Introduction
Newborn size – commonly determined by measuring weight, length and head circumference immediately after birth – is an important indicator of infant survival and also morbidity during childhood and adult life (Barker, Reference Barker1998; Gluckman et al., Reference Gluckman, Hanson, Cooper and Thornburg2008; Chandler-Laney et al., Reference Chandler-Laney, Gower and Fields2013). Furthermore, especially high birth weight is associated with obstetrical problems such as an increased risk of artificial induction of labour, prolonged birth, birth asphyxia and increased rates of Caesarian section (Mocanu et al., Reference Mocanu, Greene, Byrne and Zurner2000). Newborn size, however, is the result of fetal growth patterns. Therefore the identification of factors influencing fetal growth and consequently newborn size are of special interest to gynaecologists, perinatologists and public health researchers (Thame et al., Reference Thame, Osmond, Bennett, Wilks and Forrester2004).
It is well known that fetal growth is influenced by genetic and several environmental factors (Lunde et al., Reference Lunde, Melve, Gjessing, Skaerven and Irgens2007). In particular, maternal height, pre-pregnancy weight status and pregnancy weight gain have been identified as major determinants of fetal growth patterns and consequently newborn size (Kirchengast & Hartmann, Reference Kirchengast and Hartmann1998; Kirchengast et al., Reference Kirchengast, Hartmann, Schweppe and Husslein1998; Pickett et al., Reference Pickett, Abrams and Selvin2000; Brown et al., Reference Brown, Murtaugh, Jacobs and Margellos2002; Clausen et al., Reference Clausen, Burski, Oyen, Godang, Bollerslev and Henriksen2005; Thame et al., Reference Thame, Osmond, Bennett, Wilks and Forrester2004; Cedergren, Reference Cedergren2006; Johansson et al., Reference Johansson, Linne, Rössner and Neovius2007; Kabali & Werler, Reference Kabali and Werler2007; Crane et al., Reference Crane, White, Murphy, Burrage and Hutchens2009; Dietz et al., Reference Dietz, Callaghan and Sharma2009; Choi et al., Reference Choi, Park and Shin2011; Han et al., Reference Han, Lutsiv, Mulla, Rosen, Beyene and McDonald2011; Mitra et al., Reference Mitra, Misra, Nayak and Sahoo2012; Hanieh et al., Reference Hanieh, Ha, Simpson, Thuy, Khuong and Thoang2014). Only a few studies have documented an impact of paternal height and weight status on intrauterine growth patterns (Wilcox et al., Reference Wilcox, Newton and Johnson1995; Klebanoff et al., Reference Klebanoff, Mednich, Schulsinger, Secher and Shiono1998; Wills et al., Reference Wills, Chinchwadkar, Joglekar, Natekar, Yajnik, Fall and Kinare2010, Albouy-Llaty et al., Reference Albouy-llaty, Thiebaugeorges, Goua, Magnin, Schweitzer and Forhan2011). Maternal and paternal height reflect above all the genetic potential for growth (Wills et al., Reference Wills, Chinchwadkar, Joglekar, Natekar, Yajnik, Fall and Kinare2010). Maternal height, however, also reflects the early environmental conditions of the mother (Wills et al., Reference Wills, Chinchwadkar, Joglekar, Natekar, Yajnik, Fall and Kinare2010). In contrast, maternal pre-pregnancy weight status and pregnancy weight gain are major indicators of the actual environmental conditions.
Several studies have shown an association between maternal height, maternal pre-pregnancy weight and pregnancy weight gain with newborn size (Kirchengast & Hartmann, Reference Kirchengast and Hartmann1998, Reference Kirchengast and Hartmann2003). It is well established that newborn size is positively correlated with maternal pre-pregnancy weight status as well as pregnancy weight gain (Takimoto et al., Reference Takimoto, Sugiyama, Fukuoka, Kato and Yoshiike2006; Kalanda, Reference Kalanda2007; Catalano et al., Reference Catalano, Mele, Landon, Ramin, Reddy and Casey2014). Newborn size, however, is the result of intrauterine growth patterns. Consequently, an association between maternal somatic factors and fetal growth patterns can be assumed. Fetal growth patterns are mostly estimated by ultrasound scans. The parameters most frequently measured in utero are crown–rump length, femur length, biparietal diameter, head circumference, transverse abdominal diameter and abdominal circumference (Harada et al., Reference Harada, Tanikawa, Nakajima, Iwamoto, Mio, Terakawa and Maeda1992; Davis et al., Reference Davis, Cutter, Goldenberg, Hoffman, Cliver and Brumfield1993; Marsal et al., Reference Marsal, Perrson, Larsen, Lilja, Selbing and Sultan1996; Lee et al. Reference Lee, Balasubramaniam, Deter, Hassan, Gotsch and Kusanovic2009; Albouy-Llaty et al., Reference Albouy-llaty, Thiebaugeorges, Goua, Magnin, Schweitzer and Forhan2011).
Unfortunately only very few studies have focused on the associations between maternal factors and intrauterine growth patterns estimated by prenatal ultrasound measurements (Schwärzler et al., Reference Schwärzler, Bland, Holden, Campbell and Ville2004; Thame et al., Reference Thame, Osmond, Bennett, Wilks and Forrester2004; Drooger et al., Reference Drooger, Troe, Borsboom, Hofman, Mackenbach and Moll2005; Ay et al., Reference Ay, Kruithof, Bakker, Steegers, Wittemman and Moll2009; Sarris et al., Reference Sarris, Bottomley, Daemen, Pexsters, Timmerman, Bourne and Papageorghiou2010; Lampl et al., Reference Lampl, Gotsch, Kusanovic, Gomez, Nien, Frongillo and Romero2010; Wills et al., Reference Wills, Chinchwadkar, Joglekar, Natekar, Yajnik, Fall and Kinare2010; Albouy-Llaty et al., Reference Albouy-llaty, Thiebaugeorges, Goua, Magnin, Schweitzer and Forhan2011). The impact of maternal height and weight on fetal growth has predominantly been analysed in multiparous (Goldenberg et al., Reference Goldenberg, Davis, Cliver, Cutter, Hoffman, Dubard and Copper1993) and high-risk women (de Joung et al., Reference De Jong, Gardosi, Baldwin, Francis, Dekker and van Gejin1998; Hinkle et al., Reference Hinkle, Johns, Albert, Kim and Grantz2015). In general it could be shown that maternal characteristics are associated with fetal size in the second and third trimester (Leung et al., Reference Leung, Pang, Daljit, Leung, Poon, Wong and Lau2008; Salpou et al., Reference Salpou, Kiserud, Rasmussen and Johnsen2008). No significant correlation between maternal weight status and crown–rump length could be proved for the first trimester (Sarris et al., Reference Sarris, Bottomley, Daemen, Pexsters, Timmerman, Bourne and Papageorghiou2010).
The aims of the present study were, first, to analyse the association patterns between maternal height and pre-pregnancy weight status with fetal growth parameters during the first, second and third trimesters of pregnancy. In a second step the association patterns between maternal height, pre-pregnancy weight status and gestational weight gain with newborn size were tested.
Methods
Data set
This retrospective study was based on a data set of 4261 singleton births taking place at the Danube Hospital (SMZ Ost) in Vienna, Austria, between 2005 and 2013. Although over this period 17,430 births were recorded at the hospital, only for 7590 births had all three prenatal ultrasound examinations been performed. Furthermore, the following strict inclusion criteria were defined for inclusion in the study, reducing the sample to 4261: all recommended prenatal check-ups of the mother–child passport had been performed; healthy primiparae mothers of Austrian or Central European origin; term delivery (39th and 40th weeks of gestation); a minimum maternal age of 17 years at the time of giving birth; the delivery of a single infant without congenital malformations; no registered maternal diseases such as diabetes mellitus or nephropathy before and during pregnancy; no hypertension (BP<150/90 mmHg); and no protein or glucose in the urine. Pregnancies resulting from IVF were also strictly excluded. Additionally, drug or alcohol abuse were defined as exclusion criteria, but this amounted to less than 0.5% of the mothers.
The Viennese Danube Hospital is one of the largest public birth clinics in Vienna. In general, pre-, peri- and postnatal care is highly developed in Austria. More than 40 years ago the so-called mother–child passport – a highly sophisticated monitoring system of pregnancy, and intrauterine and postnatal development – was developed. Seven prenatal check-ups starting at the 8th week of gestation and eight postnatal check-ups of the child between birth and the fourth year of life are provided free of charge. The prenatal examinations are mainly performed in the consulting rooms of gynaecologists or at the clinic where birth was scheduled to take place. Postnatal check-ups are carried out by paediatricians.
All data collected at the individual check-ups are documented at the hospital and in the mother–child passport, which belongs to the mother. A completed mother–child passport is rewarded by the government with a financial payment. The introduction of this pre- and postnatal monitoring system has reduced neonatal and child mortality dramatically in Austria (Waldhoer et al., Reference Waldhoer, Haidinger, Langasser and Tuomilehto1996). In the present study, data from three sonographic examinations (one at each trimester) and birth outcomes were analysed. All these sonographic examinations were carried out at the Danube hospital.
Prenatal examinations: fetal biometry
Gestational age was calculated as the number of weeks from the beginning of the last menstrual bleeding to the date of delivery (=duration of amenorrhoea). Fetal growth patterns were reconstructed from the results of the three ultrasound examinations. The first examination took place at the 11th or 12th week of gestation (first trimester), the second at the 20th/21th gestational week (second trimester) and the third at the 32th/33th week of gestation (third trimester). Consequently the fetuses could vary in age up to at least 2 weeks at each trimester. Therefore the raw measurements were adjusted to a single gestation for each trimester separately before using them for statistical analyses.
The transabdominal ultrasound examinations were performed by a limited number of trained specialists (fewer than fifteen) using Voluson 730 and Voluson S6 (GE 8) ultrasonography. The following routine sonographic measurements, performed according to Hadlock’s criteria (Hadlock et al., Reference Hadlock, Harrist, Deter and Park1982a,Reference Hadlock, Harrist, Deter and Parkb,Reference Hadlock, Harrist, Deter and Parkc), were documented. At the first scan (11th or 12th gestational week) crown–rump length was determined. At the second (20th or 21th gestational week) and third examinations (32th or 33th weeks of gestation) biparietal diameter, fronto-occipital diameter, head circumference, abdominal transverse diameter, abdominal anterior–posterior diameter, abdominal circumference and femur length were measured. Crown–rump length was defined as the distance between the top of the head (crown) to the bottom of the buttocks (rump). Femur length was measured from the greater trochanter to the lateral condyle. Biparietal diameter was defined as the distance from the proximal outer table to the distal outer table of the skull at the level of the thalamus. Fronto-occipital diameter follows a line extending from a point just above the root of the nose to the most prominent portion of the occipital bone. Head circumference is the measurement around the calvarium excluding soft tissues. Transverse and anterior–posterior abdominal diameters were taken at the level of the stomach and the bifurcation of the main portal vein into its right and left branches. Abdominal circumference was measured at the level of the liver and stomach, including the left portal vein at the umbilical region (Hadlock et al., Reference Hadlock, Harrist, Deter and Park1982a,Reference Hadlock, Harrist, Deter and Parkb,Reference Hadlock, Harrist, Deter and Parkc, Reference Hadlock, Harrist, Shah and Park1984; Kurmanavicius et al., Reference Kurmanavicius, Wright, Royston, Wisser, Huch, Huch and Zimmermann1999a,Reference Kurmanavicius, Wright, Royston, Zimmermann, Huch, Huch and Wisserb; Snijders & Nicolaides, Reference Snijders and Nicolaides1994; Abdella et al., Reference Abdella, Ahmed and Moustafa2014).
Newborn characteristics
Immediately after birth the following parameters were taken directly from the newborn: birth weight in grams using a digital infant scale, birth length in centimetres using a standard measurement board for infants and head circumference in centimetres using a tape. The ponderal index (kg/m3) of the newborn was calculated (Roje et al., Reference Roje, Banovic, Tadin, Vucinovic, Capkun and Baraisic2004). A low birth weight was defined as <2500 g and a high birth weight (macrosomia) as >4000 g, according to WHO recommendations (WHO, 1980).
Maternal parameters
Exclusively primiparae women aged between 17 and 48 years (mean=28.3; SD=5.6) were enrolled in the study. Besides, medical anamnesis, civil status and nicotine consumption levels of the pregnant women were obtained by interview at the first prenatal visit (8th week of gestation). Nicotine consumption was assessed as follows: not smoking; 1–10 cigarettes per day; 11–20 cigarettes per day; and more than 20 cigarettes per day. Additionally the maternal somatometric parameters height and pre-pregnancy weight were collected at the first prenatal visit according to the recommendations of Knussmann (Reference Knussmann1988). Height was measured to the nearest 0.5 cm using a standard anthropometer. Pre-pregnancy weight was obtained by interview using the retrospective method. Additionally, body weight was measured to the nearest 0.1 kg on a balance beam scale. Since during the first 13 weeks of gestation an extremely small weight gain of only 1.7% has been reported in literature (Gueri et al., Reference Gueri, Jutsum and Sorhaindo1982), a combination of the retrospective method and weight determination at the 8th week of gestation was used. Consequently, pre-pregnancy weight was calculated as the mean value of the retrospective estimated weight and the weight at the 8th week of gestation. Additionally, maternal weight was measured before delivery (at the end of pregnancy). The weight gain during pregnancy was calculated by subtraction of pre-pregnancy weight from body weight before delivery. Maternal pre-pregnancy weight status was determined by the body mass index (BMI in kg/m2) using height and pre-pregnancy weight. To classify maternal weight status the cut-offs published by the WHO were used (WHO, 1995): underweight, BMI <18.50 kg/m2; normal weight, BMI 18.50–24.99 kg/m2; overweight, BMI 25.00–29.99 kg/m2; obesity, BMI >30.00 kg/m2.
Obstetric characteristics
Mode of delivery, spontaneous delivery versus Caesarean section and the intrauterine position of the infant at the time of delivery (head presentation, pelvic presentation) were documented. The most frequent indications for Caesarean delivery were fetal distress and dystocia. Caesarean sections on maternal request are not carried out at the Viennese Danube Hospital.
Statistical analysis
Statistical analyses were carried out using SPSS for Windows (Version 22). The Kolmogoroff–Smirnov test was performed in order to test somatometric variables for normal distribution. The results of this test indicated that normal distribution for all maternal as well as fetal and newborn parameters can be assumed. Partial correlations (maternal age and gestational week held constant) were calculated to test the correlations between maternal somatometric parameters and fetal biometry as well as newborn size. Multiple regression analyses were performed to test the associations between maternal height, pre-pregnancy weight status and fetal biometry adjusted for maternal age, nicotine consumption and fetal sex. Regression models included maternal height and pre-pregnancy weight status simultaneously. Multiple regression analyses were performed to test the associations between maternal height, pre-pregnancy weight status and gestational weight gain with newborn size, adjusted for nicotine consumption and newborn sex. All three maternal measurements were included simultaneously into the regression model. A p-value below 0.05 was considered statistically significant.
Results
Maternal characteristics
The maternal age, family status, smoking behaviour, height, pre-pregnancy weight status and gestational weight gain of the 4261 study women are presented in Table 1. The vast majority of the women (95.2%) were between the ages of 20 and 39 years when giving birth; 3.2% were younger than 20 years and only 1.5% were older than 40 years. About 50% of the sample women were married at the time they gave birth. Fifteen per cent continued smoking during pregnancy. The mean pre-pregnancy BMI of the women was 23.19 (SD 4.42), and ranged from 17.83 to 52.73 kg/m2. The majority of the women (65.5%) corresponded to the WHO definition of normal weight. Only 7.3% were classified as underweight, but more than 25% were overweight or obese before pregnancy. The mean gestational weight gain was 14.6 kg (SD 5.6), and ranged from −3.0 kg to 52.7 kg.
Table 1 Maternal characteristics (descriptive statistics), N=4261

Fetal and newborn characteristics
Fetal and newborn characteristics are presented in Table 2. The number of male newborns (50.4%) was slightly higher than that of females (49.6%). The majority of the newborns (90.0%) corresponded to the definition of normal weight (2500–3999 g). Only 1.7% were classified as small for gestational age (<2500 g), while 8.4% were macrosomic (≥4000 g). The rate of Caesarean section delivery was 15.9%. The vast majority showed a head presentation (94.7%), while only 5.7% showed a pelvic presentation.
Table 2 Fetal and newborn characteristics (descriptive statistics), N=4261

1.scan, 1st trimester scan; 2.scan, 2nd trimester scan; 3.scan, 3rd trimester scan.
Maternal somatic factors and fetal growth
In order to test the correlation patterns between maternal height and pre-pregnancy weight status and fetal biometry, partial correlations (with maternal age held constant) were calculated. As demonstrated in Table 3, maternal height correlated significantly positively from the first trimester onwards. Significant correlations were found with crown–rump length at the first trimester, with all fetal parameters at the second trimester and femur length as well as head parameters at the third trimester. No significant correlations could be observed between maternal height and fetal abdominal dimensions at the third trimester. Pre-pregnancy weight status correlated significantly positively with femur length and the fronto-occipital diameter at the second trimester and all fetal parameters at the third trimester. The results of the partial correlations were corroborated by those of the multiple regression analyses. As shown in Table 4, maternal height was significantly positively related to crown–rump length at the first trimester independent of maternal age and fetal sex. As shown in Table 5, maternal height was significantly positively related with all fetal parameters at the second trimester independent of fetal sex and maternal age, and femur length and the head parameters at the third trimester. Pre-pregnancy weight status was significantly positively associated with femur length at the second trimester and all fetal parameters at the third trimester.
Table 3 Correlations between maternal height, pre-pregnancy weight, pre-pregnancy BMI and fetal biometryFootnote a

a Partial correlation maternal age and gestational week (for each trimester separately)=constant.
Table 4 Association patterns between maternal age, stature, pre-pregnancy weight status, nicotine consumption and fetal sex with crown–rump length (dependent variable) at 11th to 12th weeks gestation using multiple regression analysis

B, regression coefficient; CI, confidence interval; ns, non-significant.
Table 5 Association patterns between maternal age, stature, pre-pregnancy weight status, nicotine consumption and fetal sex with fetal biometry at 20th/21th and 32th/33th weeks gestation using multiple regression analysis
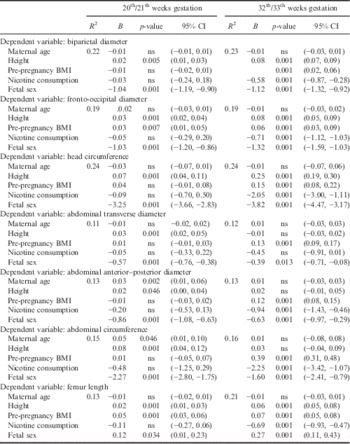
B, regression coefficient; CI, confidence interval; ns, non-significant.
Maternal somatic factors and newborn size
Maternal height, pre-pregnancy weight status and gestational weight gain correlated highly significantly with newborn birth weight, birth length and head circumference (see Table 6). These findings were corroborated by the results of the multiple regression analyses. Maternal height, pre-pregnancy weight status and gestational weight gain were significantly positively associated with all parameters of newborn size. A negative association was found between nicotine consumption and newborn size. Furthermore, female sex was significantly associated with smaller newborn dimensions (Table 7).
Table 6 Correlations between maternal height, pre-pregnancy weight and pre-pregnancy BMI with newborn sizeFootnote a
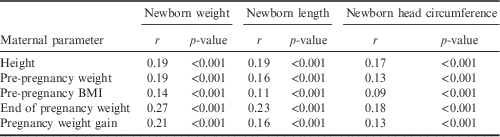
a Partial correlation maternal age=constant.
Table 7 Association between maternal parameters, nicotine consumption, newborn sex and newborn size using multiple regression analysis

B, regression coefficient; CI, confidence interval; ns, non-significant.
Discussion
The present study examined the association between maternal height and pre-pregnancy weight status and fetal growth parameters during the first, second and third trimesters in 4261 singleton pregnancies taking place in Vienna, Austria. Furthermore, the relationship between maternal height, pre-pregnancy weight status as well as gestational weight gain and newborn size was analysed.
The study has certain limitations. The main shortcoming is that fetal biometry was taken only once per trimester. Furthermore, fetal measurements and newborn size parameters were taken by several different investigators (although all were well trained). The strength of the study is the large data set comprising fifteen fetal and three newborn measurements from 4261 children. However, this large sample size may result in some significant but small correlations. In this way the large sample size potentially weakens the results.
In general it could be shown that maternal height was significantly related to fetal growth parameters independent of maternal age, maternal nicotine consumption and fetal sex from the first trimester onwards. Maternal height correlated significantly with crown–rump length at the 11th/12th week of gestation, while in contrast no association between maternal pre-pregnancy weight status and fetal growth during the first trimester could be observed. This result corresponds to that of Sarris et al. (Reference Sarris, Bottomley, Daemen, Pexsters, Timmerman, Bourne and Papageorghiou2010), who also found no significant association between maternal weight status and crown–rump length during the first trimester. These findings suggest a greater genetic and lesser environmental influence on growth patterns during the first trimester because maternal height reflects, above all, the genetic potential for growth (Wills et al., Reference Wills, Chinchwadkar, Joglekar, Natekar, Yajnik, Fall and Kinare2010). Pre-pregnancy weight status, in contrast, can be interpreted as an indicator of environmental conditions before and during early pregnancy. The minor association between environmental conditions and growth during the first trimester may be due to the fact that the first trimester is characterized by an increase in cell numbers, the formation of embryonic tissues and organogenesis, while the embryo grows only slowly in length (Meire, Reference Meire1986; Bogin, Reference Bogin1999).
The rate of growth of the fetus increases during the second trimester (Bogin, Reference Bogin1999). The present study found a statistically significant relationship between maternal height and all fetal parameters. Again, genetic factors, reflected in maternal height, were significantly related to all fetal growth parameters. Pre-pregnancy weight status, in contrast, was significantly related to femur length and the fronto-occipital diameter only. In particular, fetal abdominal dimensions were not found to be related to pre-pregnancy weight status. Consequently, intrauterine environmental factors – reflected by pre-pregnancy weight status – seem to play a minor role in fetal growth during the second trimester. This may be due to the fact that growth in length exceeds the growth in weight during the second trimester.
During the third trimester growth in fetus weight takes place at a relatively faster rate (Bogin, Reference Bogin1999) and the development and maturation of several physiological systems takes place preparing the fetus for the transition to extrauterine life. In this study maternal height was found to be significantly positively associated with femur length, and with the three fetal head parameters. In contrast, no significant association was found between maternal height and fetal abdominal dimensions. Pre-pregnancy weight status, however, was found to be significantly positively associated with femur length, the head dimensions and the three abdominal dimensions. This finding indicates that fetal growth during the third trimester is significantly associated with intrauterine environmental conditions. Maternal pre-pregnancy weight status and gestational weight gain are indicators of the energetic situation before and during pregnancy. Maternal undernutrition has adverse effects on fetal growth (Jeric et al., Reference Jeric, Roje, Strinic, Mestrovic and Vulic2013; Field et al., Reference Field, Anthony, Engle, Archibeque, Kreisler and Han2015). Fetal growth depends mainly on the delivery of oxygen and nutrients across the placenta (Aye et al., Reference Aye, Powell and Jansson2013). The ability of the placenta to supply essential nutrients to the fetus depends on the nutritional status of the mother, utero-placental blood flow and the expression and function of the trophoblast nutrient transporter (Aye et al., Reference Aye, Powell and Jansson2013). Recently evidence has emerged that the adipocyte-derived adiponectin plays a key role in the regulation of maternal, placental and fetal physiology. Adiponectin levels increase in early pregnancy (Mazaki-Tovi et al., Reference Mazaki-Tovi, Knety, Pariente, Hemi, Wiser and Schiff2007) and decline over the second half of gestation (Catalano et al., Reference Catalano, Hoegh, Minium, Huston-Presley, Bernard and Kalhan2006). It is assumed that high levels of adiponectin in early pregnancy enhance the maternal accretion of nutrients and lower levels of adiponectin in later gestation promote allocation of nutrients to the fetus (Aye et al., Reference Aye, Powell and Jansson2013). Consequently adiponectin is thought to be a key factor in fetal growth regulation stimulated by maternal body fat, i.e. maternal BMI.
As for the association between maternal somatometrics and fetal growth patterns, the findings of the present study are only partly in accordance to those of previous studies. On the one hand the results are quite similar to those of Albouy-Llaty et al. (Reference Albouy-llaty, Thiebaugeorges, Goua, Magnin, Schweitzer and Forhan2011), who described a strong association between maternal BMI and fetal abdominal circumference, as well as a significant impact of maternal stature on fetal femur length. Furthermore, the positive association found between maternal stature as well as pre-pregnancy weight status and fetal head dimensions in the third trimester is quite similar to the findings of Goldenberg et al. (Reference Goldenberg, Davis, Cliver, Cutter, Hoffman, Dubard and Copper1993), who reported positive associations between head circumference at 31 weeks, 36 weeks and at birth. On the other hand, Wills et al. (Reference Wills, Chinchwadkar, Joglekar, Natekar, Yajnik, Fall and Kinare2010) found only weak associations between maternal somatometrics and fetal dimensions. Their study population, however, consisted of rural Indian women, who were described as short, light and thin, while the present sample consisted exclusively of women of Central European origin. Additionally, more than 25% of these women were overweight or obese.
Newborn size, characterized by birth weight, birth length and head circumference, was found to be highly significantly related to maternal height, pre-pregnancy weight status and gestational weight gain. The positive impact of maternal height on newborn size is in accordance with the observation of Pickett et al. (Reference Pickett, Abrams and Selvin2000), who reported that increasing maternal height was associated with increasing newborn weight. Furthermore, a significant association between gestational weight gain and newborn size was documented in the present study. In general, newborn size increased with increasing gestational weight gain independent of maternal age, pre-pregnancy weight status and maternal stature. This finding is in accordance with those of some previous studies, which yielded a strong relationship between low gestational weight gain and small for gestational age infants (Kirchengast & Hartmann, Reference Kirchengast and Hartmann1998, Reference Kirchengast and Hartmann2003; Siega-Riz et al., Reference Siega-Riz, Viswanathan, Moos, Deierlein, Mumford and Knaack2009; Han et al., Reference Han, Lutsiv, Mulla, Rosen, Beyene and McDonald2011; Savitz et al., Reference Savitz, Stein, Siega-Riz and Herring2011; Drehmer et al., Reference Drehmer, Duncan, Kac and Schmidt2013; Galjaard et al., Reference Galjaard, Pexsters, Devlieger, Guelinckx, Abdallah and Lewis2013). Furthermore, a positive influence of gestational weight gain on fetal growth was documented by Hinkle et al. (Reference Hinkle, Johns, Albert, Kim and Grantz2015), who reported a significant impact of maternal weight gain on fetal growth and consequently newborn size.
From the results of the present study it can be concluded that a significant association between maternal size and fetal growth patterns is detectable from the first trimester onwards. Genetic factors reflected by maternal height are significantly associated with fetal growth patterns during the first and second trimester. Consequently fetal growth is accelerated among taller mothers during the first and second trimesters. During the third trimester environmental factors – reflected by maternal weight status – increase in importance for intrauterine growth. In general taller mothers and heavier mothers give birth to larger infants.
Acknowledgment
The authors are gratefully indebted to an anonymous reviewer for constructive criticism and valuable comments which improved the study markedly.









