cytosolic and nuclear extracts:
• preparation of cytosolic and nuclear extracts: to obtain a high purity nuclear fraction, practically without cytosolic contamination Reference García-Bueno, Bioque, MacDowell, Santabarbara, Martinez-Cengotitabengoa and Moreno[24] in order to ensure isolation of active components of transcription factors a widely utilized method was employed;
• western blot analysis: the protein levels of inducible nitric oxide synthase (iNOS), cyclooxygenases 1 and 2 (COX-1, COX-2), NLRP3 inflammasome, and the inhibitory subunit of NFκB, IκBα, in the cytosolic extracts and the protein levels of NFκB, the gamma isoform of peroxisome proliferator-activated nuclear receptors, PPARγ, and the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in the nuclear extracts from PBMC samples were quantified by western blot (WB) analysis.
2.3.4 Biochemical analyses in plasma and serum
Prostaglandin levels: plasma levels of COX byproducts PGE2 and 15d-PGJ2 were measured by enzyme immunoassay (EIA) using reagents in kit form (Prostaglandin E2 EIA Kit-Monoclonal, Cayman Chemical Europe, Tallinn, Estonia; and 15-deoxy-Δ12,14- Prostaglandin J2 ELISA Kit, DRG Diagnostics, Marburg, Germany, respectively) following manufacturer's instructions.
Lipid peroxidation: this was assayed by thiobarbituric acid reactive substances (TBARS) assay (Cayman Chemical Europe), based on the reaction of malondialdehyde (MDA) and thiobarbituric acid (TBA) at high temperature (95°C) and acidic conditions. The MDA-TBA adduct formed was measured colorimetrically at 530–540nm (Synergy 2).
Hcy and CRP: both were determined at the Hospital Universitario Central de Asturias (HUCA) laboratory. Plasma levels of homocysteine (Hcy) were obtained by inmunochemiluminescence (Inmulite 2000 system, SIEMENS) and serum levels of CRP by immunoturbidimetric analysis (CRPLX, Roche/Hitachi Cobas c501).
2.4 Statistical analyses
Demographic variables are described as means (SD) and percentages. The outliers of the immune-inflammatory markers were identified by SPSS in the box plots by deleting the individual data points and removed before any statistical analysis. The number of outliers was different for each inflammatory and oxidative-nitrosative marker. The most frequently marker removed was 15d-PGJ2for SCH and HC while for BD disorder was PGE2. Differences between SCH patients and both control groups (BD patients and HC) were assessed using an analysis of covariance (ANCOVA) when the assumptions were met; otherwise, a Quade test Reference Quade[47] — a non-parametric alternative — was used. As there were statistically significant differences among the three groups in age and gender, we controlled for these variables when comparing them. In addition, when comparing SCH and BD patients, we controlled for other potential confounders (length of illness in years, number of cigarettes per day, and body mass index [BMI]). A post hoc analysis (Bonferroni) was done to determine which groups had significant differences.
Finally, to determine the potential differential biomarkers for the psychopathological, cognitive, and functional dimensions of SCH, partial correlations with Bonferroni correction were used in which we controlled for potential confounders (age, length of illness in years, number of cigarettes per day, BMI, and number of antipsychotics). In addition, to control for gender, these same partial correlations were done for men and women separately.
3 Results
3.1 Demographic and clinical characteristics of the sample of SCH patients
The majority were male, never married, living with their family of origin, had a primary or secondary level of education level and a work status of “not working” (Table 1). The mean length of illness was 13.85 (10.9) years, and 56.1% of the patients had a more than 10-year history.
Table 1 Demographic and clinical characteristics of the sample of patients with schizophrenia.
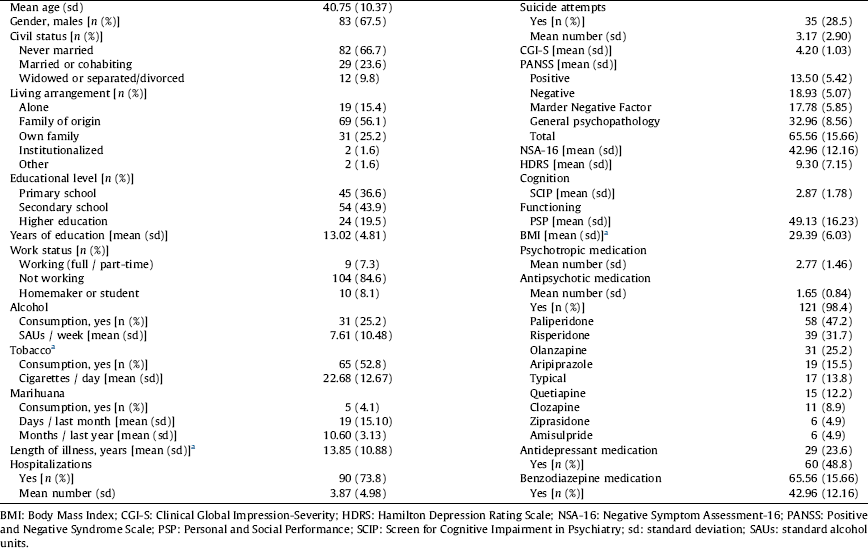
a Patients with BD: 45.1% smokers [mean of cigarettes per day among smokers: 17.17 (10.49)], mean length of illness 20.58 (11.85) years and BMI 28.98 (4.95).
3.2 Pharmacological treatment of SCH and BD patients
The pharmacological treatment of SCH patients is described in Table 1. Regarding BD patients, 100% were under mood stabilizers (42.6% lithium, 38.2% quetiapine, 27.7% valproic acid, 5% lamotrigine and 1% oxcarbamacepine), 35.3% were under antipsychotics (11.8% olanzapine, 6.9% aripiprazole, 5.9% paliperidone, 2% paliperidone one-month long-acting injectable, 5.9% risperidone and 3% others), 49% antidepressants (28.6% SSRI, 8.8% SNRI, 3.9% tricyclics and 8.8% others) and 61.8% benzodiazepines.
3.3 Biomarkers of inflammation in SCH patients compared with BD patients and HC
Table 2 and Figs. 1 and 2 show the comparisons among the three groups after controlling age and gender. Regarding the pro-inflammatory parameters, expression of NFκB, a crucial inflammatory transcription factor, was higher in SCH than in BC and in HC (P<0.001, P=0.003, respectively), although no difference was found between BD and HC. COX-1 expression was statistically higher in both patient groups than in HC (P<0.0001), although no differences were found between SCH and BD. Similarly, one of its soluble products, PGE2, was higher in SCH when compared with BC and with HC (P<0.001). Plasma levels of CPR described a gradient among the three groups, that was statistically higher in SCH and BD patients than in HC (P<0.0001, P=0.048), and levels were higher in SCH than in BD patients (P=0.031).
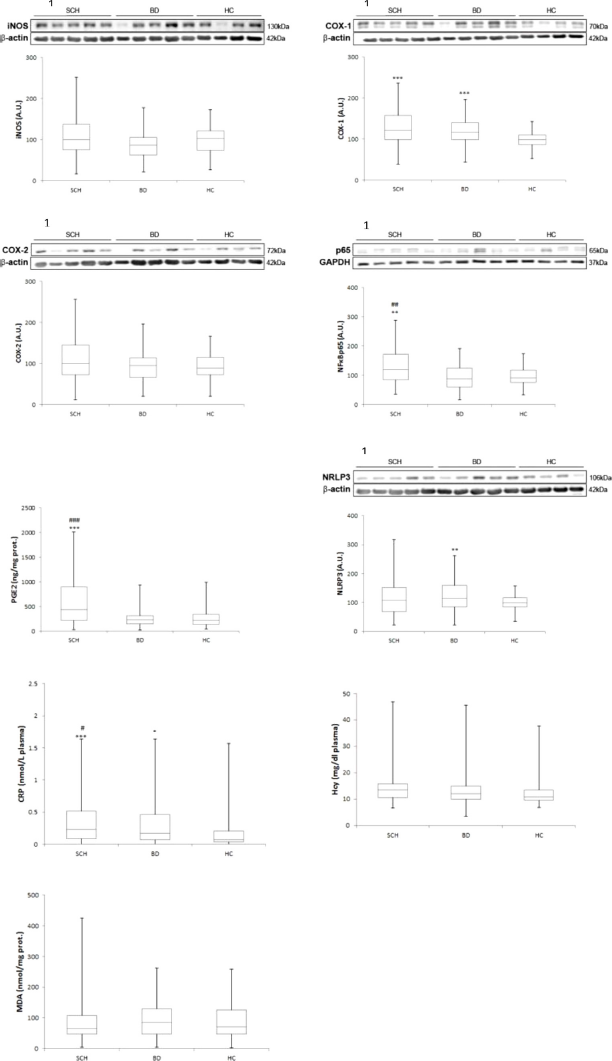
Fig 1. Mean differences (SD) on inflammatory / oxidative components in plasma and PBMC in patients with schizophrenia compared with bipolar disorder and healthy controls. Western blot analysis of iNOS, COX-1, COX-2, NFkB p65 and NLRP3. Plasma levels of CRP, HCy and MDA. AU, arbitrary units. ANCOVA and Quade test were used. 1Densitometric analysis of the proteins studied and with the corresponding housekeeping proteins used as loading control (in cytosolic and nuclear extracts). *: compared with HC; * P≤0.05; ** P≤0.005; *** P≤0.0005. #: compared with BD; # P≤0.05; ## P≤0.005; ### P≤0.0005.

Fig 2. Mean differences (SD) on antiinflammatory / antioxidant components in plasma and PBMC in patients with schizophrenia compared with bipolar disorder and healthy controls. Western blot analysis of PPARg, IkBa and NRF2. Plasma levels of 15d-PGJ2. AU, arbitrary units. ANCOVA and Quade test were used. 1Densitometric analysis of the proteins studied and with the corresponding housekeeping proteins used as loading control (in cytosolic and nuclear extracts). *: compared with HC; * P ≤ 0.05; ** P ≤ 0.005; *** P ≤ 0.0005. #: compared with BD; # P ≤ 0.05; ## P ≤ 0.005; ### P ≤ 0.0005.
Table 2 Comparisons of inflammatory and oxidative-nitrosative markers among groups after controlling for age and gender.
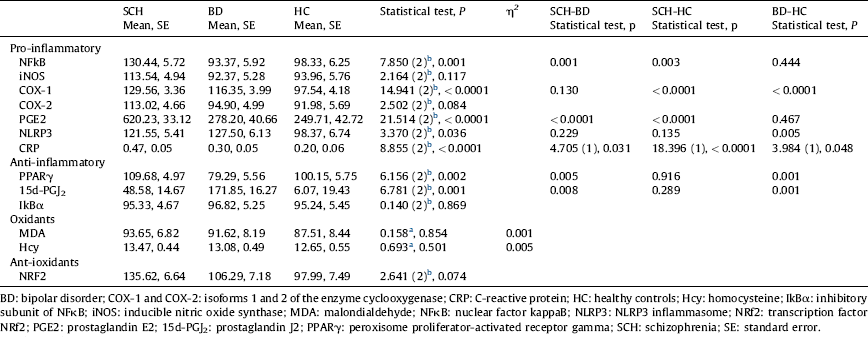
a ANCOVA.
Regarding anti-inflammatory parameters (nuclear expression of PPARγ, a transcription factor that mitigates inflammation, and plasma levels of 15d-PGJ2, an anti-inflammatory PG and one of the endogenous PPARγ ligands), no differences were found between SCH patients and HC. However, BD patients showed changes in these anti-inflammatory pathways: lower PPARγ and higher 15d-PGJ2 levels than SCH patients (P=0.005; P=0.008) and HC (P=0.001; P=0.001).
No between-group differences were found in other oxidative/antioxidative parameters.
3.4 Biomarkers of inflammation in SCH compared with BD
The results of the comparison between SCH and BD after controlling for age, gender, length of illness, number of cigarettes per day, and BMI are shown in Table 3. As can be observed, the changes in inflammatory markers in SCH (NFκB, P=0.001, PGE2, P<0.0001) and anti-inflammatory markers in BD (PPARγ, P=0.041, 15d-PGJ2, P=0.028) remain statistically significant. Interestingly, after controlling for these new confounders, the inducible isoforms of the specific inflammatory enzymes iNOS and COX-2 show statistically higher levels in SCH than BD (P=0.019; P=0.040). However, the differences in expression of constitutive COX-1 and plasma CRP levels between SCH and BD disappear.
Table 3 Comparisons of inflammatory and oxidative-nitrosative markers between SCH and BD after controlling for age, gender, length of illness, cigarettes/day, and BMI.
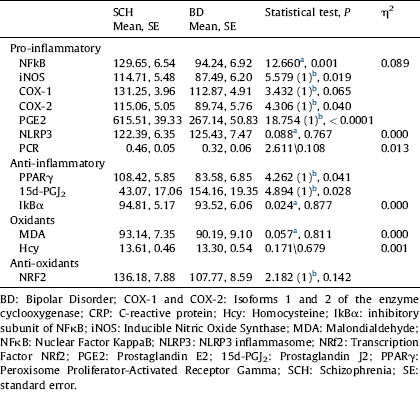
a ANCOVA.
b Quade test. Covariables: age, gender, length of illness, cigarettes/day, and BMI.
3.5 Potential differential inflammatory biomarkers of the psychopathological, cognitive, and functional dimensions of SCH
In males, there was a slight positive correlation of the positive dimension with PPARγ (r=0.27, P<0.05) and the four measures of the negative dimension with Hcy (PANSS negative r=0.34, P<0.01; PANSS Marder Negative Factor r=0.38, P<0.01; NSA global r=0.27, P<0.05; NSA total r=0.38, P<0.01). Furthermore, the cognitive dimension negatively correlated with PPARγ (r=−0.30, P<0.05) (Table 4). After Bonferroni correction only the correlations between Hcy and PANSS Negative, PANSS Marder Negative Factor and NSA total remained significant. None of the immune-inflammatory biomarkers correlated with the depressive dimension or functioning.
Table 4 Partial correlation coefficients between psychopathological, cognitive, and functional dimensions of SCH and inflammatory and oxidative-nitrosative biomarkers after controlling for age, length of illness, cigarettes/day, and BMI (males).
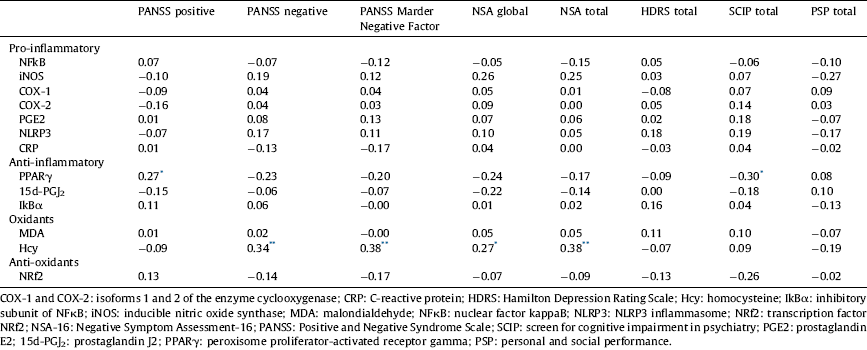
* <0.05.
** <0.01.
In females, the positive dimension positively correlated with Hcy (r=0.44, P<0.05) and, as in males, the cognitive dimension negatively correlated with PPARγ (r=−0.44, P<0.05) (Table 5). However, after Bonferroni correction none of the correlations were significant.
Table 5 Partial correlation coefficients between psychopathological, cognitive, and functional dimensions of SCH and inflammatory and oxidative-nitrosative biomarkers after controlling for age, length of illness, cigarettes/day, and BMI (females).

* <0.05.
** <0.01.
4 Discussion
Our study adds support to the hypotheses of an inflammatory process underlying the pathophysiology of SCH, as we found a significant increase in the levels of crucial intra- and intercellular inflammatory response components: NFκB, iNOS, COX-2, and PGE2 in SCH patients compared with BD and HC. Furthermore, unlike the previous literature on the subject, our results allow us to suggest that these alterations may be considered a specific pro-inflammatory biomarker pattern for SCH, since we included patients with another SMD (BD) as a control group.
Regarding pro-inflammatory parameters, after controlling for age and gender, we found significantly higher levels of COX-1 in SCH and BD patients compared with HC. Therefore, COX-1 may be considered a biomarker of SMD. However, the little literature there is on this constitutive form of cyclooxygenase did not find significant differences in its levels in the post-mortem frontal cortex of SCH patients vs. HC Reference Yagami, Koma and Yamamoto[48]. We also identified a significant increase in other pro-inflammatory mediators, NFκB and PGE2, in SCH patients compared with BD patients and HC, suggesting that these mediators may be specific markers of SCH. The activity of these two markers has also been found to be significantly increased in FEP Reference García-Bueno, Bioque, Mac-Dowell, Barcones, Martinez-Cengotitabengoa and Pina-Camacho[21, Reference García-Bueno, Bioque, MacDowell, Santabarbara, Martinez-Cengotitabengoa and Moreno24]. In patients with established SCH, PGE2 levels were significantly higher than in HC Reference Kaiya, Uematsu, Ofuji, Nishida, Takeuchi and Nozaki[22, Reference Martínez-Gras, Pérez-Nievas, García-Bueno, Madrigal, Andrés-Esteban and Rodríguez-Jiménez23], while another study did not detect statistically significant differences between SCH and BD patients Reference Vuksan-Cusa, Sagud and Jakovljević[49].
Previous studies on the widely used and nonspecific marker of CRP levels have also found higher levels in SCH than HC Reference Fernandes, Steiner, Bernstein, Dodd, Pasco and Dean[25–Reference Fawzi, Fawzi and Said30] independent of antipsychotic treatment Reference Fawzi, Fawzi and Said[30] and disease progression Reference Fawzi, Fawzi and Said[30]. The novelty of the present study is that this marker shows a differential gradient among the three groups, with significantly higher levels in patients with SCH than BD and HC, and also significantly higher levels in patients with BD than HC.
The comparison between patients with SCH and BD allowed us to control for some additional relevant confounding variables. In this case, the previous markers of SCH, NFκB and PGE2, remained statistically significantly increased, and two new inducible pro-inflammatory and pro-oxidant enzymes, iNOS and COX-2, reached statistical significance. On the contrary, the difference in CRP levels between SCH and BD disappeared, suggesting that it may be related to other associated inflammatory conditions, such as smoking, obesity, or chronicity. In general, our results indicate that the inflammatory component is more pronounced in SCH than in BD, as patients with SCH showed significantly higher NFκB, PGE2, iNOS, and COX-2 activity. It could be argued that our BD patients using lithium may have decreased levels of these biomarkers through the inhibition of GSK-3 Reference Noma, Takahashi-Yanaga, Arioka, Mori and Sasaguri[50]. However, when we compared them between BD patients with and without lithium we did not find any statistical significance (data not shown). Interestingly, after controlling for relevant confounding variables, specific intra- and intercellular inflammatory parameters remain significant in SCH, whereas other more nonspecific parameters disappear, supporting their possible role as trait biomarkers.
Regarding anti-inflammatory mediators, although previous studies in acutely decompensated chronic SCH Reference Martínez-Gras, Pérez-Nievas, García-Bueno, Madrigal, Andrés-Esteban and Rodríguez-Jiménez[23] and FEP Reference García-Bueno, Bioque, Mac-Dowell, Barcones, Martinez-Cengotitabengoa and Pina-Camacho[21, Reference García-Bueno, Bioque, MacDowell, Santabarbara, Martinez-Cengotitabengoa and Moreno24] found significantly lower levels of 15d-PGJ2 and PPARγ, we found no significant differences between SCH patients and HC. A possible explanation could be a difference in functioning of the pro-/anti-inflammatory system over the stages/phases of SCH, i.e., while in the early and acute phases of the disorder, the anti-inflammatory system is able to fight against activation of the pro-inflammatory system, in the late stages when negative symptoms predominate this ability is lost. Another explanation could be BMI. While in the studies of Martínez-Gras et al. (2011) and García-Bueno et al. (2013; 2014) mean BMI was normal (24.9kg/m2), the cohort studied here had a mean BMI of 29.4, at the upper limit of overweight. Although more studies are needed, the results presented here point toward a possible role of negative symptoms and increased BMI in the lack of anti-inflammatory system response in chronic SCH. Interestingly, such a response is still present in BD patients, with lower levels of PPARγ and higher levels of 15d-PGJ2 than the other two groups, which may represent compensatory mechanism.
With respect to Hcy, although it has been suggested that high levels are a risk factor for SCH Reference Nishi, Numata, Tajima, Kinoshita, Kikuchi and Shimodera[33, Reference Geller, Friger, Sela and Levine51–Reference Numata, Kinoshita, Tajima, Nishi, Imoto and Ohmori53], some studies, including our own research, find no differences between SCH patients and HC Reference Di Lorenzo, Amoretti, Baldini, Soli, Landi and Pollutri[35, Reference Wysokinski and Kloszewska36].
Regarding our aim of determining the potential differential pathways of the psychopathological (positive, negative, and depressive), cognitive, and functional dimensions of SCH, we found PPARγ was associated with the positive and cognitive dimensions. Higher levels of this anti-inflammatory parameter were related to lower cognitive impairment in both genders. Given the lack of pharmacological approaches to treating this dimension, this finding is of special importance and to our knowledge, this is the first report to show this relationship in chronic SCH patients. These results are in line with studies that have found a relationship between inflammation and poor cognitive performance Reference Ribeiro-Santos, Lucio Teixeira and Salgado[54]. Levels of inflammatory cytokines and higher levels of CRP have been related to cognitive impairment in SCH Reference Dickerson, Stallings, Origoni, Boronow and Yolken[55–Reference Schwarz, Izmailov, Spain, Barnes, Mapes and Guest60]. In SCH patients, PPARγ-dependent endogenous counterbalancing mechanisms seem to be active, compensating the inflammation and probably exerting the pro-energetic, neuroprotective, and antiexcitotoxic profile previously described in in vivo experimental models Reference Garcia-Bueno, Caso, Perez-Nievas, Lorenzo and Leza[61]. In FEP patients, after controlling for the possible effects of confounding factors, better performance on sustained attention tasks is associated with higher levels of anti-inflammatory signaling, which may suggest that this is also a protective factor for cognition Reference Cabrera, Bioque, Penadés, González-Pinto, Parellada and Bobes[62]. In addition, a negative association has been reported between cognitive impairment and oxidative stress Reference Martinez-Cengotitabengoa, Mac-Dowell, Leza, Mico, Fernandez and Echevarria[20].
With regard to Hcy, high levels have been related to the severity of psychopathology Reference Misiak, Frydecka, Slezak, Piotrowski and Kiejna[63, Reference Narayan, Verman, Kattimani, Ananthanarayanan and Adithan64], PANSS total score Reference Song, Fan, Li, Kennedy, Pang and Quan[65], and the acute phase of the disorder Reference Petronijevic, Radonjic, Ivkovic, Marinkovic, Piperski and Duricic[66]. In our case, Hcy levels were related to the positive dimension in females and the negative dimension in males, so these data are difficult to interpret, and more studies will be necessary. In the case of women, these data seem to be in line with studies finding a relationship between higher levels of immune-inflammatory parameters and positive symptoms Reference Fernandes, Steiner, Bernstein, Dodd, Pasco and Dean[25, Reference Dimitrov, Lee, Yantis, Valdez, Paredes and Braida67, Reference Kurian, Le-Niculescu, Patel, Bertram, Davis and Dike68] or acute stages of the disorder Reference Miller, Buckley, Seabolt, Mellor and Kirkpatrick[69]. Contrary, some studies have found a positive correlation between Hcy levels and the severity of negative symptoms Reference Bouaziz, Ayedi, Sidhom, Kallel, Rafrafi and Jomaa[34, Reference Misiak, Frydecka, Slezak, Piotrowski and Kiejna63, Reference Petronijevic, Radonjic, Ivkovic, Marinkovic, Piperski and Duricic66].
Some limitations of this study should be noted. First, there are some issues concerning the information obtained from the sample. In this sense, the lack of clinical information (cigarettes/day and BMI) of the HC group could overestimate the biomarkers differences found between this group and the others. Furthermore, physical comorbidities were asked directly to patients, so this information could be not as accurate as expected. Secondly, it is a cross-sectional study, so we cannot make a causal argument for the observed associations. Thirdly, as SCH and BD differ with respect to age (younger age of onset and thus younger age for a similar length of illness in SCH versus BD) and gender (a greater proportion of females with BD than SCH), it was not possible to match the three groups for these variables. Thus, we had to control for these confounding variables as covariates. Moreover, the sample included stable SCH patients on outpatient maintenance treatment, so it is possible that these results are specific to this subgroup of patients, jeopardizing the generalizability of our results to other subgroups with SCH, such us unstable patients. Another potential weakness of the study is antipsychotics as a confounding variable because they have an anti-inflammatory effect and they can induce changes in the weight and metabolism. To that end, we controlled the number of antipsychotics that each patient to mitigate this effect, because the study design did not allow us to control either for type of antipsychotic or equivalent dose of haloperidol, given the different effects of the antipsychotics on endocrine-metabolic and immune-inflammatory biomarkers. Finally, assessment of the SCH dimensions was not as precise as we would have liked, especially with regard to the cognitive and depressive dimensions. Cognition was assessed with a screening test, the SCIP, instead of a more powerful cognitive battery, and the depressive dimension with the HDRS, which is a scale that also includes anxiety symptoms, making it possible to overestimate depressive symptoms.
Key strengths deserve mention. First, we used two control groups, healthy subjects and another SMD with the aim of controlling and thereby increasing the specificity of our results. Secondly, we included a broad spectrum of inflammatory markers in both PBMC and plasma samples, allowing in-depth insights into relationships between multiple components of the pro- and anti-inflammatory signaling pathways. Thirdly, for assessing the negative dimension of the SCH, in addition to the PANSS negative subscale, we used its Marder Negative Factor and one more specific scale, the NSA. Finally, we excluded people with physical comorbidities or treatment with immunosuppressive or anti-inflammatory drugs, which could interfere with the status of the study's immune-inflammatory biomarkers.
With regard to implications for clinical practice, although more scientific evidence is needed, this study provides further evidence of the inflammatory hypothesis of SMD. It proposes a specific cluster of inflammatory biomarkers for established SCH (NFκB, iNOS, COX-2, and PGE2) that differentiates it from BD. Moreover, some biomarkers may be related to the positive, negative, and cognitive dimensions of SCH. In future, their pharmacological modulation may constitute a promising therapeutic target.
Authors’ contributions
LG-A, MPG-P, PSM and JB designed the study. LG-A, MPG-P, LF-T, LG-B, and PASM acquired the data. JCL and JRC perform biochemical analyses. LG-A and MPG-P conducted statistical analyses. LG-A and MPG-P wrote the 1st draft of the manuscript. The rest reviewed it and gave the final approval.
Disclosure of interest
Julio Bobes has received research grants and served as consultant, advisor or speaker for the companies: AB-Biotics, Adamed, Almirall, AstraZeneca, Bristol-Myers Squibb, Ferrer, Glaxo- Smith-Kline, Hoffman La Roche, Janssen-Cilag, Lilly, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Sanofi-Aventis, Servier, Shering-Plough and Shire, research funding from the Spanish Ministry of Economy and Competiveness–Centro de Investigación Biomedica en Red area de Salud Mental (CIBERSAM) and Instituto de Salud Carlos III-, Spanish Ministry of Health, Social Services and Equality – Plan Nacional sobre Drogas- and the 7th Framework Program of the European Union.
Leticia Garcia-Alvarez has received honoraria from the 7th Framework Program European Union and has served as speaker for Pfizer.
Maria Paz Garcia-Portilla has been a consultant to and/or has received honoraria/grants from Alianza Otsuka-Lundbeck, CIBERSAM, European Union (7th Framework Program), Hoffman La Roche, Instituto de Salud Carlos III, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Servier, Roche, and Rovi. Celso Iglesias-García has received grant/research support from Esteve, Grupo Ferrer Internacional, S.A., Otsuka Pharmaceuticals SA, Pfizer SLU, and Roche Farma, S.A.
Leticia González Blanco was supported by a grant from the Spanish Foundation of Psychiatry and Mental Health. Also, this author has received speaker fees and travel expenses for attending conferences from Janssen-Cilag, Otsuka, Lundbeck and Pfizer.
Pilar A. Saiz has been a consultant to or has received honoraria or grants from Adamed, AstraZeneca, Brainpharma, Bristol-Myers Squibb, CIBERSAM, Esteve, European Comission, Ferrer inCode, GlaxoSmithKline, Instituto de Salud Carlos III, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Pfizer, Plan Nacional Sobre Drogas, Rovi and Servier.
All other researchers declare that they have no competing interest.
Acknowledgments
The authors wish to thank for Sandra Rodríguez-Maus for her excellent technical assistance and Sharon Grevet for her English assistance. They were sponsored by research grants.
This work was partly supported by the CIBERSAM, the Spanish Ministry of Economy and Competitiveness (MINECO-SAF 2016/75500-R to JCL), Instituto de Salud Carlos III (Ref. PI13/02263 to JB and PI1402037 to PGP) and Fondos Europeos de Desarrollo Regional (FEDER).
JRC is a postdoctoral Ramón y Cajal Researcher (Spanish Ministry of Economy, Industry and Competitiveness).










Comments
No Comments have been published for this article.