Patients with a severe mental illness (SMI), mostly schizophrenia, other psychotic disorders, major depression or bipolar disorder, have almost twice the normal risk of premature death from cardiovascular disease, Reference Laursen, Wahlbeck, Hällgren, Westman, Ösby and Alinaghizadeh1 are more likely to have metabolic syndrome, Reference Zimmet, Magliano, Matsuzawa, Alberti and Shaw2 and have a life expectancy that is shortened by up to 30 years compared with the general population. Reference Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen and Asai3 The increased mortality risk is associated with side-effects of antipsychotic medication as well as unhealthy but modifiable lifestyle behaviours. Reference McEvoy, Meyer, Goff, Nasrallah, Davis and Sullivan4
Lifestyle interventions in patients with SMI have previously been shown to reduce body weight Reference McGinty, Baller, Azrin, Juliano-Bult and Daumit5 and waist circumference, and to improve cardiometabolic risk factors such as serum triglyceride levels, fasting glucose and insulin concentrations. Reference Bruins, Jörg, Bruggeman, Slooff, Corpeleijn and Pijnenborg6,Reference Cabassa, Ezell and Lewis-Fernández7 These studies included mostly out-patients Reference Daumit, Dickerson, Wang, Dalcin, Jerome and Anderson8,Reference Brar, Ganguli, Pandina, Turkoz, Berry and Mahmoud9 or individuals with a first-episode of schizophrenia, Reference Alvarez-Jimenez, Gonzalez-Blanch, Vazquez-Barquero, Pérez-Iglesias, Martínez-García and Pérez-Pardal10,Reference Wu, Zhao, Jin, Shao, Fang and Guo11 whereas studies in patients with SMI admitted to sheltered or clinical care facilities are scarce. In addition, sustainability of effects is questionable.
Most lifestyle interventions are designed to stimulate individuals to change their diet and physical activity behaviour and involve counselling, goal-setting and weight monitoring. The challenge of these programmes is that they are highly dependent on individual patients' interests, motivation and capacities, which are reduced in people with SMI because of negative symptoms and cognitive deficits. Reference Hassapidou, Papadimitriou, Athanasiadou, Tokmakidou, Pagkalos and Vlahavas12 In residential facilities, the setting is important as well since facilities are often characterised by an ‘obesogenic’ environment as a result of an abundant provision of unhealthy food products and a lack of daily activities. Reference Swinburn, Egger and Raza13,Reference Faulkner and Cohn14 An approach focusing on the obesogenic environment, ‘making the healthy choice the easy choice’ by educating staff how to change daily practice with regard to healthy nutrition and physical activities in the facility, may lead to sustainable changes for all residential patients with SMI. Two studies have addressed the obesogenic environment of residential patients with SMI by modifying food delivery Reference Cohn, Grant and Faulkner15 or adjusting the offered food combined with nutritional counselling and exercise sessions, Reference Melamed, Stein-Reisner, Gelkopf, Levi, Sivan and Ilievici16 and reported promising improvements in patients' somatic health. However, these studies lacked a control group Reference Cohn, Grant and Faulkner15 or had a small sample size. Reference Melamed, Stein-Reisner, Gelkopf, Levi, Sivan and Ilievici16 Another approach that may work well for the SMI population is the ‘small change approach’. This approach aims for modest lifestyle changes leading to modest, but sustainable weight loss in the long term. Reference Sbrocco, Nedegaard, Stone and Lewis17
We developed the Effectiveness of Lifestyle Interventions in PSychiatry (ELIPS) trial. Reference Looijmans, Jörg, Schoevers, Bruggeman, Stolk and Corpeleijn18 In this trial, we designed a lifestyle intervention to improve cardiometabolic health of patients with SMI living in residential facilities by stimulating a healthy lifestyle via small but sustainable changes in the obesogenic environment. The ELIPS trial is a pragmatic randomised controlled trial (RCT), designed to offer tailored, scalable and implementable interventions. Reference Treweek and Zwarenstein19 This means that already in the trial phase, the intervention was aimed at and implemented by regular staff members in daily care. We expected stable or improved cardiometabolic health in the intervention group compared with deteriorated cardiometabolic health in the control group. In addition, we explored whether the intervention effect depends on gender, age and type of facility.
Method
The ELIPS study protocol was published Reference Looijmans, Jörg, Schoevers, Bruggeman, Stolk and Corpeleijn18 and will be briefly explained below. The Medical Ethical Committee for Research in Mental Health Care (Metigg) concluded that the study protocol and use of anonymised data from Routine Outcome Monitoring (ROM; below) was in accordance with the Declaration of Helsinki and (inter)national regulations, and that the study did not fall under the scope of the Medical Research Involving Human Subjects Act, thereby waiving informed consent. The trial was registered in the Dutch Trial Registry (NTR2720, www.trialregister.nl).
Participants and recruitment
Patients with SMI from all sheltered and long-term clinical care teams (n = 29 teams, 20–65 patients per team) of two mental health organisations in The Netherlands were included in the study from September 2010 until December 2011 if they participated in the annual ROM (below) (Fig. 1). Long-term clinical care facilities deliver direct, all-day intensive professional care. Sheltered facilities provide supported living, a combination of housing and services in the community. Exclusion criteria were age below 18, pregnancy, Korsakoff syndrome or inability to participate in tests. In total 240 patients per arm were needed to detect a clinical relevant change of −5% in waist circumference (α = 0.05, power 0.90), taking into account estimated ROM screenings performed in a well-established infrastructure.
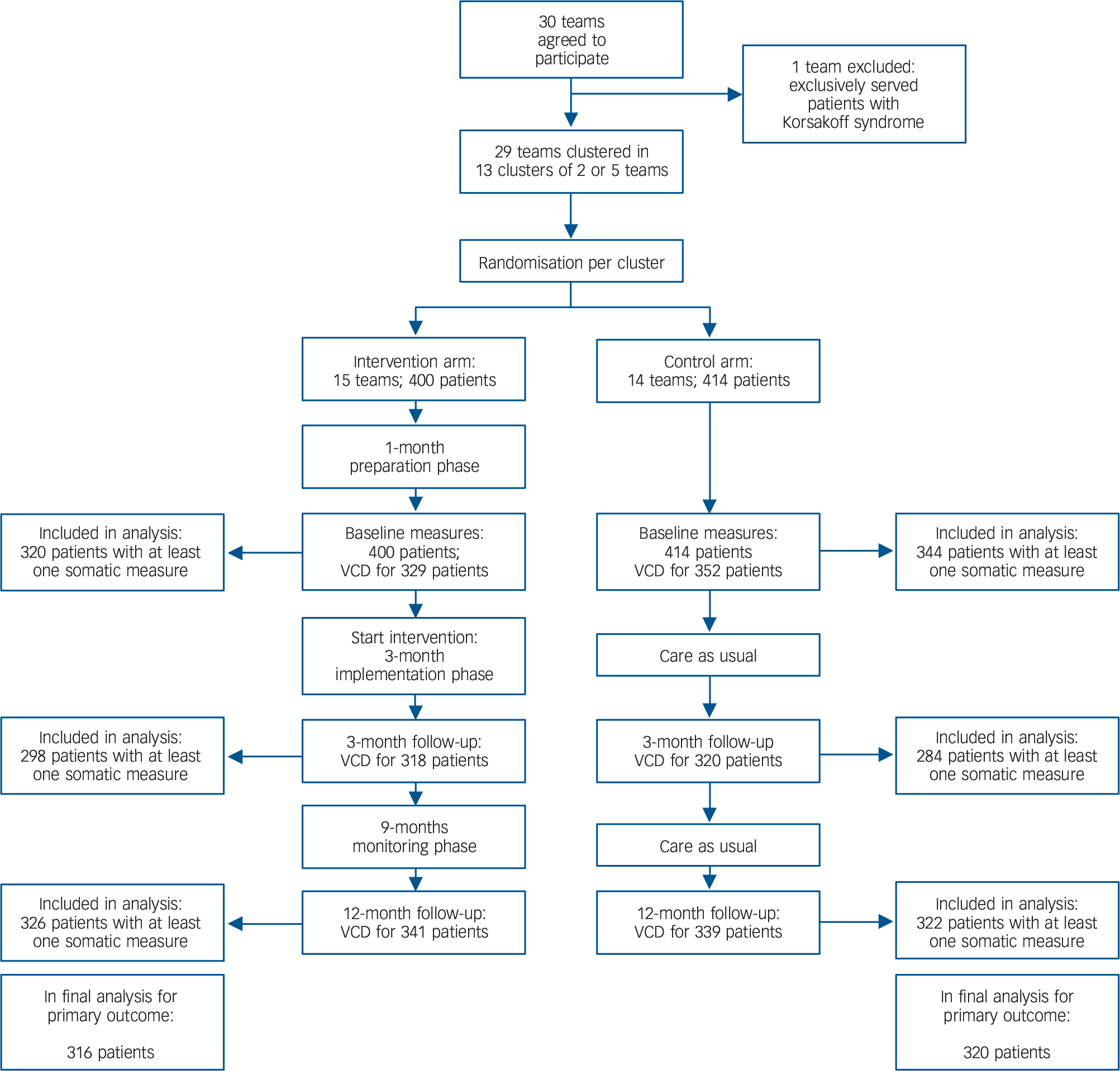
Fig 1 Flow chart of patients in the Effectiveness of Lifestyle Interventions in Psychiatry (ELIPS) trial.
A total of 736 patients have at least one physical measure at baseline or 12-months follow-up and were included in the analysis (not retraceable in flow chart). VCD, valid care data.
Intervention
The ELIPS intervention was directed at nursing teams and addressed the obesogenic environment of patients; see ELIPS study protocol Reference Looijmans, Jörg, Schoevers, Bruggeman, Stolk and Corpeleijn18 for examples from practice. The intervention consisted of a preparation, implementation and monitoring phase. In the 1-month preparation phase, lifestyle coaches introduced themselves to staff and patients, screened the environment and teams' daily routines, and listed patients' and teams' preferences and sites' logistic possibilities. Lifestyle coaches created a team-tailored lifestyle plan based on listed preferences and possibilities and four pre-established ELIPS lifestyle goals: (a) to stimulate physical activity; (b) to increase supply/availability of healthy food products; (c) to organise at least one activity per week focused on a healthy diet; and (d) to improve the obesogenic environment at an organisational level. In the 3-months implementation phase, lifestyle coaches implemented the planned ELIPS lifestyle activities as described in the team-tailored lifestyle plan. Lifestyle coaches first demonstrated activities to staff, then carried out the activities together with staff and finally supervised staff while they carried out the activities. Lifestyle coaches trained teams to create a healthy environment and stimulate healthy behaviours in patients. At the end of the implementation phase, teams set goals to achieve in the 9-month monitoring phase. In the monitoring phase, a lifestyle coach visited all intervention teams twice and discussed with the team and team leader whether goals were achieved, which barriers in achieving the goals were encountered and discussed options to tackle these barriers. Also, the researchers organised one benchmark meeting for all intervention team leaders where difficulties in achieving team goals were discussed and tips, tricks and successful examples were shared
Lifestyle coaches were trained for 2 days about the ELIPS lifestyle programme, motivational interviewing techniques and the patient population. Lifestyle coaches were fulfilling the final of 4 years of education to become professional lifestyle coaches. Because lifestyle coaches were still in training, each team had two lifestyle coaches at its disposal, who were appointed by the research team. Per week, lifestyle coaches spent on average 8h on activities with patients (6 contact hours and about 2h preparation time) and 8 h on training of staff and organisational aspects, such as developing information materials, meetings with staff and project management.
Patients in the control condition received care as usual. Lifestyle initiatives in control teams were documented. Randomisation was performed at team level using a randomised block design with 12 clusters of two comparable teams and 1 cluster of five comparable teams based on mental healthcare organisation, type of facility, case-load size and location (urban or rural). Teams were randomised with 55 into the intervention or control arm with computerised random number generator by a non-participating research nurse.
Measurements and outcomes
Primary outcome was waist circumference at 3 and 12 months after baseline. Secondary outcomes were body mass index (BMI; kg/m2) and metabolic syndrome z-score. Information on age, gender, diagnosis and medication use was derived from patient records. Physical health data were collected by trained nurses in annual ROM screenings, part of the ongoing PHAMOUS (Pharmacotherapy Outcome and Monitoring Survey) cohort. Reference Bruins, Pijnenborg, Bartels-Velthuis, Visser, van den Heuvel and Bruggeman20 Annual ROM screenings are standard care in both organisations and were rescheduled 1–2 weeks before the start of the intervention (baseline measure) and 3 and 12 months thereafter (follow-up measurements). Patients received a small fee for the additional 3-months ROM screening (€5.00/$5.38). ROM nurses were masked to intervention allocation. Waist circumference was measured twice in the standing position at the end of an expiration using a flexible non-stretching tape halfway between iliac crest and lowest rib. Weight was measured by calibrated scales (Seca, model 813, Hamburg, Germany) in light clothing without shoes or jackets. Height was measured without shoes. Systolic and diastolic blood pressure (BP) were measured using an automated blood pressure monitor (BOSO medicus control, Jungingen, Germany) in the sitting position after 5 min rest. Patients visited a (hospital) laboratory to collect a fasting blood sample for levels of lipids (total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides) and glucose metabolism (glucose, haemoglobin A1c (HbA1c)). If not fasting, this was routinely indicated on the form by the nurse.
The metabolic syndrome was defined as the presence of three or more of the following criteria: Reference Grundy, Cleeman, Daniels, Donato, Eckel and Franklin21 (a) waist circumference ⩾88/102 cm (female/male); (b) systolic BP ⩾130 and/or diastolic BP ⩾85 mmHg or receiving antihypertensive medication; (c) HDL-cholesterol < 1.03/1.3 mmol/L (female/male; divide by 0.0259 to convert to mg/dL) or receiving lipid-lowering medication; (d) triglycerides ⩾1.7 mmol/L (divide by 0.0113 to convert to mg/dL) or receiving lipid-lowering medication; and (e) fasting glucose ⩾6.1 mmol/L (divide by 0.0555 to convert to mg/dL), Reference Forouhi, Balkau, Borch-Johnsen, Dekker, Glumer and Qiao22 receiving antihyperglycemic medication or reporting a diagnosis for diabetes. When fasting glucose levels were not available (baseline: 46%, n = 342; 3 months: 53%, n = 392; 12 months: 46%, n = 342), patients were considered to fulfil the glucose-risk criterion if they reported having diabetes (9.6%, n = 71) or if HbA1c ⩾6.0%. 23 The individual components were standardised into z-scores (with HDL-cholesterol z-score multiplied by – 1) Reference Eisenmann24,Reference Bakker, Gansevoort and de Zeeuw25 and the sum divided by 5 was used as a continuous variable for the degree of metabolic syndrome. BP was standardised using mean arterial pressure (MAP). Antipsychotic medication was categorised in three groups according to the strength of the side-effects on cardiometabolic health (none, mild or strong) based on the literature (see online Table DS1). 26,27
Statistical analyses
Data were analysed according to the intention-to-treat principle using SPSS version 22. A P < 0.05 was considered statistically significant. Results are reported as mean (95% confidence interval). Differences in frequency distributions were tested with chi-square or Mann-Whitney U-tests. For testing main differences between intervention and control, a likelihood-based general linear mixed model was applied, using a subject-specific model to adjust for clustering of patients within teams using an ‘unstructured’ error structure, and controlling for the block design. For all analyses, the outcomes over time per patient formed the first level of the model, the patient formed the second level, and team formed the third level and cluster as random factor. Since it is possible that intervention effects on somatic outcomes differ between implementation (first 3 months) and monitoring phase (9 months thereafter), time was coded as two dummy variables. Group (intervention or control), time, and group × time interactions were entered in the model as fixed factors with adjustment for age, gender, type of facility and antipsychotic medication. As a secondary analysis, clinically relevant change was studied, defined as a change of at least 5% in waist circumference. Finally, we studied the intervention effect within prespecified subgroups (gender, age groups and type of facility).
Results
The 29 teams were randomised into 14 control and 15 intervention teams, resulting in 814 eligible patients (Fig. 1). Of these, 736 (90%) had at least one physical measurement at baseline or at 12 months and were included in the analyses. The majority of patients were men (63.2%), the mean age was 48.3 years (s.d. = 12.6) ranging from 20 to 85 years (Table 1). Most patients were overweight or obese (65.9%) and 58.4% met the criteria for metabolic syndrome (Table 2). In the intervention group, 46% of the patients lived in long-term clinical facilities compared with 36% in the control group (P<0.01). This yielded a significantly higher age and more psychotic disorders in the intervention group.
Table 1 Baseline characteristics of participants in the Effectiveness of Lifestyle Interventions in Psychiatry (ELIPS) study
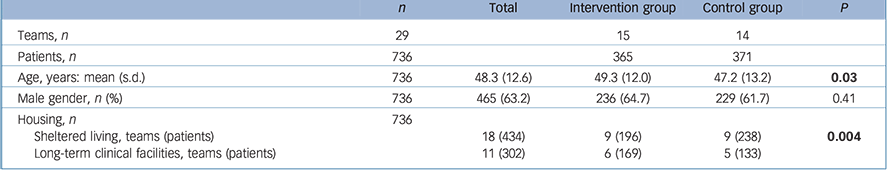
| n | Total | Intervention group | Control group | P | |
|---|---|---|---|---|---|
| Teams, n | 29 | 15 | 14 | ||
| Patients, n | 736 | 365 | 371 | ||
| Age, years: mean (s.d.) | 736 | 48.3 (12.6) | 49.3 (12.0) | 47.2 (13.2) | 0.03 |
| Male gender, n (%) | 736 | 465 (63.2) | 236 (64.7) | 229 (61.7) | 0.41 |
| Housing, n | 736 | ||||
| Sheltered living, teams (patients) | 18 (434) | 9 (196) | 9 (238) | 0.004 | |
| Long-term clinical facilities, teams (patients) | 11 (302) | 6 (169) | 5 (133) | ||
Table 2 Baseline clinical characteristics of participants in the Effectiveness of Lifestyle Interventions in Psychiatry (ELIPS) study a
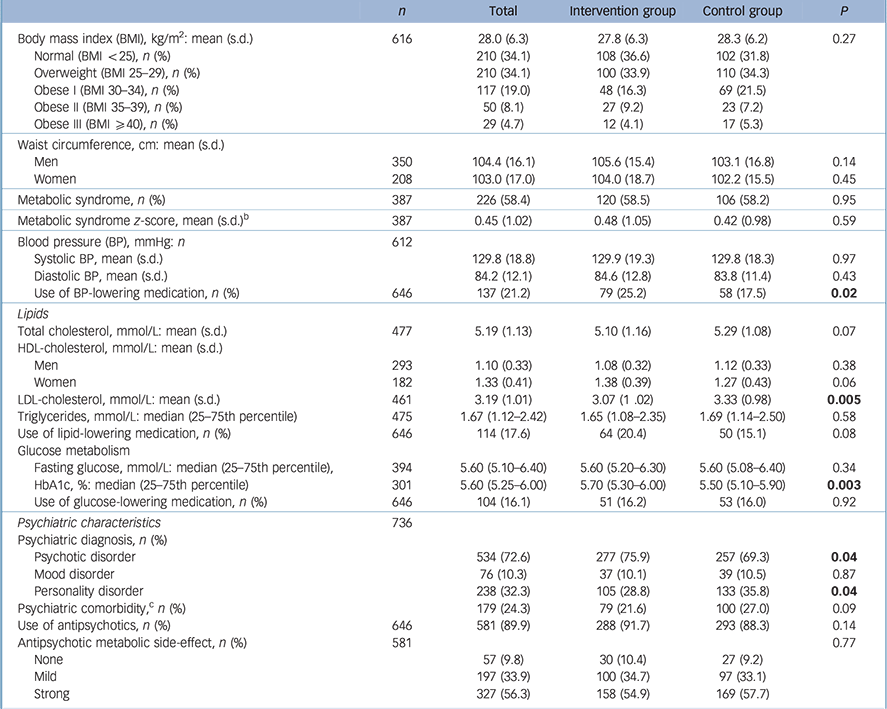
| n | Total | Intervention group | Control group | P | |
|---|---|---|---|---|---|
| Body mass index (BMI), kg/m2: mean (s.d.) | 616 | 28.0 (6.3) | 27.8 (6.3) | 28.3 (6.2) | 0.27 |
| Normal (BMI <25), n (%) | 210 (34.1) | 108 (36.6) | 102 (31.8) | ||
| Overweight (BMI 25–29), n (%) | 210 (34.1) | 100 (33.9) | 110 (34.3) | ||
| Obese I (BMI 30–34), n (%) | 117 (19.0) | 48 (16.3) | 69 (21.5) | ||
| Obese II (BMI 35–39), n (%) | 50 (8.1) | 27 (9.2) | 23 (7.2) | ||
| Obese III (BMI ⩾40), n (%) | 29 (4.7) | 12 (4.1) | 17 (5.3) | ||
| Waist circumference, cm: mean (s.d.) | |||||
| Men | 350 | 104.4 (16.1) | 105.6 (15.4) | 103.1 (16.8) | 0.14 |
| Women | 208 | 103.0 (17.0) | 104.0 (18.7) | 102.2 (15.5) | 0.45 |
| Metabolic syndrome, n (%) | 387 | 226 (58.4) | 120 (58.5) | 106 (58.2) | 0.95 |
| Metabolic syndrome z-score, mean (s.d.) b | 387 | 0.45 (1.02) | 0.48 (1.05) | 0.42 (0.98) | 0.59 |
| Blood pressure (BP), mmHg: n | 612 | ||||
| Systolic BP, mean (s.d.) | 129.8 (18.8) | 129.9 (19.3) | 129.8 (18.3) | 0.97 | |
| Diastolic BP, mean (s.d.) | 84.2 (12.1) | 84.6 (12.8) | 83.8 (11.4) | 0.43 | |
| Use of BP-lowering medication, n (%) | 646 | 137 (21.2) | 79 (25.2) | 58 (17.5) | 0.02 |
| Lipids | |||||
| Total cholesterol, mmol/L: mean (s.d.) | 477 | 5.19 (1.13) | 5.10 (1.16) | 5.29 (1.08) | 0.07 |
| HDL-cholesterol, mmol/L: mean (s.d.) | |||||
| Men | 293 | 1.10 (0.33) | 1.08 (0.32) | 1.12 (0.33) | 0.38 |
| Women | 182 | 1.33 (0.41) | 1.38 (0.39) | 1.27 (0.43) | 0.06 |
| LDL-cholesterol, mmol/L: mean (s.d.) | 461 | 3.19 (1.01) | 3.07 (1.02) | 3.33 (0.98) | 0.005 |
| Triglycerides, mmol/L: median (25–75th percentile) | 475 | 1.67 (1.12–2.42) | 1.65 (1.08–2.35) | 1.69 (1.14–2.50) | 0.58 |
| Use of lipid-lowering medication, n (%) | 646 | 114 (17.6) | 64 (20.4) | 50 (15.1) | 0.08 |
| Glucose metabolism | |||||
| Fasting glucose, mmol/L: median (25–75th percentile), | 394 | 5.60 (5.10–6.40) | 5.60 (5.20–6.30) | 5.60 (5.08–6.40) | 0.34 |
| HbA1c, %: median (25–75th percentile) | 301 | 5.60 (5.25–6.00) | 5.70 (5.30–6.00) | 5.50 (5.10–5.90) | 0.003 |
| Use of glucose-lowering medication, n (%) | 646 | 104 (16.1) | 51 (16.2) | 53 (16.0) | 0.92 |
| Psychiatric characteristics | 736 | ||||
| Psychiatric diagnosis, n (%) | |||||
| Psychotic disorder | 534 (72.6) | 277 (75.9) | 257 (69.3) | 0.04 | |
| Mood disorder | 76 (10.3) | 37 (10.1) | 39 (10.5) | 0.87 | |
| Personality disorder | 238 (32.3) | 105 (28.8) | 133 (35.8) | 0.04 | |
| Psychiatric comorbidity, c n (%) | 179 (24.3) | 79 (21.6) | 100 (27.0) | 0.09 | |
| Use of antipsychotics, n (%) | 646 | 581 (89.9) | 288 (91.7) | 293 (88.3) | 0.14 |
| Antipsychotic metabolic side-effect, n (%) | 581 | 0.77 | |||
| None | 57 (9.8) | 30 (10.4) | 27 (9.2) | ||
| Mild | 197 (33.9) | 100 (34.7) | 97 (33.1) | ||
| Strong | 327 (56.3) | 158 (54.9) | 169 (57.7) | ||
a. SI unit conversion factors: to convert total cholesterol, high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol to mg/dL, divide values by 0.0259; to convert triglycerides to mg/dL, divide values by 0.0113; to convert fasting glucose to mg/dL, divide values by 0.0555.
b. The means and standard deviations of the patients ranging within healthy reference values were used to standardise HDL-cholesterol (1.1–2.0 mmol/L in women and 0.9–1.7 mmol/L in men), triglycerides (⩽2.2 mmol/L) and fasting glucose (⩽7.1 mmol/L) or haemoglobin A1c (HbA1c) (<8.0%).
c. Two or more of the defined diagnoses.
After 3 months of lifestyle intervention, the intervention group showed a significant decrease in waist circumference of 1.51 cm (95% CI −2.99 to −0.04) compared with the control group (Table 3). After 12 months, the waist circumference in the intervention group remained lower than in the control group (1.28 cm −2.79 to 0.23) although no longer statistically significant. Metabolic syndrome z-score decreased by 0.22 s.d. (95% CI −0.38 to −0.06) in the intervention compared with the control group after 3 months, of which most of the effect was attributable to a significant decrease of 0.48 s.d. in glucose z-score (95% CI −0.87 to −0.09) and of 0.09 s.d. in waist circumference z-score (95% CI −0.18 to −0.01) in the intervention group. The effect on metabolic syndrome z-score was not sustained after 12 months. We found no intervention effects on BMI. In general, changes in waist circumference and BMI over time varied widely between teams in both the intervention and control group (Fig. 2).
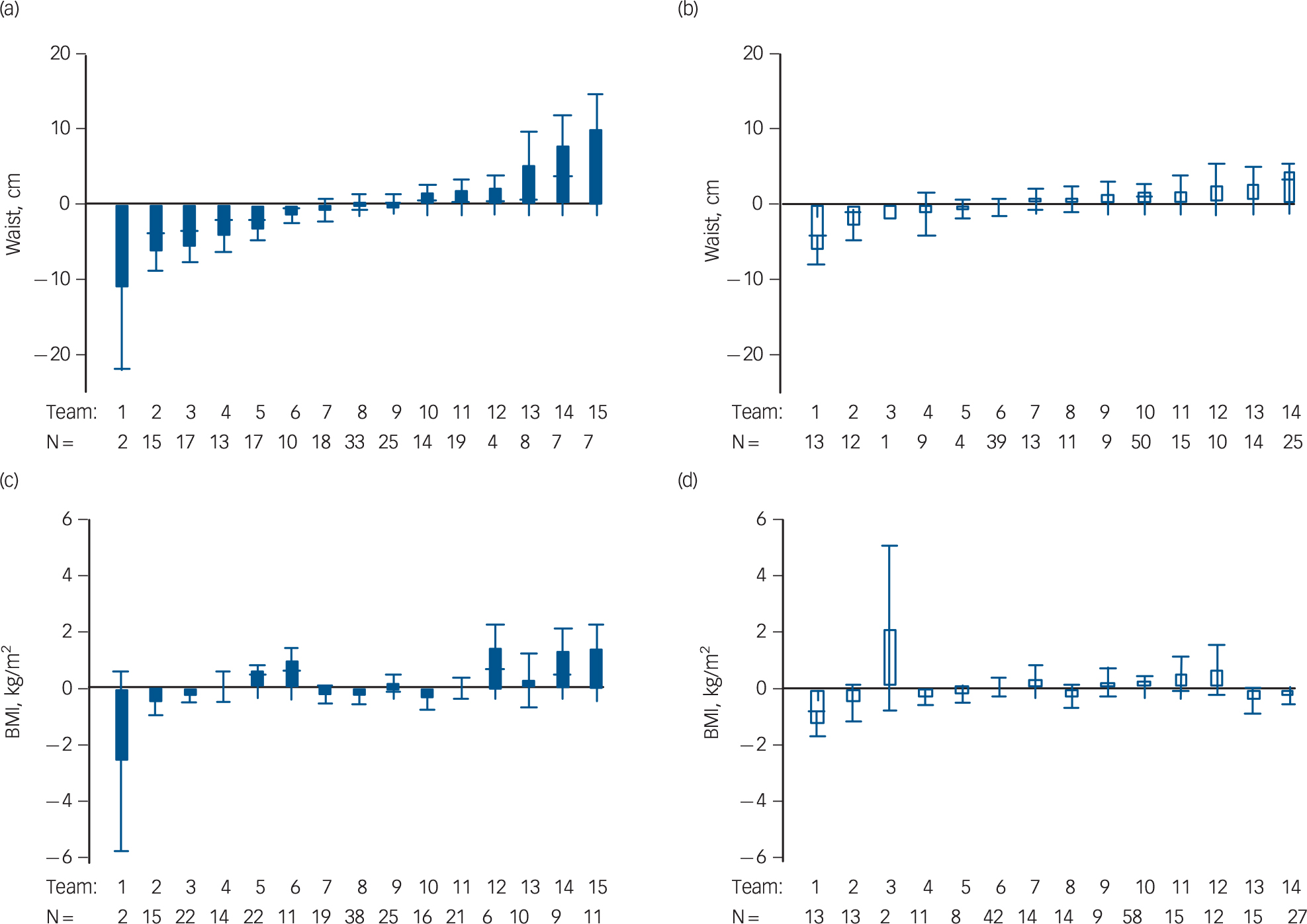
Fig. 2 Heterogeneity between teams in mean changes in waist circumference and body mass index (BMI) after 12 months.
Mean change in waist circumference (cm) and BMI (kg/m2) from baseline to 12 months post-baseline per team with n patients. Waist change in the (a) intervention and (b) control groups; BMI change in the (c) intervention and (d) control groups. Error bars represent standard errors of the mean.
Table 3 Somatic outcomes after 3 and 12 months of lifestyle intervention in in-patients with serious mental illness: results of general linear mixed models analyses with adjustment for age, gender, type of facility and antipsychotic side-effects
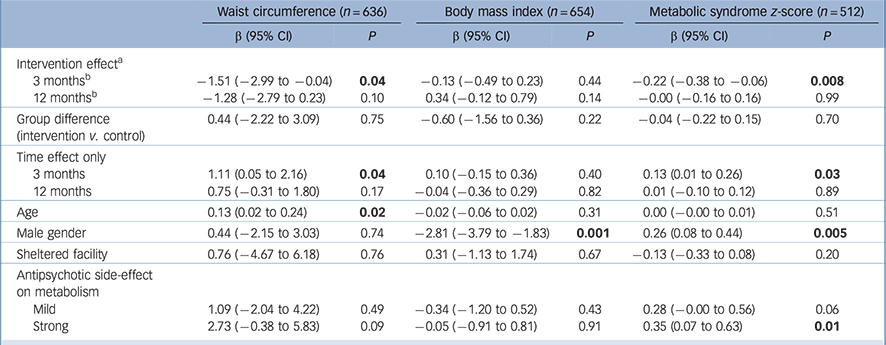
| Waist circumference (n = 636) | Body mass index (n = 654) | Metabolic syndrome z-score (n = 512) | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Intervention effect a | ||||||
| 3 months b | −1.51 (−2.99 to −0.04) | 0.04 | −0.13 (−0.49 to 0.23) | 0.44 | −0.22 (−0.38 to −0.06) | 0.008 |
| 12 months b | −1.28 (−2.79 to 0.23) | 0.10 | 0.34 (−0.12 to 0.79) | 0.14 | −0.00 (−0.16 to 0.16) | 0.99 |
| Group difference (intervention v. control) |
0.44 (−2.22 to 3.09) | 0.75 | −0.60 (−1.56 to 0.36) | 0.22 | −0.04 (−0.22 to 0.15) | 0.70 |
| Time effect only | ||||||
| 3 months | 1.11 (0.05 to 2.16) | 0.04 | 0.10 (−0.15 to 0.36) | 0.40 | 0.13 (0.01 to 0.26) | 0.03 |
| 12 months | 0.75 (−0.31 to 1.80) | 0.17 | −0.04 (−0.36 to 0.29) | 0.82 | 0.01 (−0.10 to 0.12) | 0.89 |
| Age | 0.13 (0.02 to 0.24) | 0.02 | −0.02 (−0.06 to 0.02) | 0.31 | 0.00 (−0.00 to 0.01) | 0.51 |
| Male gender | 0.44 (−2.15 to 3.03) | 0.74 | −2.81 (−3.79 to −1.83) | 0.001 | 0.26 (0.08 to 0.44) | 0.005 |
| Sheltered facility | 0.76 (−4.67 to 6.18) | 0.76 | 0.31 (−1.13 to 1.74) | 0.67 | −0.13 (−0.33 to 0.08) | 0.20 |
| Antipsychotic side-effect on metabolism |
||||||
| Mild | 1.09 (−2.04 to 4.22) | 0.49 | −0.34 (−1.20 to 0.52) | 0.43 | 0.28 (−0.00 to 0.56) | 0.06 |
| Strong | 2.73 (−0.38 to 5.83) | 0.09 | −0.05 (−0.91 to 0.81) | 0.91 | 0.35 (0.07 to 0.63) | 0.01 |
a. The control group is the reference group.
b. Group × time.
In the intervention group, 20.1% of the participants had a clinically relevant improvement (⩾−5% waist circumference) and 20.6% had a clinically relevant deterioration (⩾+5% waist circumference) in waist circumference after 12 months. In the control group this was 17.8% and a substantially higher 29.3%, respectively (P = 0.075).
To investigate whether subgroups differed in their response to the intervention, stratified analyses were performed for gender, age groups and housing facility. The intervention effect was most pronounced in males (waist circumference: −2.42 cm (95% CI −4.10 to −0.74) and metabolic syndrome z-score: −0.33 s.d. (95% CI −0.55 to −0.10)) and younger participants (metabolic syndrome z-score ⩽43 years: −0.31 s.d. (95% CI −0.58 to −0.05) after 3 months (online Table DS2). The decrease in waist circumference and metabolic syndrome z-score in the intervention group was strongest in participants living in sheltered facilities, after 3 (waist circumference: −1.68 cm (95% CI −3.34 to −0.01); metabolic syndrome z-score: −0.31 s.d. (95% CI −0.51 to −0.11)) and 12 months (waist circumference: −2.63 cm; 95% CI −4.28 to −0.98) whereas those in the intervention group in long-term clinical facilities showed a small increase in metabolic syndrome z-score (0.25 s.d. (95% CI 0.00–0.49)) after 12 months.
For comparability with lifestyle intervention studies including patients with SMI with a BMI ⩾25 kg/m2, Reference Daumit, Dickerson, Wang, Dalcin, Jerome and Anderson8 we performed sensitivity analyses in this subgroup. Findings remain the same: waist circumference was reduced by 1.79 cm (95% CI −3.50 to −0.08) at 3 months and a trend for reduced waist circumference of 1.59 cm (95% CI −3.34 to 0.16) was found at 12 months. Metabolic syndrome z-scores decreased by 0.20 s.d. (95% CI −0.40 to −0.01) after 3 months. Again, no intervention effects on BMI were found.
Discussion
Main findings
This large, multisite, RCT showed that changing the obesogenic environment of patients with SMI in residential settings into a healthier environment significantly reduced waist circumference and degree of metabolic syndrome after 3 months of intervention compared with care as usual. The magnitude of these effects decreased when, after 3 months, staff took over the lifestyle activities. This shows that improving the obesogenic environment can evoke beneficial changes without targeting patients directly, but sustainability remains a challenge.
An innovative feature of the ELIPS intervention is the focus on the obesogenic environment as opposed to directly and solely targeting patients' dietary and/or sedentary behaviours. Reference Swinburn, Egger and Raza13 Moreover, structural changes in the environment will affect every patient, irrespective of their personal interest in improving their lifestyles. Changing the environment using a small change approach indeed resulted in a small improvement: 1.5 cm in waist circumference over 3 months. If this can be sustained over a longer period of time, it will however, lead to clinically relevant improvements in adiposity and thereby reducing risk of cardiovascular disease and diabetes. Reference Farin, Abbasi and Reaven28,Reference Fox, Despres, Richard, Brette and Deanfield29 Earlier studies found that for every 5 cm increase in waist circumference the risk of death increased 13% for females and 17% for males. Reference Pischon, Boeing, Hoffmann, Bergmann, Schulze and Overvad30
Comparison with previous studies
Two earlier RCTs focused on residential patients, targeting both patients and staff with a 12-month lifestyle intervention with the structural guidance of external coaches. Reference Forsberg, Björkman, Sandman and Sandlund31,Reference Hjorth, Davidsen, Kilian, Pilgaard Eriksen, Jensen and S⊘rensen32 Forsberg and colleagues did not find improvements in waist circumference or glucose levels. Reference Forsberg, Björkman, Sandman and Sandlund31 This was, however, a small study (n = 41). Hjorth and colleagues showed a reduction of 3.1 cm in waist circumference and stabilised fasting glucose levels v. increased glucose levels in controls, which is comparable with our finding of reductions in waist circumference and fasting glucose z-score. The improvement in glucose metabolism is consistent with studies showing that lifestyle interventions are effective at preventing type 2 diabetes in the general population. Reference Roumen, Blaak and Corpeleijn33 The beneficial effect on fasting glucose suggests that lifestyle improvements, possibly via increased physical activity, improve insulin resistance. Reference Goodyear, Laurie, Kahn and Barbara34 Changes in physical activity may result in changes in body composition with reduced fatness and increased muscle mass, Reference Hjorth, Davidsen, Kilian, Pilgaard Eriksen, Jensen and S⊘rensen32 which may explain the significant effects on waist circumference but not BMI (Fig. 2). A meta-analysis of lifestyle interventions for SMI in both in- and out-patients confirmed our results on waist circumference (Cohen's d = −0.37, 95% CI −0.60 to −0.13) and fasting glucose (Cohen's d = −0.24, 95% CI −0.32 to −0.10). Reference Bruins, Jörg, Bruggeman, Slooff, Corpeleijn and Pijnenborg6 Studies using individual and/or group counselling sessions, for example Daumit and colleagues Reference Daumit, Dickerson, Wang, Dalcin, Jerome and Anderson8 and McKibbin et al, Reference McKibbin, Patterson, Norman, Patrick, Jin and Roesch35 found a significant decrease of 2.0–3.7 cm in waist circumference after 6 months of exercise, weight management or psychoeducational sessions. Thus, a lifestyle intervention in residential patients with SMI focusing on the obesogenic environment may yield results comparable to interventions targeting patients directly with individual or group counselling.
Possible factors influencing intervention implementation and sustainability
Changes in waist circumference varied widely between teams (Fig. 2). This is most likely related to the ease with which teams implemented and sustained new lifestyle habits. Structural aspects played a role, such as environmental features of the facility (for example physical activity opportunities in urban v. rural settings Reference Kaczynski and Henderson36 ), available budget (for example for healthy food products) and availability of staff members (for example nurses being scheduled to organise activities). Furthermore, logistic changes (for example altering the type of bread offered during lunch) were possibly more easily implemented than cultural changes (for example offering walk-and-talk therapy Reference Doucette37 instead of sitting in the counselling office). Perhaps of greater influence were attitudes of staff: nurses differed in their experience of conflicting priorities (such as a high workload), conflicts with role definitions (for example nurses are not dieticians or physical therapists) and conflicts with own health behaviours (for example setting a good example by not ordering pizza during night shifts). Reference Lean, Leavey, Killaspy, Green, Harrison and Cook38,Reference Bradshaw, Wearden, Marshall, Warburton, Husain and Pedley39
The pragmatic character of the ELIPS trial allowed the intervention to be tailored to the resources of the facility. Moreover, regular staff implemented the intervention in everyday practice after 3 months of lifestyle coaching, giving a clear indication of what is attainable in ‘real-world’ settings. The design of the study, consisting of an implementation and a monitoring (support) phase, demonstrated the difficulty of sustaining achieved improvements. Despite involvement of regular staff in organising lifestyle activities and embedding lifestyle activities in teams' working routine, the magnitude of effects achieved at 3 months decreased in the 9 months thereafter, when staff members were less frequently guided by lifestyle coaches. This is in line with findings from a meta-analysis of lifestyle interventions by Bradshaw et al. Reference Bradshaw, Wearden, Marshall, Warburton, Husain and Pedley39 and the study of Daumit and colleagues Reference Daumit, Dickerson, Wang, Dalcin, Jerome and Anderson8 where initial significant effects on waist circumference were no longer significant when the frequency of sessions decreased and trained staff members took over most of the activities of lifestyle coaches. So, improvements in waist circumference and glucose levels are within reach, but sustainability might be achieved only when staff members are guided on a regular basis by a lifestyle coach whose primary responsibility is to promote the patients' lifestyle. The frequency of these guiding contacts needed to sustain or maximise results in the long term, should be explored, but likely needs to exceed two visits in 9 months.
The ELIPS intervention seemed to be especially beneficial for men and patients living in sheltered facilities. Perhaps the lifestyle activities, possibly the physical activities, were more appealing to male than female participants. Staff in long-term clinical care facilities might have experienced more obstacles in changing routines, anticipating dysregulation of the most severely ill patients. However, these results need to be interpreted with caution as subanalyses inevitably contained fewer patients than needed according to the power calculation, which was based on the comparison of intervention and control groups only.
Strengths and limitations
Strengths and limitations of the study are related to the pragmatic character of the RCT. The control condition was less controlled than it would have been in an explanatory trial. Reference Treweek and Zwarenstein19 Despite being in the control condition, staff members or patients may have taken initiative to work on a more healthy lifestyle, following the trend in society. The intervention condition would have differed less between facilities if we had not used a team-tailored lifestyle plan. Using an implementation approach, however, largely increases the external validity of the study results. Our inclusion strategy further increased the external validity by avoiding selection bias of participating patients. Reference Treweek and Zwarenstein19
Implications
A small change approach focusing on the obesogenic environment of patients living in sheltered or long-term care facilities has the potential to produce clinically relevant reductions in adiposity and thereby reduce cardiometabolic risk. However, our small results indicate that changing the obesogenic environment alone is not enough. It should be considered a prerequisite for improving patients' health Reference Swinburn, Egger and Raza13 and be part of an integrated approach of multiple targets, including sensible pharmaceutical strategies. A next step would be to develop a scalable (nursing) programme for maintenance of healthy changes and initiatives in the facilities, that is effective, affordable and sustainable in the long term.
Funding
This work was supported by a grant from ZonMW (). We received in-kind support from GGZ Friesland, Lentis and the Rob Giel Research Center.
Acknowledgements
We thank all patients, teams and organisations who participated in this trial. We thank Jacqueline Cambier, Msc, Human Movement Science and expert by experience, for her helpful remarks on the design of the study and advice in obtaining funding. We also thank Dr Ellen visser for data management and all helpful instructions. Neither received any compensation for their contribution.








eLetters
No eLetters have been published for this article.