There is convergent evidence that basal ganglia structures and their connections, especially with frontal cortices, are implicated in obsessive–compulsive disorder (OCD) pathogenesis. Consistent neuroimaging data, Reference Radua and Mataix-Cols1 and more recent neurosurgical Reference de Koning, Figee, van den Munckhof, Schuurman and Denys2 and neurophysiological Reference Guehl, Benazzouz, Aouizerate, Cuny, Rotge and Rougier3 evidence, suggests that basal ganglia structures are involved, particularly with regard to their connections with the frontal cortices in the ‘corticostriatal’ loops that are postulated to be aberrant in OCD psychopathology. Reference Cavedini, Gorini and Bellodi4 It has also recently been suggested that OCD and its related disorders should be classified separately from the other anxiety disorders, partly because of the weight and consistency of neurobiological findings. Reference Stein, Fineberg, Bienvenu, Denys, Lochner and Nestadt5 Sydenham chorea that follows rheumatic fever, is also thought to be a basal ganglia disorder and has high rates of OCD with new onset obsessive–compulsive symptoms seen in up to 70% of acute cases. Reference Asbahr, Negrao, Gentil, Zanetta, da Paz and Marques-Dias6 An autoimmune post-streptococcal aetiology is generally accepted for Sydenham chorea and evidence of streptococcal infection is part of the diagnostic criteria.
There is now growing evidence for a range of neuropsychiatric disorders associated with post-streptococcal autoimmunity. Specific antibodies directed against basal ganglia antigens have been isolated (anti-basal ganglia antibodies (ABGA)) from individuals with Sydenham chorea, and are thought to be a component of the immune response to infection with group A streptococcus. Reference Martino and Giovannoni7 Such cases often have evidence of streptococcal infection with raised anti-streptolysin-O titres (ASOT). Sydenham chorea was the first such disorder in which these antibodies were characterised Reference Church, Cardoso, Dale, Lees, Thompson and Giovannoni8 but an increasingly broad range of conditions have been found to be associated with ABGA; from discrete neurological conditions such as dystonia, Reference Edwards, Trikouli, Martino, Bozi, Dale and Church9 to ‘classical’ neuropsychiatric disorders such as Tourette syndrome Reference Church, Dale, Lees, Giovannoni and Robertson10 and to the rarer syndromes of encephalitis lethargica Reference Dale, Church, Surtees, Lees, Adcock and Harding11 and paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Reference Swedo, Leonard, Garvey, Mittleman, Allen and Perlmutter12 High rates of obsessive–compulsive symptoms are found in these disorders, with onset of symptoms coinciding with the onset of the disorder.
We set out to test the hypothesis that there is an increased incidence of ASOT or ABGA positivity in adults with OCD compared with a control group of individuals with either depression or schizophrenia. We also examined for differences in clinical variables between the two groups according to ABGA status, hypothesising that a younger age at onset of symptoms, the presence of comorbid tics or Tourette syndrome and a history of throat infections may be associated with ABGA positivity.
Method
Participants
Participants were selected from specialist clinics according to existing diagnoses that were confirmed by the research team. Eligibility criteria were a diagnosis of OCD (or schizophrenia or unipolar affective disorder for participants in the control group) by DSM-IV 13 criteria.
Ninety-six participants with OCD (the OCD group) were recruited during 2006–7 from the UK National OCD specialist out-patient and in-patient services at: (a) Springfield Hospital, The South West London and St George's Trust, London, (b) Queen Elizabeth II Hospital, Hertfordshire Partnership NHS Foundation Trust, Welwyn Garden City, and (c) Bethlem Royal Hospital, The South London and Maudsley NHS Foundation Trust, London. Clinical data were collected on the same day as phlebotomy.
Non-contemporaneous controls were recruited from two sites. This group was made up of 33 people with chronic depression (unipolar affective disorder) (Maudsley Hospital, London) and 17 people with chronic schizophrenia (Addenbrooke's Hospital, Cambridge). These participants were recruited from specialist clinics to represent disorders similar in chronicity and severity to OCD.
A power calculation informed sample sizes. The findings in childhood OCD revealed ABGA positivity of 42% compared with 4% in a neurological control group. Reference Dale, Heyman, Giovannoni and Church14 We made a conservative assumption that adults would be half as likely as children to be ABGA positive (21%) and that the psychiatric control group might have a similar positivity to the neurological control group at 4%. Based on these assumptions the OCD group need 100 participants and the control group 50 participants to achieve a power of 80% at standard significance levels of 5%.
The UK National Research Ethics Service (study reference number: 06/Q1702/48) approved this study. Written informed consent was obtained from each participant.
Clinical measures
The following clinical information was collected from the OCD group on the day of phlebotomy. Psychiatric, medical, family and treatment (including medication and psychotherapy response) histories were recorded. Treatment resistance was defined as treatment with a serotonin reuptake inhibiting medication and either an antipsychotic or a full cognitive–behavioural therapy (CBT) course without complete and sustained remission while undergoing treatment. Participants were asked whether they had previously been diagnosed with confirmed streptococcal infections, and symptoms compatible with tics and Tourette syndrome. A history of Sydenham chorea or rheumatic fever was assessed.
Current obsessive–compulsive symptoms were assessed using a self-rating symptom scale (the Obsessive-Compulsive Inventory Revised version, OCI-R), Reference Foa, Huppert, Leiberg, Langner, Kichic and Hajcak15 and an observer-rated scale, the Yale–Brown Obsessive-Compulsive Schedule (Y–BOCS) Reference Goodman, Price, Rasmussen, Mazure, Fleischmann and Hill16 including the symptom checklist (Y–BOCS-SC). Current depression was measured using the Montgomery–Åsberg Depression Rating Scale (MADRS). Reference Montgomery and Åsberg17 Current and past psychiatric comorbidity was evaluated using the Mini International Neuropsychiatric Interview (MINI). Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller18
Serological analysis
Anti-basal ganglia antibodies assays were performed by T.R.J.N. with methods previously described; Reference Church, Cardoso, Dale, Lees, Thompson and Giovannoni8,Reference Dale, Candler, Church, Wait, Pocock and Giovannoni19 western immunoblotting was used to detect antibodies against recombinant commercial antigens of pyruvate kinase, neuronal-specific enolase and aldolase C (the antigens for ABGA). The Invitrogen mini-gel, Nu-PAGE system (Invitrogen, Carlsbad, USA) was used with lithium dodecyl sulphate buffer. The gel was transferred to nitrocellulose (BioRad, Hemel Hempstead, UK) and the proteins blocked with 2% milk proteins. Blots were loaded onto manifolds and serum samples (diluted 1:300) were incubated overnight before being washed with 0.9% saline with 0.2% milk proteins and 0.025% tween. Secondary antibodies were then added and incubated for 2 h; for assay positive control lanes rabbit anti-goat and rabbit anti-mouse antibodies were used and for sample lanes rabbit anti-Human immunoglobulin G, FAB fragments. Blots were washed again and developed with 4-chloro-1-napthol for 15–30 min and interpreted by an experienced observer (A.J.C.) masked to case–control status. Anti-streptolysin-O titres were performed by T.R.J.N. using latex agglutination kits (Spectrum Diagnostics Ltd, Cairo, Egypt).
Statistical analyses
All statistical tests were performed with SPSS 15.0 software on Windows. Fisher's exact or chi-squared tests were used to compare ABGA and ASOT positivity between OCD and control groups. Clinical characteristics in the ABGA positive and negative participants were compared using chi-squared, Fisher's exact or t-tests (according to the nature of the variable) and also using logistic regression.
Association between OCD symptom dimensions and ABGA status was also investigated. This was done with logistic regression using both the OCI-R and the Y–BOCS-SC, Reference Goodman, Price, Rasmussen, Mazure, Fleischmann and Hill16 which can be used to generate symptom dimensions for individuals according to the specific combination of symptoms each person has. The OCI-R items can be grouped into six dimensions: washing, obsessing, hoarding, ordering, checking or neutralising. Reference Foa, Huppert, Leiberg, Langner, Kichic and Hajcak15
In the Y–BOCS-SC, symptoms are organised in 13 major categories plus 2 additional miscellaneous categories. We coded symptoms in two different ways. First, if a participant endorsed any of the individual symptoms under each of the 13 major symptom categories during their lifetime, a score of 1 was given (‘lifetime present’). If none of the individual items was endorsed, that particular category was scored 0 (‘lifetime absent’). In addition, the number of endorsed lifetime symptoms under each of the 13 major symptom categories of the Y–BOCS-SC was summed in order to take full advantage of the variance within the data. This method offers an approximation to the severity of each symptom type. Reference Leckman, Grice, Boardman, Zhang, Vitale and Bondi20 An algorithm was used to calculate four symptom dimension scores, based on results of a recent meta-analysis. Reference Bloch, Landeros-Weisenberger, Rosario, Pittenger and Leckman21 Scores of the contamination obsessions and cleaning compulsions were summed to form the ‘contamination/cleaning’ dimension. Scores of the aggressive, sexual, religious and somatic obsessions as well as checking compulsions were summed to form the ‘forbidden thoughts’ dimension. Symmetry obsessions and ordering/arranging, counting and repeating compulsions were summed to form the ‘symmetry/order’ dimension. Finally, hoarding obsessions and compulsions were summed to form the ‘hoarding’ symptom dimension. Scores on the resulting four dimensions were used as independent variables in the analyses.
Results
Demographic comparisons
A total of 45.8% of the OCD group and 36.0% of the control group were male and this difference was not significant (chisquared text P = 0.25). The mean age of the OCD group was 42.4 years (s.d. = 13.3, range 18–80, Table 1) and the mean age of the control group was 45.72 years (s.d. = 13.1, range 20–73), and again this difference was not significant (t-test P = 0.16).
Serological analyses
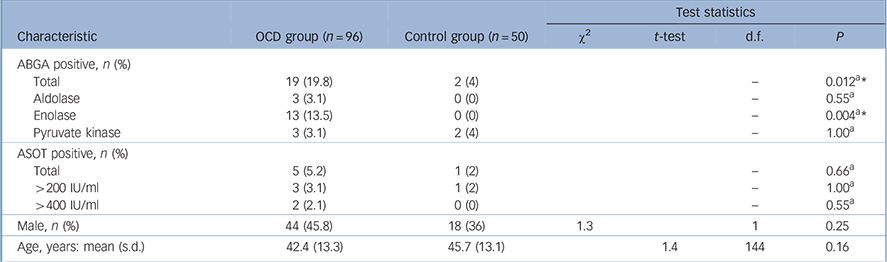
Rates of positivity for ABGA were 19.8% in the OCD group, which were significantly raised compared with 4% in the control group (Fisher's exact P = 0.012, Table 1). The enolase antigen accounted for the majority (13/19) of the positive ABGAs in the OCD group. No individuals were positive for more than one antigen. Only 1 of the 19 ABGA positive participants in the OCD group was also positive for ASOT and neither of the 2 ABGA positive controls had a positive ASOT. Increased rates of ASOT positivity were found in the OCD group compared with the control group (particularly titres >400 IU/ml) but the rates of positivity in both the OCD and control groups were low and the differences were not significant.
TABLE 1 Comparison between obsessive–compulsive disorder (OCD) and control group for key variables
| Test statistics | ||||||
|---|---|---|---|---|---|---|
| Characteristic | OCD group (n = 96) | Control group (n = 50) | σ2 | t-test | d.f. | P |
| ABGA positive, n (%) | ||||||
| Total | 19 (19.8) | 2 (4) | – | 0.012Footnote a Footnote * | ||
| Aldolase | 3 (3.1) | 0 (0) | – | 0.55Footnote a | ||
| Enolase | 13 (13.5) | 0 (0) | – | 0.004Footnote a Footnote * | ||
| Pyruvate kinase | 3 (3.1) | 2 (4) | – | 1.00Footnote a | ||
| ASOT positive, n (%) | ||||||
| Total | 5 (5.2) | 1 (2) | – | 0.66Footnote a | ||
| >200 IU/ml | 3 (3.1) | 1 (2) | – | 1.00Footnote a | ||
| >400 IU/ml | 2 (2.1) | 0 (0) | – | 0.55Footnote a | ||
| Male, n (%) | 44 (45.8) | 18 (36) | 1.3 | 1 | 0.25 | |
| Age, years: mean (s.d.) | 42.4 (13.3) | 45.7 (13.1) | 1.4 | 144 | 0.16 | |
ABGA, anti-basal ganglia antibody; ASOT, anti-streptolysin-O titre.
a. Fisher's exact test.
* Significant at P<0.05.
Other analyses
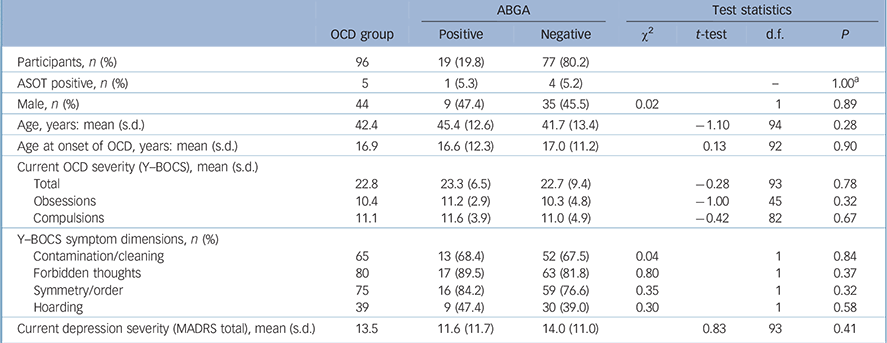
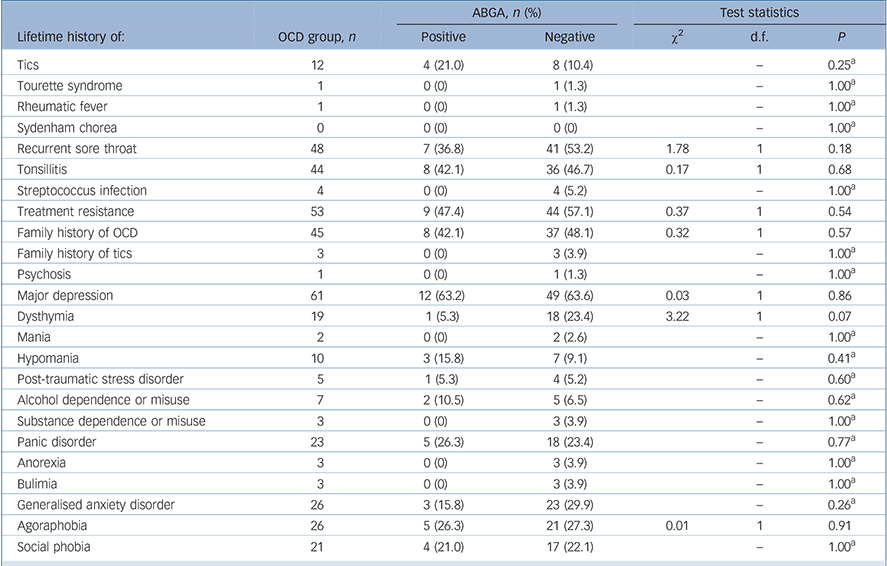
The mean age at onset of OCD was 16.9 years (s.d. = 11.4, range 2–70) and mean Y–BOCS (severity) scores were 22.8 (s.d. = 8.9, range 3–40, Table 2). There was no association between ABGA positivity and age, gender, ASOT titres, age at onset of OCD, severity of OCD (Y–BOCS or OCI-R scores), treatment resistance, presence of psychiatric comorbidity on MINI screen, severity of depression (MADRS score), history of tics or Tourette syndrome, rheumatic fever, sore throats, tonsillitis or streptococcal infections. Indeed, one participant had a history of rheumatic fever but was found to be ABGA negative. Logistic regression was also used to look for associations and none were found. No consistent significant associations were found between ABGA positivity and symptom dimensions of OCD (OCI-R and Y–BOCS-SC) using the methodology described. Table 3 details the association between ABGA status and the lifetime history for various conditions in the OCD group.
TABLE 2 Characteristics of obsessive–compulsive disorder (OCD) group according to presence of anti-basal ganglia antibodies (ABGA)
| ABGA | Test statistics | ||||||
|---|---|---|---|---|---|---|---|
| OCD group | Positive | Negative | σ2 | t-test | d.f. | P | |
| Participants, n (%) | 96 | 19 (19.8) | 77 (80.2) | ||||
| ASOT positive, n (%) | 5 | 1 (5.3) | 4 (5.2) | – | 1.00Footnote a | ||
| Male, n (%) | 44 | 9 (47.4) | 35 (45.5) | 0.02 | 1 | 0.89 | |
| Age, years: mean (s.d.) | 42.4 | 45.4 (12.6) | 41.7 (13.4) | –1.10 | 94 | 0.28 | |
| Age at onset of OCD, years: mean (s.d.) | 16.9 | 16.6 (12.3) | 17.0 (11.2) | 0.13 | 92 | 0.90 | |
| Current OCD severity (Y–BOCS), mean (s.d.) | |||||||
| Total | 22.8 | 23.3 (6.5) | 22.7 (9.4) | –0.28 | 93 | 0.78 | |
| Obsessions | 10.4 | 11.2 (2.9) | 10.3 (4.8) | –1.00 | 45 | 0.32 | |
| Compulsions | 11.1 | 11.6 (3.9) | 11.0 (4.9) | –0.42 | 82 | 0.67 | |
| Y–BOCS symptom dimensions, n (%) | |||||||
| Contamination/cleaning | 65 | 13 (68.4) | 52 (67.5) | 0.04 | 1 | 0.84 | |
| Forbidden thoughts | 80 | 17 (89.5) | 63 (81.8) | 0.80 | 1 | 0.37 | |
| Symmetry/order | 75 | 16 (84.2) | 59 (76.6) | 0.35 | 1 | 0.32 | |
| Hoarding | 39 | 9 (47.4) | 30 (39.0) | 0.30 | 1 | 0.58 | |
| Current depression severity (MADRS total), mean (s.d.) | 13.5 | 11.6 (11.7) | 14.0 (11.0) | 0.83 | 93 | 0.41 | |
ASOT, anti-streptolysin-O titre; Y–BOCS, Yale–Brown Obsessive Compulsive Scale; MADRS, Mongomery–Åsberg Depression Rating Scale.
a. Fisher's exact test.
Discussion
Findings from other studies
There is some preliminary evidence in other studies of both childhood and adult OCD being associated with anti-brain antibodies (including ABGA), particularly in those with neuropsychiatric comorbidity. An increased incidence of ABGA (42%) was found in 50 children with OCD, particularly in those with comorbid Tourette syndrome or tics, compared with multiple control groups. Reference Dale, Heyman, Giovannoni and Church14 A smaller study of 32 children found some consistent but non-significant findings, Reference Morer, Lazaro, Sabater, Massana, Castro and Graus22 and another study found no increase in anti-brain antibodies in 13 children with OCD alone or in 23 children with OCD plus chronic tic disorder, but did find some evidence for increased incidence in 20 children with both OCD and PANDAS. Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer23
TABLE 3 Lifetime history of various conditions in the obsessive–compulsive disorder (OCD) group according to presence of anti-basal ganglia antibodies (ABGA)
| ABGA, n (%) | Test statistics | |||||
|---|---|---|---|---|---|---|
| Lifetime history of: | OCD group, n | Positive | Negative | σ2 | d.f. | P |
| Tics | 12 | 4 (21.0) | 8 (10.4) | – | 0.25Footnote a | |
| Tourette syndrome | 1 | 0 (0) | 1 (1.3) | – | 1.00Footnote a | |
| Rheumatic fever | 1 | 0 (0) | 1 (1.3) | – | 1.00Footnote a | |
| Sydenham chorea | 0 | 0 (0) | 0 (0) | – | 1.00Footnote a | |
| Recurrent sore throat | 48 | 7 (36.8) | 41 (53.2) | 1.78 | 1 | 0.18 |
| Tonsillitis | 44 | 8 (42.1) | 36 (46.7) | 0.17 | 1 | 0.68 |
| Streptococcus infection | 4 | 0 (0) | 4 (5.2) | – | 1.00Footnote a | |
| Treatment resistance | 53 | 9 (47.4) | 44 (57.1) | 0.37 | 1 | 0.54 |
| Family history of OCD | 45 | 8 (42.1) | 37 (48.1) | 0.32 | 1 | 0.57 |
| Family history of tics | 3 | 0 (0) | 3 (3.9) | – | 1.00Footnote a | |
| Psychosis | 1 | 0 (0) | 1 (1.3) | – | 1.00Footnote a | |
| Major depression | 61 | 12 (63.2) | 49 (63.6) | 0.03 | 1 | 0.86 |
| Dysthymia | 19 | 1 (5.3) | 18 (23.4) | 3.22 | 1 | 0.07 |
| Mania | 2 | 0 (0) | 2 (2.6) | – | 1.00Footnote a | |
| Hypomania | 10 | 3 (15.8) | 7 (9.1) | – | 0.41Footnote a | |
| Post-traumatic stress disorder | 5 | 1 (5.3) | 4 (5.2) | – | 0.60Footnote a | |
| Alcohol dependence or misuse | 7 | 2 (10.5) | 5 (6.5) | – | 0.62Footnote a | |
| Substance dependence or misuse | 3 | 0 (0) | 3 (3.9) | – | 1.00Footnote a | |
| Panic disorder | 23 | 5 (26.3) | 18 (23.4) | – | 0.77Footnote a | |
| Anorexia | 3 | 0 (0) | 3 (3.9) | – | 1.00Footnote a | |
| Bulimia | 3 | 0 (0) | 3 (3.9) | – | 1.00Footnote a | |
| Generalised anxiety disorder | 26 | 3 (15.8) | 23 (29.9) | – | 0.26Footnote a | |
| Agoraphobia | 26 | 5 (26.3) | 21 (27.3) | 0.01 | 1 | 0.91 |
| Social phobia | 21 | 4 (21.0) | 17 (22.1) | – | 1.00Footnote a | |
a. Fisher's exact test.
A study of 74 adults with OCD found a significant increase in positivity of ASOT compared with controls with major depression, indicating a possible link to streptococcal infections, and a non-significant increase in anti-neuronal positivity. Reference Maina, Albert, Bogetto, Borghese, Berro and Mutani24 A study of 23 adults with OCD found an increased incidence of ABGA (and antibodies against the thalamus) in the cerebrospinal fluid (CSF), but not the serum, compared with healthy controls. Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar25 None of these studies in adults used the same methodology for detecting ABGA as our study.
Three antigens for ABGA have since been described Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller18 and are neuronal surface glycolytic enzymes (pyruvate kinase, enolase and aldolase C), and they share significant homology with streptococcal proteins. This is consistent with the hypothesis of ‘molecular mimicry’, which postulates that pathogens have evolved surface molecules similar to host proteins that are presumed to aid avoidance of detection or destruction by the host immune system. Reference Kirvan, Swedo, Kurahara and Cunningham26 Pyruvate kinase has also been identified as an antigen for ABGA in a series of participants with Tourette syndrome. Reference Kansy, Katsovich, McIver, Pick, Zabriskie and Lombroso27 We had no a priori hypothesis to expect enolase to constitute the majority of the positive results and if this finding is replicated it would warrant specific investigation with respect to OCD pathogenesis. It should also be noted that other groups have found evidence of other potential antigens (such as tubulin, ganglioside and the dopamine receptor) in the same range of clinical syndromes and these may also be relevant to OCD and would clearly also warrant further investigation. Reference Murphy, Kurlan and Leckman28
The occurrence of these specific anti-basal ganglia antibodies is rare in control populations, Reference Martino, Church and Giovannoni29 although rates of exposure to streptococci, that are postulated to stimulate antibody production, are relatively common. As both autoimmune disorders Reference Atassi and Casali30 and OCD Reference Westenberg, Fineberg and Denys31 are known to be partly genetic, it is possible that a particular genetic background, in conjunction with exposure to streptococcal infection, represents one trajectory for the development of OCD. There may also be variables in the strain of streptococcus that influence the autoimmune response in those infected. There are some initial animal models for the proposed autoimmune hypothesis, which more directly attempt to establish a pathogenic role for streptococcal autoantibodies. Reference Yaddanapudi, Hornig, Serge, De Miranda, Baghban and Villar32 It should be noted that in this study we did not find an increase in ASOT positivity in the OCD group compared with the control group nor did we find an association between ABGA positivity and ASOT positivity, although assessing exposure to streptococcal bacteria by single measurements of ASOT is thought to be highly problematic, Reference Johnson, Kurlan, Leckman and Kaplan33 particularly if the onset of clinical variables was many years ago, as was the case with all the participants in our study.
If exposure to streptococcal infection is established as a risk factor for the development of OCD in certain susceptible individuals, there may be potential for novel or modified treatments. In a small group of children with the PANDAS form of post-streptococcal neuropsychiatric illness, obsessive–compulsive symptoms significantly improved with removal of the circulating antibodies by plasmapheresis. Reference Perlmutter, Leitman, Garvey, Hamburger, Feldman and Leonard34 There have also been trials of prophylactic antibiotics in the PANDAS groups, with the expectation that prevention of recurrent infection should prevent repeated rises in autoantibody titre, and in turn prevent recurrence of psychiatric symptoms. To date there has been one positive Reference Snider, Lougee, Slattery, Grant and Swedo35 and one negative Reference Garvey, Perlmutter, Allen, Hamburger, Lougee and Leonard36 trial, and the current clinical consensus is that there is insufficient evidence for antibiotic prophylaxis to represent standard clinical practice in this group of children. Indeed, trials of treatment in children with PANDAS have suggested that their obsessive–compulsive symptoms respond to the conventional evidence-based treatments of CBT and/or serotonin reuptake inhibiting medication. Reference Storch, Murphy, Geffken, Mann, Adkins and Merlo37 However, it should be noted that PANDAS remains controversial as a clinical entity. Reference de Oliveira and Pelajo38
We found no support for our hypotheses that ABGA positivity might be associated with a history of recurrent throat infections or the presence of comorbid tics, Tourette syndrome or other impulse control disorders such as trichotillomania (which was screened for in the Y–BOCS checklist). Indeed, no such associations were found with any clinical variables, including those such as age at onset and treatment resistance that might be expected given the studies of ABGA in PANDAS and OCD in children. This could relate to the low incidence of some of these clinical variables and the study may therefore have been underpowered to pick up such associations. It is interesting to note that similarly there were no distinguishing features in a recent study of three people with first-episode psychosis that were positive for anti-brain autoantibodies. Reference Zandi, Irani, Lang, Waters, Jones and McKenna39 It is therefore possible that there might be no way of determining a possible autoimmune aetiology from clinical features and this could have wider reaching implications for the management of these conditions. We did not assess cognitive function in this study and it would be interesting to assess this in future work to see whether the type and severity of deficits differs between patients who are antibody positive or antibody negative.
Limitations
This study is limited by possible bias in that the samples were non-contemporaneously recruited and analysed and therefore not masked to the person performing the analysis. However, the rating of the blots, which is the part of the analysis most sensitive to bias, was masked. The study is further limited by its relatively small sample size. However, this study has sufficient power to detect the hypothesised differences between the OCD group and the control group based on previous studies. As the OCD group studied were recruited from specialist OCD services, they may not be generalisable to milder or less chronic and less treatment-resistant cases of OCD. However, despite this, at the time of recruitment the OCD group had mean Y–BOCS scores of 22.8, which is on the border of mild and moderate impairment. Replication of this result in other OCD populations and in other laboratories is therefore required, ideally with larger sample sizes.
Clinical implications
This study provides preliminary evidence that a significant proportion of unselected adults with OCD are associated with anti-basal ganglia antibodies. The association found does not imply causality; further work is also required to determine whether these antibodies are causally related to the development of obsessive–compulsive symptoms and do not represent an epiphenomenon secondary to another aetiological process. It is now increasingly recognised that to prove a disorder is antibody-mediated, extracellular antigen binding needs to be demonstrated – a preliminary study has recently found such evidence for individuals who were ABGA positive and had Sydenham chorea but was not found for individuals with PANDAS. Reference Brilot, Merheb, Ding, Murphy and Dale40 Further work is needed in establishing whether ABGAs are pathogenic or just a marker of inflammation and infection.
It would be premature for these findings to suggest additional investigations or different treatments in adults with OCD, especially as this study finds no correlation of clinical features with ABGA. People with OCD, particularly those who have suffered recurrent streptococcal infection, may be interested that this bacterial exposure could be one factor in their vulnerability to develop OCD.
Funding
The South London & Maudsley NHS Foundation Trust, the Institute of Psychiatry, London, and the Department of Neuroinflammation, Institute of Neurology, London.
Acknowledgements
We thank the individuals who have participated in this study and the clinicians who have assisted with sample collection at each site (Ashlesha Bagadia, Andrew Papadopolous, Shashi Rani, Rani Samuel and Professor Anne Farmer). We also thank Miles Chapman, for his help with ABGA assays, and Helen Galley, for help with data entry.






eLetters
No eLetters have been published for this article.