INTRODUCTION
Mobility exists in different forms: people can move to another area or country [Reference Nunn1, Reference Decosas2], they can visit relatives and attend ceremonies, and they can undertake work-related travel, such as trading, truck driving, and seasonal work [Reference Pickering3–Reference Lagarde, Pison and Enel5]. Mobility is often related to risky sexual behaviour, and consequently to the spread of HIV and other sexually transmitted diseases (STDs) [Reference Nunn1, Reference Lurie6, Reference Lydie7]. Studies in Senegal and South Africa found that seasonal or temporary migrants reported more sexual partners [Reference Lagarde, Pison and Enel5] and had a higher HIV prevalence than residents [Reference Lurie6]. In a study in rural Tanzania, immigrants, including those that had immigrated several years earlier, had more risky behaviour than people who were born in the area [Reference Mmbaga8]. Most of the long-distance truck drivers in Kenya reported having sex with commercial sex workers (CSWs), with one quarter being infected with HIV [Reference Bwayo4]. Social and living conditions make migrants and travellers more prone to casual and commercial sex [Reference Chirwa9, 10]. Partners remaining at home also reported more casual partners [Reference Kishamawe11]. Mobile groups may form a small part of the population, but due to their high-risk behaviour and increased HIV prevalence, they may play an important role in the spread of HIV.
The role of condom promotion and health education in reducing HIV transmission has been established for many years [Reference Weller and Davis12–Reference Stoneburner and Low-Beer15]. Although there is no available data, we can speculate that mobile people are often not reached in these interventions. Recently arrived immigrants may not know where to buy condoms or where to find health clinics. Travellers away from home may not use condoms and may more easily engage in sex with a new partner [Reference Lydie7, Reference Lagarde16]. This potential risk of non-participation in HIV control, together with increased risk behaviour in mobile people, may make mobile groups important targets for the prevention of HIV transmission.
Spread and control of HIV can be studied with dynamic modelling [Reference Anderson and Garnett17]. Two models have incorporated the relationship between migration and HIV infection [Reference Coffee, Lurie and Garnett18, Reference Walker19]. To our knowledge there are no models that explicitly model travel. The microsimulation model STDSIM [Reference Van der Ploeg20, Reference Korenromp21] has been used to study the results of the Mwanza and Rakai trials [Reference Korenromp22, Reference White23], to explore the contrasting HIV epidemics in East and West Africa [Reference Freeman24, Reference Orroth25], and to study the possible impact of male circumcision [Reference White26]. In the current study, we used STDSIM to study the importance of mobile groups on HIV spread and to what extent non-participation of mobile groups may decrease the impact of interventions. Moreover, we explored the potential impact of interventions targeted at specific groups of travellers.
METHODS
Modelling mobility in STDSIM
We used the microsimulation model STDSIM, which simulates the natural history and transmission of HIV and other STDs in a population over time [Reference Van der Ploeg20, Reference Korenromp27]. In STDSIM, individuals are part of heterosexual networks consisting of (steady and casual) sexual relationships, one-off contacts, and men's contacts with CSWs. Different interventions to control the spread of HIV and other STDs can be modelled in STDSIM. In earlier simulations with STDSIM, mobility was not considered [Reference White23, Reference Korenromp28]. For the present study, we included migration and travel to model mobility in detail. The different mobility parameters and their quantification are explained below. Mobility parameters were based on data or, if this was lacking, on assumptions based on expert opinion (Table 1).
Table 1. STDSIM parameter values concerning mobilityFootnote *
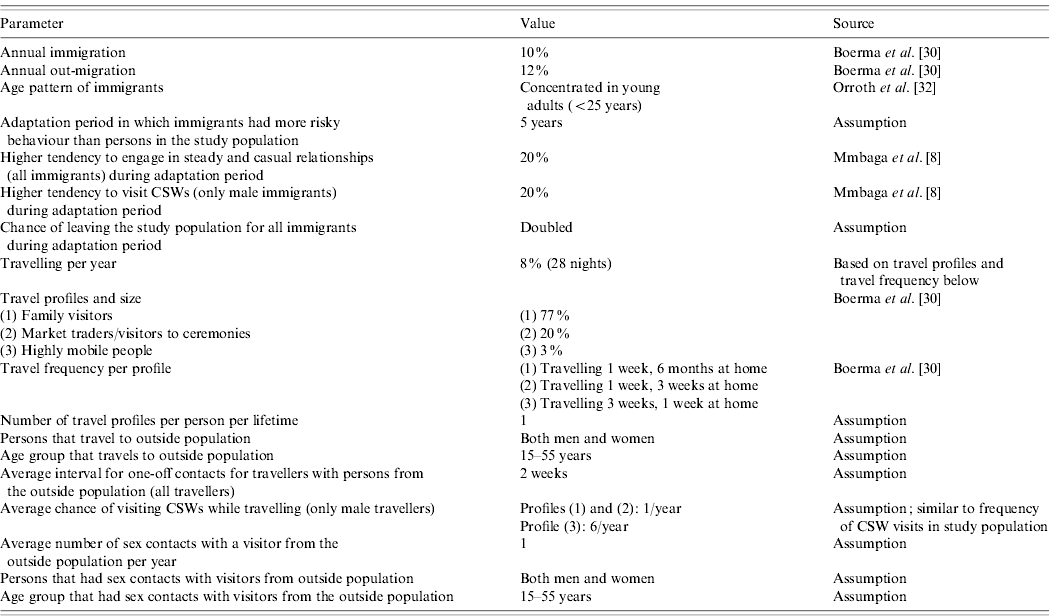
CSW, Commercial sex worker.
* Parameter values concerning natural history, transmission, treatment and interaction of STD/HIV infections can be found in White et al. [Reference White23].
We simulated an HIV epidemic in Kisesa, a rural area in Mwanza region in Tanzania [Reference Wambura29]. Starting from an existing quantification of STDSIM for the HIV epidemic in another part of Mwanza region [Reference White23, Reference Korenromp28], almost all parameters on demography, STD biology and sexual behaviour could be kept the same. The values of these parameters and their sources can be found in White et al. [Reference White23]. However, to compensate for the higher risk due to the new mobility patterns, the overall rate of starting new sexual relationships and the level of visits to CSWs needed to be downward-adjusted by 20% to arrive at the same HIV prevalence. Furthermore, quantifications for condom use were changed, since condom use had increased by the early 1990s. In Kisesa, 8% of men reported always using a condom during sex with a casual partner, and an additional 17% sometimes used a condom [Reference Boerma30]. In women, these figures were 7% and 6%, respectively. We therefore set condom use during casual sex at 10% from 1990 onwards. Condom use in commercial sex contacts was assumed to be twice as high (20%). Data from Kenya from 1999 showed that 34–56% of clients and 75% of CSWs reported always or usually using condoms during commercial sex [Reference Voeten31]. Therefore, we assumed that condom use increased to 50% in 2000. Analogously, we assumed that condom use during casual sex increased from 10% in 1990 to 25% in 2000.
As source for immigrants, visitors and sexual partners for travellers, we first created an ‘outside’ population with STDSIM. This outside population was also defined as a rural population with the same risk behaviour as the study population, and the parameters for those of the existing Mwanza quantification without mobility [Reference White23, Reference Korenromp28]. HIV prevalence in men and women in the outside population was pre-set to be identical to the simulated HIV prevalence in the study population. Individuals of this population were stored in a database system from which STDSIM could sample immigrants, visitors and sexual partners for travellers. The database system contains information for each year of the simulation.
Mobility defined and quantified
Two mobile groups were defined: immigrants (people from the outside population who recently moved into the study population), and travellers (people living in the study population travelling to the outside population). These mobile groups are non-exclusive: immigrants can be travellers, and vice versa.
We modelled 10% immigration annually, based on migration rates found in Kisesa [Reference Boerma30]. Migration was concentrated in young adults [Reference Orroth32]. Based on the study by Mmbaga et al. [Reference Mmbaga8], we assumed that immigrants who move into the study population have more risky behaviour in the first 5 years after immigration, i.e. all immigrants had a 20% higher tendency to engage in steady and casual relationships and male immigrants were 20% more likely to visit CSWs. During these first 5 years the chance for recent immigrants to leave the population was twice as high as for non-migrants. We further assumed that after these 5 years, immigrants adapted to the existing sexual behaviour norms of the study population they started to live in.
Based on data from Kisesa [Reference Boerma30], we defined the following three travel profiles: (1) those who visit family and relatives (77%), travelling for a period of 1 week followed by half a year at home. (2) Traders who visit nearby markets and towns to sell their products, and people who attend ceremonies (20%), they are away from home for 1 week followed by 3 weeks at home. (3) Highly mobile people (3%), such as truck drivers, who are away from home for 3 weeks followed by 1 week at home. These assumptions imply 8% travelling (28 nights per year) divided over three travel profiles. People were assumed to be in one travel profile during their lifetime. Both men and women, aged 15–55 years, travelled according to one of these profiles. Travellers had sexual contacts with steady or casual partners while at home, just like the rest of the study population, but one-off contacts with persons from the outside population while travelling. We assumed that the average interval for one-off contacts was 2 weeks for each group following a Poisson process. Besides this, male travellers could also visit CSWs during travel, with six visits per year for highly mobile men and one visit per year for other travellers.
Besides immigration and travelling, there are two other forms of mobility in our model: out-migration (people who leave the study population), and visiting (people from the outside population that have sexual contacts with random persons in the study population). Based on Kisesa data [Reference Boerma30], 12% out-migration was modelled per year. Furthermore, we assumed an average of one sex contact per person per year with a visitor from the outside population for the 15–55 years age group. Visitors had sexual contact with persons of the same age group and both men and women were at risk of having sexual contacts with visitors (based on a fully random process).
HIV interventions
We simulated two interventions, both starting in 2009. First, condom promotion campaigns: we assumed that condom promotion increased condom use from 25% to 50% (casual contacts) and from 50% to 75% (CSW contacts) from 2009 onwards. The second intervention was health education aimed at partner reduction. From 2009 onwards, health education was assumed to reduce the number of sexual partners and CSW visits by 25%.
To study the impact of non-participation of mobile people, we modelled four scenarios for each intervention. In the first scenario, both immigrants and travellers participated in the intervention of interest, similar to the rest of the population. In the second and third scenario, immigrants (during their adaptation period of 5 years), or travellers (while away from home), did not participate at all, while the other group participated as in the first scenario. In the fourth scenario, both mobile groups did not participate. Non-participation of travellers while away from home was defined as non-adherence to the intervention when travelling; travellers did participate in the intervention of interest when at home.
Targeting of control at travellers was modelled through a combined intervention campaign, consisting of health education and condom promotion. The campaign started in 2009 and was targeted at two travel groups in STDSIM: highly mobile people (3%) and market traders/visitors to ceremonies (20%), thus in total at 23% of the population. Targeting is by definition adapted to a certain group and is likely to result in higher participation than general population interventions. We therefore assumed that health education resulted in 50% reduction in the average number of sexual partners and CSW visits. Condom promotion increased condom use to 75% during casual contacts and to 95% during commercial sex while away from home. This targeted campaign was compared to the HIV interventions in the general population in which both mobile groups did not participate (described above).
In a sensitivity analysis, we explored the impact of travellers having a 2–4 times more risky behaviour than in the baseline assumptions. The chance of visiting a CSW was increased 2–4 times for male travellers, and the time interval for one-off contacts was decreased 2–4 times for both male and female travellers. Results of each scenario were averaged over 100 runs to reduce the influence of random fluctuations associated with stochastic modelling. All results concern the general adult population aged 15–55 years, except where the available data reported HIV prevalence for those aged 15–45 years (see Fig. 1).
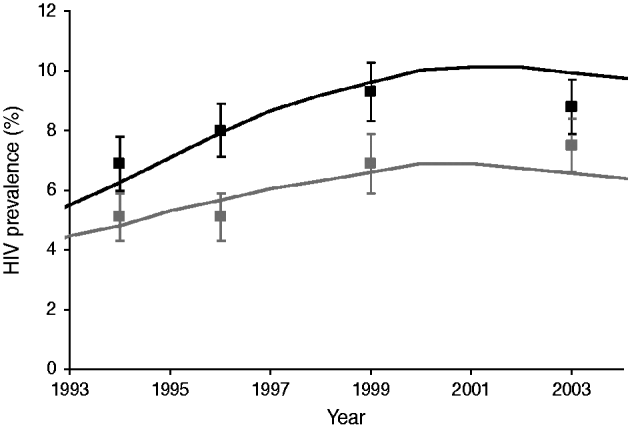
Fig. 1. Simulated and observed HIV prevalence (%) in men and women aged 15–45 years in Kisesa, Tanzania. Black lines and symbols=women; grey lines and symbols=men; markers indicate observed data with 95% confidence intervals; lines indicate the model prediction in the study population.
RESULTS
In Figure 1, data for adults aged 15–45 years is shown for both simulated and empirical data. Simulated HIV prevalence in men and women fitted data from Kisesa reasonably well [Reference Wambura29]. Both model and data showed higher prevalences for women and an increasing trend until 2000. After the increase in condom use in 2000, HIV prevalence showed a slightly downward trend. This trend continued until the end of the simulation period in the baseline situation without HIV interventions (Fig. 2).
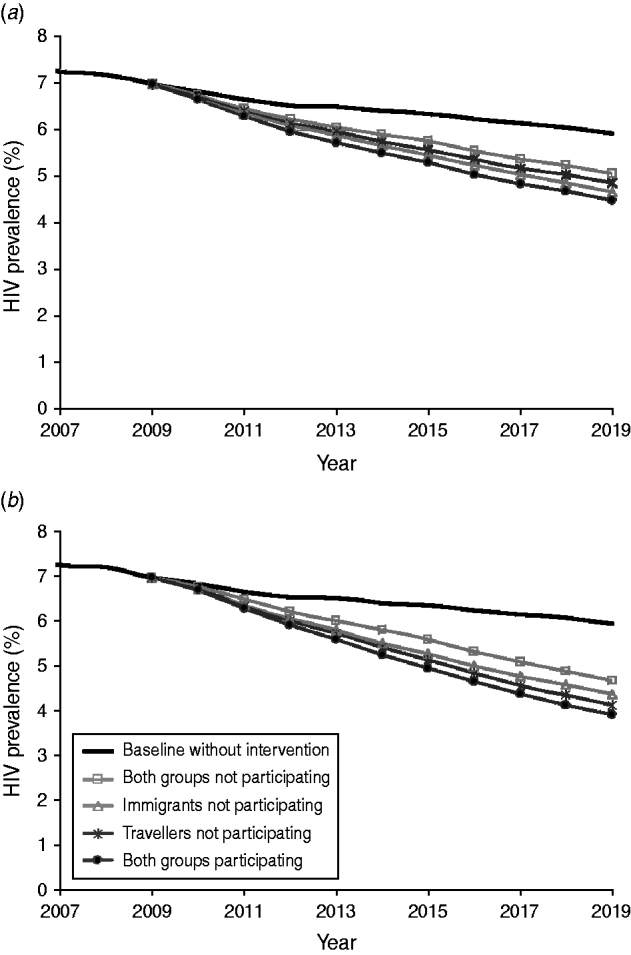
Fig. 2. Impact of participation and non-participation of mobile groups on the effectiveness of (a) the condom promotion intervention and (b) the health education intervention. HIV interventions started in 2009.
The sexual risk behaviour and HIV prevalence per mobile group resulting from our assumptions is presented in Table 2. Men aged 15–55 years had on average 41 sex acts per year. For women, this number was 40. About one fifth of the population consisted of recent immigrants (i.e. migrated <5 years ago). Young male immigrants (aged 15–25 years) had casual sex more often than young non-migrants, resulting in a higher HIV prevalence. After age 25 years, most men had steady relationships. Immigrants aged ⩾25 years still had a higher HIV prevalence, due to slightly more casual sex and CSW visits. Female immigrants showed similar patterns, although young immigrant women were more often married. At age ⩾25 years HIV prevalence was similar for recent immigrant and non-migrant women. Mobile groups are non-exclusive: migrants and non-migrants can also travel, and are on average away for 8% of the time. Migrants and non-migrants had on average two one-off contacts while away from home, and 0·14 CSW contacts if male. The total number of sex contacts was lowest in the group of travellers most often away (Table 2). However, their sexual behaviour was more risky, since sexual contacts within steady relationships occurred less often and contacts with one-off and CSW partners occurred much more frequently. This leads to the highest HIV prevalence in people who travel most: 8·1% in highly mobile men and 11·4% in highly mobile women.
Table 2. Sexual risk behaviour and HIV prevalence for men and women in 2009, grouped by mobility status
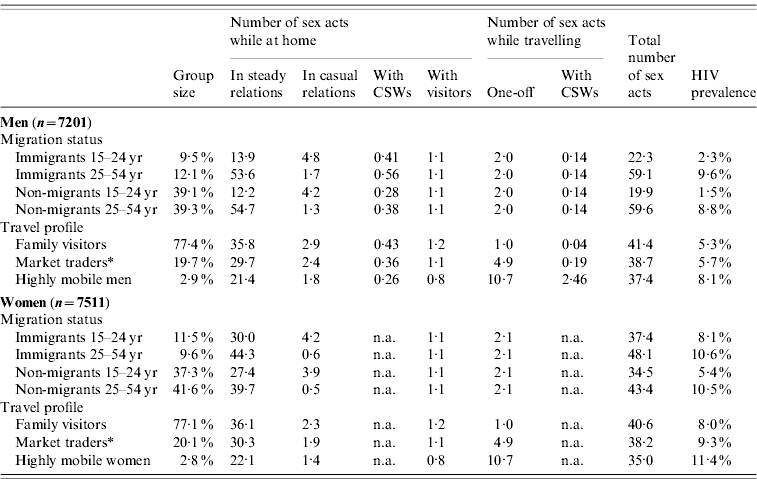
CSW, Commercial sex worker; n.a., non-applicable.
* This group of travellers also includes those attending ceremonies such as marriages and funerals.
Table 3 gives the overall number of averted HIV cases in a 10-year period for the different interventions. The effectiveness of condom promotion and health education if both mobile groups participated was estimated at 19% and 26% prevented HIV cases, respectively. Non-participation of immigrants and travellers while away from home could reduce the impact of both interventions by about 40% (Table 3). Figure 2 illustrates the HIV prevalence over time for the different scenarios for condom promotion (Fig. 2a) and health education (Fig. 2b). By 2019, HIV prevalence could decrease from 5·9% without intervention to 4·5% with condom promotion and to 3·9% with health education. Non-participation of one or both mobile groups will lead to reduced effectiveness of these interventions (Fig. 2).
Table 3. Averted HIV cases/100 000 HIV-negative adult person-years in the period 2010–2019, and the impact of non-participation of mobile groups

The baseline situation without any intervention resulted in 1538 HIV cases in the period 2010–2019. Non-participation of mobile groups means that immigrants do not participate in the 5 years after immigration, and that travellers do not adhere to the intervention while away from home.
* 295 corresponds to a 19% decrease in HIV cases; 401 corresponds to a 26% decrease in HIV cases.
Table 4 shows the impact of the combined targeted intervention in travel groups compared to HIV interventions in the general population. With the targeted campaign the number of new HIV cases in the period 2010–2019 was reduced by 11%. This is only slightly less than campaigns with health education or condom promotion in the general population.
Table 4. The impact of targeting travellers on averted HIV cases and reduction in HIV incidence for the period 2010–2019
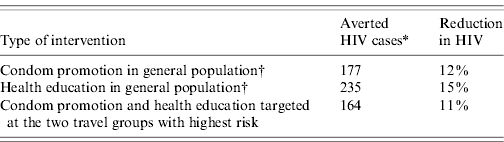
The baseline situation without any intervention resulted in 1538 HIV cases in the period 2010–2019.
* Averted HIV cases/100 000 HIV-negative adult person-years.
† Mobile groups do not participate in general population interventions.
Sensitivity analysis showed that condom promotion was more sensitive to the risk behaviour of travellers than health education. Due to non-participation of both mobile groups condom promotion effectiveness was reduced by 40% in the baseline situation, by 47% with two times more risky behaviour, and by 56% when risk behaviour was four times more risky. The effectiveness of health education was reduced by 41%, 43%, and 45%, respectively. Overall, results did not change drastically if the risk behaviour of travellers away from home was increased.
DISCUSSION
With our assumptions, immigrants and travellers who were more often away from home had considerably more HIV infections than non-migrants and people who travelled less often. This was mainly due to young immigrants more often having casual sex, and frequent travellers engaging more frequently in risky sex with one-off contacts and CSWs. We also showed that mobility could considerably reduce the effect of different HIV intervention strategies. If recent migrants and travellers do not participate in the intervention of interest, the effectiveness could be reduced by 40%. On the other hand, targeting of these interventions at travellers can have a substantial impact on HIV incidence, comparable to the same, but less stringent, interventions in the general population.
Since mobility is an important risk factor for HIV infection, it should not be neglected in modelling studies on HIV transmission and control [Reference Nunn1, Reference Decosas and Adrien33, Reference Quinn34]. Until now, only a few modelling studies on HIV or other STDs have taken migration into account [Reference Coffee, Lurie and Garnett18, Reference Walker19]. Coffee et al. [Reference Coffee, Lurie and Garnett18] used a deterministic model to evaluate the interactions between migration, sexual behaviour and HIV infection in South Africa. They showed that migration increases HIV prevalence by enhanced risk behaviour, and less so by linking geographically separate epidemics. Walker et al. [Reference Walker19] used a deterministic model to study the impact of migration on declining HIV epidemics. They found that trends in HIV prevalence are influenced when migration rates change and when people migrating have different HIV risks compared to the rest of the population. Our study is the first to explore both migration and travelling behaviour.
The impact of non-participation of mobile groups on the effectiveness of HIV interventions may be explained by the simple fact that this relatively large group of persons is non-participating, and less so by their increased risk. To study how much is explained by random non-participation of people, we compared this random non-participation with non-participation of immigrants. Results for condom promotion are largely explained by non-participation per se. In contrast, for health education it does matter who is non-participating: reduction in effectiveness was more than doubled if immigrants were non-participating, i.e. 25% (Table 3) vs. 11% (not shown). This can be explained by the increased risk behaviour of immigrants in the first 5 years.
It is less straightforward to compare non-participation of travellers with equal-sized random non-participation, since travellers are assumed to only keep to their pre-intervention behaviour when they are away from home. Although not modelled, it is expected that it does matter who is non-participating. The main reason is the type of sex partners when away from home. These are one-off contacts and CSW visits (for male travellers). CSWs are more often HIV infected, therefore the likelihood of getting HIV is higher. One-off contacts may also form a risk, since each sex contact will be with a different partner and having multiple sex partners is a risk factor for HIV infection [Reference Malamba35].
We assumed that persons in the study population would on average have one sexual contact with a visitor per year. These sexual contacts with visitors were randomly distributed in the 15–55 years age group, thereby including those usually living in monogamous relationships. This assumption may have led to some overestimation of the predicted impact of contacts with visitors on the spread of HIV, since one study indicated that these contacts, especially for men, are usually with unmarried persons [Reference Boerma30].
In most HIV prevention strategies, health education campaigns are combined with condom campaigns, and possibly with other strategies such as voluntary counselling and testing. In our model simulations, interventions were kept separate to calculate the impact of non-participation per intervention.
Mobile groups can be missed in HIV surveys and prevention programmes. These persons are highly vulnerable to becoming HIV infected due to their sexual risk behaviour and social circumstances. Prevention programmes can be expanded by making antiretroviral treatment and condoms available and easy to find and affordable for all people in an area, including new immigrants.
Another option is to specifically target mobile groups. Often it will not be possible to reduce mobility behaviour, so interventions should try to change risk behaviour of travellers and immigrants. Examples of such targeted interventions are condom distribution and education activities at truck stops [Reference Nyamuryekung'e36]. In our exploration of targeting travellers, we observed that the impact on HIV incidence could be substantial. We assumed that travellers who are targeted in order to reduce their number of sexual partners and to increase condom use in risky contacts would only do so when they are travelling, and not when they are at home. It is, however, likely that they will also change their behaviour while at home. Therefore, the expected impact of targeting may even be higher than the 11% found.
The targeting campaign involving 23% of the population may have a considerable impact, and it may cost less than general interventions since fewer people need to participate. The main challenge will be to reach these people. Options to involve travellers include the provision of health education at truck and bus stops, or along main routes. A study along the trans-African highway in Kenya and Uganda showed that availability of condoms along the route may increase uptake [Reference Morris37]. Another possibility of making contact with travellers may be at ceremonies such as funerals or marriages. These ceremonies may involve large groups of people and can last for several days, for example disco funerals in Kenya [Reference Njue38]. Disco funerals provide opportunities to engage in risky sex, involving casual sex, sometimes with multiple partners and mostly without condoms. Although the privacy of the family of the deceased should of course be respected, it may be good to discuss HIV risks and prevention strategies in these settings. Disc-jockeys can be used as peer educators and condoms may be freely available [Reference Njue38].
In conclusion, this modelling study showed that non-participation of mobile groups can considerably reduce the effectiveness of HIV control strategies. It will be worthwhile to further explore to what extent migrants and travellers are non-participating in prevention programmes. In areas with high levels of mobility and substantial non-participation of mobile people, impact of HIV interventions may considerably be improved by actively addressing these people. Moreover, specific targeting of these groups may form an interesting prevention strategy.
ACKNOWLEDGEMENTS
This work was supported by the European Commission (Contract B7.6211/99/010). The authors are grateful to Dr Richard White and Dr Eline Korenromp for their advice in using the STDSIM model.
DECLARATION OF INTEREST
None.








