Kawasaki disease is an acute acquired vasculitis disease with specific predilection to inflammatory changes in the coronary arteries. Kawasaki disease is the most common acquired heart disease in children in developed countries. Reference Newburger, Takahashi and Burns1 Despite standard management in the acute phase, coronary artery aneurysms develop in approximately 4% of patients and may be more prevalent with delayed or missed treatment. Reference McCrindle, Rowley and Newburger2 While most coronary aneurysms resolve without intervention, larger aneurysms confer a significant risk for sudden death from thrombosis in the subacute phase of the disease; long-term vascular wall changes contribute to luminal narrowing, calcifications, and thrombosis that lead to acute myocardial ischaemia or ischaemic cardiomyopathy. Reference McCrindle, Manlhiot and Newburger3 Patients with large coronary aneurysms who are at high risk for coronary complications meet indication for chronic anticoagulation therapy according to the American Heart Association guideline recommendations. Reference McCrindle, Rowley and Newburger2
Due to both platelets and humoral clotting factors promoting thrombus formation within aneurysmal coronaries, recommended treatment includes a combination of antiplatelet and anticoagulant therapy. Reference McCrindle, Rowley and Newburger2 Standard-of-care anticoagulation with warfarin (targeting INR 2-3) and low-molecular-weight heparin have comparable efficacy, and selection is driven by secondary risk factors and patient preferences. Reference Monagle and Newall4 Direct oral anticoagulant agents have garnered particular interest as an alternative therapy in children given and the latest American Heart Association guidelines recognised their potential role for this specific indication. Reference McCrindle, Rowley and Newburger2 Compared to warfarin or low-molecular-weight heparin, direct oral anticoagulants are suggested to have more predictable pharmacokinetics with a quicker onset of action and a shorter half-life, and they have minimal drug–food interactions and fewer drug–drug interactions. Reference Monagle and Newall4,Reference Cohen, Levy-Mendelovich and Ageno5 Direct oral anticoagulants are also suggested to have a more predictable anticoagulation effect that is independent of antithrombin levels, do not require frequent monitoring of anticoagulation effect that is challenging and stressful in children and easily administered orally Reference Jones, Monagle and Manias6 . Previous studies of warfarin treatment in children with Kawasaki disease estimated underexposure with patient time in therapeutic range of approximately 50%. Reference Monagle and Newall4,Reference Baker, Vanderpluym and Gauvreau7 For direct oral anticoagulants, several recent clinical trials established stable pharmacokinetics and safety in children when varying dosing strategies, fixed or weight-based and often changed for clinical indication, renal, or hepatic dysfunction. Reference Albisetti8 These trials suggest at least non-inferiority for direct oral anticoagulants in preventing venous thromboembolisms or Fontan-associated thromboses. Reference Young, Lensing and Monagle9–Reference McCrindle, Michelson and Van Bergen11 The ENNOBLE-ATE trial [Edoxaban for Prevention of Blood Vessels Being Blocked by Clots (Thrombotic Events) in Children at Risk Because of Cardiac Disease, NCT03395639] was a phase III multi-institutional trial assessing the use of edoxaban for anticoagulation in children under age 18 years with cardiac disease as the underlying indication to therapy. Reference Bhatt, Portman and Berger12 This study followed 167 patients, including 37 patients with history of Kawasaki disease and large coronary artery aneurysms for whom prevention of coronary arterial thrombosis was the primary goal of treatment. The study found that between 109 children on the edoxaban arm and 58 children on standard anticoagulation (24 and 13 among the Kawasaki group, respectively), there was no significant difference in safety and efficacy. Edoxaban was dosed by age and body weight, once daily. Reference Portman, Jacobs and Newburger13 Following the conclusion of the clinical trial, our centre offered patients who met indication for chronic anticoagulation the option to continue off-label edoxaban or choose standard-of-care anticoagulation. We summarise medium- and long-term outcomes of bleeding and thrombosis events among this group of patients and report steady-state pharmacokinetic and pharmacodynamic parameters.
Method
A single-centre retrospective case review was performed, evaluating all patients who started treatment with edoxaban between 8/2018 and 12/2022. This study was approved by the Institutional Review Board at Seattle Children’s Hospital. Individuals (or their guardians) chose edoxaban off-label over warfarin or enoxaparin for anticoagulation for coronary artery aneurysms associated with Kawasaki disease (or similar vasculitis) in consultation with their cardiologist in Kawasaki Clinic, whether as continuation of treatment that was started as part of the ENNOBLE-ATE trial or as a new therapy. Patients were eligible for edoxaban treatment and were included in this study if they had history of Kawasaki disease (meeting latest guideline recommendations) and evidence of coronary aneurysms with dilation to a z-score≥10 per body surface area at the time of treatment. Also included in this cohort is one patient with coronary aneurysms associated with anti-neutrophil cytoplasmic autoantibody vasculitis, which we treated similarly to Kawasaki vasculitis.
Edoxaban was dosed once daily based on a composite of age and body weight: 1.4 mg/kg until age 6 years and 1.2 mg/kg for age 6 – <12 years to a maximal dose of 45 mg. For patients aged 12 to<18 years, standard dose of 45 mg was prescribed for body weight ≥30 and <60 kg and 60 mg for weight ≥60 kg; 30 mg would be prescribed for body weight <5th percentile or if patient met another indication for dose reduction Reference Bhatt, Portman and Berger12 . Edoxaban (Savaysa, Daiichi Sankyo, Inc.) is available in 15, 30, and 60 mg tablets that can be crushed into liquid for younger patients. All patients received low-dose aspirin 3–5 mg/kg/day (maximum 81 mg/day) in addition to edoxaban.
Some participants of the ENNOBLE-ATE trial elected to test edoxaban pharmacokinetic and pharmacodynamic indices, including peak and trough concentrations. These were not previously reported as part of the trial. Edoxaban (DU-176) plasma concentration was analysed using a previously validated spectrometric method at a range of 0.764–382 ng/mL. Reference He, Gajee and Mangaraj14 Blood draws for trough and peak concentrations were performed immediately before and 1 to 2 hours after dose administration, respectively, once patient had been receiving the medication for approximately 1 month. For this study, reference edoxaban concentrations were extrapolated from expected median peak and trough concentrations in adult patients treated with edoxaban 60 mg once daily for either stroke prevention in nonvalvular atrial fibrillation or treatment of pulmonary embolism or venous thromboembolism. The median and interquartile range concentrations provided by the International Council for Standardization in Hematology include expected peak of 170–234 ng/mL (interquartile range 125–317 ng/mL) and expected trough of 19 to 36 ng/mL (interquartile range 10–62 ng/mL). Reference Gosselin, Adcock and Bates15 Values for peak and trough concentrations were intended to be used as guides to provide evidence of drug absorption and accumulation or excessive clearance, respectively, not as therapeutic targets. Anti-factor Xa levels were measured together with the peak level as additional confirmation for edoxaban activity. Reference Ono, Nishimura and Takahashi16 Glomerular filtration rate was estimated using an available creatinine level from a time closest to the drug concentration draw, using the Bedside Schwartz formula using an available online calculator. 17
Chart review and collection of key demographics and clinical data were performed. One patient was excluded as no follow-up data were available after starting the medication as they relocated out of state. Data were summarised in a descriptive manner alone for a single cohort with no control group. Kaplan–Meier curve was used to review time to thrombosis event. Reference Kaplan and Meier18 Kaplan–Meier curves were produced using R version 4.2.1. Time zero was defined as the start date of edoxaban or the date of diagnosis, respectively, and coronary artery thrombosis events observed during follow-up (diagnosed by angiogram) were counted. Subjects who did not experience an event were censored at the end of follow-up, 48 months (for time on edoxaban) and 12 years (time from diagnosis).
Results
Sixteen patients met the inclusion criteria and were treated with edoxaban for chronic anticoagulation, including 11 patients who continued this therapy after participation in the ENNOBLE-ATE trial (demographic data are summarised in Table 1). Of this cohort, 15 patients had history of Kawasaki disease (6 with Infantile Kawasaki) and 1 patient had anti-neutrophil cytoplasmic autoantibody vasculitis. Patients started edoxaban treatment at a median age of 7 years (range: 1–17 years) and continued the medication for a median of 48.8 months (range: 5.6–60.4 months). All but one patient had been receiving chronic anticoagulation with enoxaparin (n = 8) or warfarin (n = 7) for a median period of 35 months (range: 1-160 months) since their initial diagnosis and prior to starting edoxaban. All patients continued single antiplatelet therapy with aspirin. No edoxaban dose reduction was required for any patient, and none were concomitantly receiving medications known to inhibit P-glycoprotein. To our knowledge, there were no interruptions in daily therapy for more than one day, and all patients remained adherent.
Table 1. Demographics and treatment summary

Continuous variables are presented as median (range).
Steady-state edoxaban peak and trough plasma levels are available for 10 patients (Table 2). Levels of edoxaban are presented in relation to expected interquartile range for standard adult dosing (Fig. 1). Reference Gosselin, Adcock and Bates15 One patient had measured peak concentration within expected median of 170–234 ng/mL, and 7 patients had values within the expected interquartile range of 125–317 ng/mL but above expected median. Two patients had peak levels above the expected range; neither of these patients had bleeding complications. All patients had a measured trough level below expected median values of 19–36 ng/mL. Two patients were below the trough interquartile range (10 ng/mL); these patients did not experience thrombosis during the study period (and, of note, both patients who experienced thrombosis did not have peak and trough levels measured). Edoxaban half-life ranged 3.72–5.59 hours which is more than 1 standard deviation below the mean half-life of 11.5 ± 5.63 hours reported in adults receiving edoxaban 60 mg once daily. Reference Parasrampuria and Truitt19 The area under the concentration-time curve for 24-hour (AUC0–24h) remained similar to reported average AUC values of 1766 ± 435.3 ng·h/ml, with 2 patients in this cohort below 1 standard deviation of this mean. Lastly, peak anti-factor Xa activity concentrations ranged between 1.51 – >2.0 IU/mL, within 1 standard deviation above mean peak anti-factor Xa activity levels reported in adults. Reference Parasrampuria and Truitt19

Figure 1. Peak and trough edoxaban concentrations (ng/mL) are charted for each patient and plotted by weight (kg). The dotted level lines mark the suggested interquartile range for median peak levels (317 and 125 ng/mL, respectively) and for median trough levels (62 and 10 ng/mL, respectively).
Table 2. Steady-state pharmacokinetic and pharmacodynamic characteristics of edoxaban in children (n = 10)
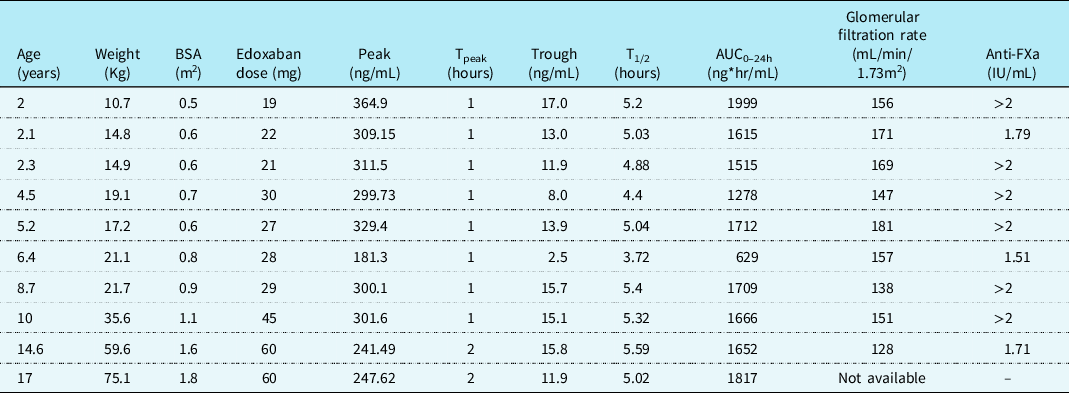
Peak and trough plasma concentration of edoxaban are presented for each patient from a single measurement for each, approximately 30 days after initiation of therapy. BSA: body surface area. TPEAK: time after dose peak level was drawn at. T1/2: medication half-life calculated by peak and trough levels. AUC0–24h: drug exposure reflected by plasma drug concentration-time curve over the 24-hour dosing period.
No significant bleeding events were reported in individuals in this cohort. One male patient experienced gross haematuria that was concurrent with adenovirus infection. As haemoglobin levels remained stable, edoxaban was not stopped and haematuria resolved spontaneously. Two patients experienced coronary artery thrombosis during the follow-up period (Fig. 2). One was an 11-year-old female with history of infantile Kawasaki disease and two large aneurysms in the right coronary artery with previously seen ectasia and calcifications, and a moderate aneurysm in the left anterior descending artery. She received edoxaban for 38 months, 45 mg daily (for body weight 44.7 kg at the time of the event). She reported symptoms of exertional chest pain and dizziness and was found to have an occlusive thrombus at the site of right coronary aneurysm (and a right-dominant system), via catheter-based angiography. The second patient was a 9-year-old male with history of Kawasaki disease diagnosed at 23 months of life. He had a known large aneurysm in the right coronary artery, and in the distal circumflex coronary arteries that developed proximal stenosis and ectasia. He received edoxaban for 8 months, 45 mg daily (for body weight 47 kg). He presented with recurring exertional chest pain, positive troponin, and ischaemic changes on exercise stress test. Angiography showed near-complete occlusion of the circumflex artery with thrombus originating in the aneurysm and extending into the origin of the posterior descending branch in a left-dominant system. Both patients were compliant with the medication by self-reporting. Kaplan–Meier estimate for a thrombosis-free rate is thus 84% at 48 months on edoxaban therapy and 88% at 10 years and 70% at 12 years from initial diagnosis of Kawasaki for patients who were on edoxaban at the time of this study, but likely on a different anticoagulation agent prior.

Figure 2. Kaplan–Meier plots for thrombosis event-free time reviewed for patients on edoxaban for chronic anticoagulation since initiating therapy with edoxaban ( a ) and since the initial diagnosis of Kawasaki disease or other vasculitis, including potential time on another anticoagulation agent prior to initiating edoxaban ( b ).
Discussion
We summarise our experience with edoxaban for chronic anticoagulation in children and adolescents at high risk for coronary artery thrombosis. This report supplements the finding of the controlled and blinded ENNOBLE-ATE trial with long-term follow-up data. In our cohort, systemic anticoagulation with edoxaban in addition to low-dose aspirin appears safe and with no significant bleeding events. The data support the conclusion of the ENNOBLE-ATE trial that edoxaban may be a good alternative to standard anticoagulation for children and is congruent with several other recent trials on the use of direct oral anticoagulants in the paediatric population. Reference Male, Lensing and Palumbo10,Reference Portman, Jacobs and Newburger13,Reference von Vajna, Alam and So20,Reference VanderPluym, Esteso and Ankola21 To our knowledge, this is the first report on direct oral anticoagulants that focuses on long-term prevention of coronary arterial thrombosis in children.
Endothelial changes, neointimal hyperplasia, and pro-thrombotic conditions in aneurysmal coronary arteries are well-described long-term sequela of large coronary artery aneurysms associated with Kawasaki disease. Patients with large coronary aneurysms are thus at risk for acute thrombotic events and ischaemic cardiomyopathy years after the diagnosis of Kawasaki disease. Reference Iemura, Ishii and Sugimura22–Reference Dionne, Ibrahim and Gebhard24 Among the 16 patients in our cohort who met indication for chronic anticoagulation and were treated with edoxaban and aspirin, 2 were diagnosed with thrombosis during the follow-up period. Thrombosis occurred 7 and 11 years after their initial diagnosis with Kawasaki, predicting 88 and 70% event-free rate at 10 and 12 years from diagnosis. This rate is largely similar to that reported by McCrindle et al. from reviewing the data for 1651 participants in the International Kawasaki Disease Registry, 440 individuals with large coronary artery aneurysms. Reference McCrindle, Manlhiot and Newburger3 Based on a median follow-up time of 5.2 years, the calculated risk for thrombosis per 10 patient-years was 18 ± 2%. In our cohort, we did not see acute myocardial ischaemia or death (as compared to prevalence of 5 ± 2% per patient for acute myocardial ischaemia and 2 ± 1% for cardiac death). Reference McCrindle, Manlhiot and Newburger3 While not reported, it is assumed that patients in the registry who had large coronary artery aneurysms were all receiving chronic anticoagulation and antiplatelet therapies similar to our cohort, as this has been the standard of care during the time of data collection, between 1999 and 2017. Reference McCrindle, Rowley and Newburger2,Reference Newburger, Takahashi and Gerber25 In our cohort, 37% of patients had history of infantile Kawasaki disease that is considered a specific risk-factor for long-term coronary artery complications. Reference Patel, Bruce and Harrington26
Direct oral anticoagulants include direct thrombin inhibitor (dabigatran) and factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) which all show favourable results in clinical trials. In the EINSTEIN-Jr trial (Oral Rivaroxaban in Children with Venous Thrombosis), rivaroxaban demonstrated safety and efficacy of bodyweight-adjusted regimens with those of standard anticoagulation in treatment of venous thromboembolism in children. Reference Young, Lensing and Monagle9,Reference Monagle, Lensing and Thelen27 Both phase II and phase III programmes demonstrated that once-daily dosing in children who weigh ≥30 kg resulted in the expected exposure when compared to adults, but twice-daily dosing was required for children ≥ 12 and <30 kg, and thrice-daily dosing required for body weight <12 kg in order to achieve values within the 5th to 95th percentile of the adult exposure range. In a recent report of a single-centre experience with apixaban in children with cardiac disease at Boston Children’s Hospital, VanderPluym et al. summarise outcomes for patients who, similar to our cohort, elected to use apixaban off-label for chronic anticoagulation (some as a continuation of treatment after participation in the multi-institutional SAXOPHONE trial – Safety of Apixaban on Pediatric Heart Disease on the prevention of Embolism). Reference VanderPluym, Esteso and Ankola21,Reference Payne, Burns and Glatz28 Apixaban was dosed twice daily for all children and adolescents at 6 different fixed doses by weight groups (or, in older patients, at increased dose for certain clinical indications). Apixaban levels, drawn 4 hours after the dose, ranged higher in older children and with a lesser variability. The authors report only 2 major bleeding events and no thrombosis events among 219 patients, 13 with Kawasaki-associated coronary aneurysms. The same group reported safety and efficacy of using apixaban for anticoagulation in children supported by ventricular assist device. Reference Kobayashi, Cetatoiu and Esteso29 Finally, a simulation pharmacokinetic model for dabigatran in paediatric population, also based on twice-daily administration as in adults, predicted stable peak and trough blood levels and anticoagulation effect. Reference Röshammar, Huang and Albisetti30
Edoxaban has favourable properties relative to other direct oral anticoagulants reported in adults, including rapid peak plasma concentration of 1–2 hours, high oral bioavailability, limited protein binding and dose-proportional linear pharmacokinetics with minimal accumulation. Reference Parasrampuria and Truitt19 Moreover, administration of a crushed edoxaban tablet, either mixed into applesauce or suspended in water, results in similar exposure compared to the intact tablet, which is advantageous in children. Edoxaban plasma concentration is in a linear relationship with the anti-Xa effect, leading to stable and predictable anticoagulation. In our cohort, pharmacokinetic and pharmacodynamic data were available for 10 patients and all received once-daily dosing. Similar to apixaban, we suggest that children have a higher fluctuation in edoxaban concentration with relatively high peak levels, above expected interquartile range in 2 of 10 patients but no major bleeding event that required interruption in treatment. High peak values in children may represent excellent absorption and oral bioavailability, perhaps specifically of eluted medicine compared to tablet. Reference Röshammar, Huang and Albisetti30 Lower than expected trough levels could suggest increased drug clearance in children that have higher glomerular filtration rate relative to adults. Higher creatinine clearance has been previously associated with lower edoxaban trough levels in adult. Reference Lin, Kuo and Ho31 Although the half-life of edoxaban estimated in our cohort was approximately 50% of that reported in adults, average AUC0–24h values were still comparable to those reported in adults suggesting appropriate exposure during the dosing interval. With only two patients having measured trough levels less than the range of expected median values, and only two patients experiencing thrombotic events, the possible higher drug clearance may not be clinically significant. While twice-daily dosing could possibly lead to better stabilisation of drug concentration, high trough levels may also be associated with a higher risk of bleeding. Future studies should determine whether the benefits of once-daily dosing outweigh the potential variability in drug exposure in younger patients.
Limitations
This single-centre review is limited by retrospective and unblinded data collection that is descriptive, with no control group for comparison. This, as well as the small number of patients in this cohort, limits the ability to draw conclusions or to recommend a practice change. Medication compliance was assessed by physician or nurse direct inquiry only and can thus be subjected to reporting bias. Our off-label dosing practice described for this cohort is similar to that used in the ENNOBLE-ATE trial, and other dosing strategies for edoxaban were not explored or compared. Pharmacokinetic and pharmacodynamic monitoring labs were obtained only once per patient and we cannot determine whether they change over time. Also, anti-factor Xa levels were obtained with peak levels and not tested at the time of trough edoxaban concentration.
Conclusions
Edoxaban may be a promising direct oral anticoagulant for children at risk for coronary artery thrombosis when given together with aspirin. It has the potential benefit of only once-daily administration. The use of direct oral anticoagulants revolutionised the practice of anticoagulation in adults and has the potential for an even larger benefit for the paediatric population. As previously demonstrated, for children with large coronary aneurysms, close monitoring and early intervention are still the mainstay of treatment, as even when adequate anticoagulation is maintained, they are at high risk for developing arterial wall changes and thrombosis. Continued long-term multi-institutional studies are needed to define the safety, efficacy, and pharmacokinetics of edoxaban or other direct oral anticoagulants for the treatment of children with Kawasaki-associated risk for coronary artery thrombosis.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors. Analysis of peak and trough plasma levels of edoxaban was performed by the drug manufacturer, Daiichi Sankyo, Tokyo, Japan.
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the Institutional Review Boards at Seattle Children’s Hospital, Seattle, Washington, USA.







