Inflammation plays an important role in acute infection and injury, but it can be harmful in chronic non-communicable diseases. Mitigation of the harmful effects of oxidative stress and resolution of inflammation is an active process regulated by biochemical mediators and receptor-signalling pathways, driven by specialised pro-resolving mediators (SPM)( Reference Serhan and Petasis 1 ). These enzymatically produced SPM exert potent receptor-mediated immuno-resolving effects on the activated immune system to resolve inflammation by restoring homoeostasis( Reference Bannenberg and Serhan 2 ). Studies have shown that SPM increase with time during the inflammatory process, acting on a number of G-coupled protein receptors, including the leukotriene B4 (LTB4) receptor to affect resolution of inflammation( Reference Serhan and Petasis 1 , Reference Tabas 3 ).
Serhan & Petasis( Reference Serhan and Petasis 1 ) first described SPM derived from the n-3 fatty acids EPA and DHA. SPM derived from EPA via the intermediate 18-hydroxyeicosapentaenoic acid (18-HEPE) are known as E-series resolvins, whereas those derived from DHA via 17-hydroxydocosahexaenoic acid (17-HDHA) are known as D-series resolvins( Reference Serhan and Petasis 1 ). Protectins (PD1) and maresins (MaR-1) are derived from DHA via the precursor 17-hydroperoxydocosahexaenoic acid (17-HpDHA) and 14-hydroxydocosahexaenoic acid (14-HDHA), respectively( Reference Serhan and Petasis 1 ).
SPM are present in biological fluids, cells and tissues and increase with time during the inflammatory process( Reference Serhan and Petasis 1 , Reference Serhan, Chiang and Dalli 4 ). To date most of the characterisation of SPM has been reported in animal models of inflammation( Reference Serhan, Chiang and Dalli 4 ). A number of SPM have been reported in human blood at concentrations previously shown to be biologically active (i.e. 0·1–0·5 nm)( Reference Mas, Croft and Zahra 5 ) but limited data are available on SPM in human tissues and their role and significance in inflammatory pathophysiology. In animal models, SPM have been shown to have antiviral properties( Reference Morita, Kuba and Ichikawa 6 ), reduce arthritic( Reference Lima-Garcia, Dutra and da Silva 7 ) and post-operative pain( Reference Huang, Wang and Serhan 8 ), colitis( Reference Bento, Claudino and Dutra 9 ), airway inflammation( Reference Koltsida, Karamnov and Pyrillou 10 , Reference Krishnamoorthy, Burkett and Dalli 11 ), ocular inflammation( Reference de Paiva, Schwartz and Gjorstrup 12 ) and pneumonia( Reference Dalli, Kraft and Colas 13 ), and promote tissue regeneration( Reference Dalli, Ramon and Norris 14 ). Studies in rodents show that maternal dietary n-3 fatty acid intake increased SPM and the upstream precursors in the placenta( Reference Jones, Mark and Keelan 15 ). However, maternal n-3 fatty acid supplementation in human studies only increased the precursors 18-HEPE and 17-HDHA in the placenta( Reference Keelan, Mas and D’Vaz 16 ).
The ‘developmental origins of health and disease’ (DOHaD) hypothesis proposes that factors that modify physiological processes during the critical developmental period in early life may exert a long-term influence on disease in adult life( Reference Barker 17 ). Thus, early life nutritional exposures may be significant determinants of the development and future health of the offspring. In this regard, maternal n-3 fatty acid supplementation in pregnancy has been shown to enhance n-3 fatty acid status of the offspring( Reference Dunstan, Mori and Barden 18 ), influence immunological outcomes by modifying offspring cytokine concentrations( Reference Dunstan, Mori and Barden 19 , Reference Dunstan, Mori and Barden 20 ) and progenitor phenotype( Reference Denburg, Hatfield and Cyr 21 ), attenuate offspring lipid peroxidation( Reference Barden, Mori and Dunstan 22 ) and improve early childhood cognitive and visual development( Reference Dunstan, Simmer and Dixon 23 ).
To our knowledge, there are no data on SPM concentrations in offspring at birth and early childhood following maternal n-3 fatty acid supplementation during pregnancy. Therefore, this study aimed to examine the effects of maternal n-3 fatty acid supplementation during pregnancy on plasma SPM and their precursors in the offspring. We hypothesised that maternal n-3 fatty acid supplementation would increase cord SPM with consequent benefit to the fetus and the offspring at birth, and that there would be a programming effect such that these benefits would be sustained to 12 years of age (12 years).
Methods
Recruitment of participants
Details relating to the study design, methodology, inclusion and exclusion criteria have been published( Reference Dunstan, Mori and Barden 19 ). In brief, the study was a single-centred, randomised (1:1), double-blind, placebo-controlled parallel study with participants stratified by parity (no previous term childbirth v. one or more), prepregnancy BMI, age and maternal allergy (allergic rhinitis or asthma). The original study aimed to investigate the effects of maternal n-3 fatty acid supplementation during pregnancy on neonatal immune development, allergy and neurodevelopment outcomes in the offspring( Reference Dunstan, Mori and Barden 18 – Reference Dunstan, Mori and Barden 20 , Reference Barden, Mori and Dunstan 22 – Reference Dunstan, Mitoulas and Dixon 25 ). A follow-up was undertaken at 12 years to assess the effects of n-3 fatty acid supplementation in pregnancy on SPM in childhood. The study was registered with the Australian Clinical Trials Registry (ACTRN00336395, https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336395).
In all, ninety-eight women with a history of allergy, and booked for delivery at St. John of God Hospital, Subiaco, Western Australia were recruited and randomised between January 1999 and September 2001. The women were otherwise healthy, non-smokers and had their fish intake restricted. Maternal allergy was defined by a positive skin prick test and a history of physician-diagnosed allergic rhinitis and/or asthma. The study was carried out in accordance with the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the St John of God Hospital and Princess Margaret Hospital Human Ethics Committees. Written informed consent was obtained from all mothers.
Intervention
The women were randomised to receive either 4×1 g fish oil or olive oil (OO) capsules as the control. Supplementation commenced at 20 weeks of gestation and ceased at delivery. The n-3 fatty acid capsules (Ocean Nutrition Ltd) provided a total of 3·7 g of n-3 fatty acids as ethyl esters with 56·0 % as DHA and 27·7 % as EPA (confirmed by GC). Placebo capsules (Pan Laboratories) contained OO with 66·6 % as n-9 oleic acid and <1 % n-3 PUFA. This dose was based on previous trials studying the effect of n-3 fatty acid supplementation in pregnancy( Reference Olsen, Dalby So̸rensen and Secher 26 ), on high-risk pregnancies( Reference Onwude, Lilford and Hjartardottir 27 ), on blood pressure( Reference Salvig, Olsen and Secher 28 ) and in our previous studies in adults( Reference Mori, Beilin and Burke 29 ). Randomisation and dispensing of capsules were conducted at a different centre and independent of those involved with co-ordinating the trial. Participants, research scientists, and the paediatrician remained blinded to the group allocation for the duration of the study. The participants completed a validated, semi-quantitative FFQ at 20- and 30-weeks gestation to monitor fish consumption. Compliance with the treatment regimen was checked by measuring the incorporation of DHA and EPA into erythrocyte cell membranes of the women at 30- and 36-weeks gestation and 6 weeks postnatally( Reference Dunstan, Mori and Barden 18 ).
Outcomes
The offspring were assessed at birth and 12 years for resolvins, protectins and maresins and their precursors. At birth, 83 infants were included in the study: eighty-one cord blood samples were available for analyses. At 12 years, fifty-eight children participated in the follow-up study: forty-three blood samples were available for analyses.
Clinical outcomes
Infants were clinically evaluated at 12 months of age (1 year), which included a detailed history and clinical examination( Reference Dunstan, Mori and Barden 19 ). The clinical outcome measures considered in this study were incidence of atopic eczema dermatitis syndrome, food allergy and allergic disease.
Blood collection and processing
Cord blood was collected at birth from the placental vessels. Peripheral blood was obtained by venepuncture after an overnight fast at 12 years, where consented and practical. Blood was collected into heparinised tubes and processed immediately after the clinic visit( Reference Dunstan, Mori and Barden 19 ). Plasma was stored at −80°C. Erythrocytes were washed before lipids were extracted with methanol–chloroform (2:1) and stored at −80°C.
Fatty acid measurements
Fatty acid ethyl esters were extracted from washed red cell membranes and assayed by GC as previously described for cord erythrocytes( Reference Mori, Burke and Puddey 30 ) and erythrocytes at 12 years( Reference Pottala, Espeland and Polreis 31 ).
Measurement of specialised pro-resolving mediators in plasma
SPM standards were purchased from Cayman Chemicals( Reference Mas, Croft and Zahra 32 , Reference Barden, Mas and Croft 33 ). 10R,17S-dihydroxy-4Z,7Z,11E,13E, 15Z,19Z-DHA (PD1) standard was a gift by Professor Charles N. Serhan (Harvard Medical School). 5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-EPA (resolvin E2 (RvE2)), (17,18S-dihydroxy-5Z,8Z,11Z,13E,15E- EPA (RvE3) and 17,18R-dihydroxy-5Z,8Z,11Z,13E,15E-EPA (18R-RvE3) standards were a gift from Professor Makoto Arita (Department of Health Chemistry, Graduate School of Pharmaceutical Sciences, University of Tokyo). 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-EPA (RvE1) and (14-hydroxy-4Z,7Z,10Z,12E,16Z,19Z-DHA (14-HDHA) were provided by Cayman Chemicals.
The assay was performed as previously described( Reference Mas, Croft and Zahra 32 , Reference Barden, Mas and Croft 33 ). In brief, 1 ml of plasma and internal standard LTB4-d4 (500 pg) were acidified with 2 ml of 100 mm sodium acetate pH 3, applied to solid phase extraction cartridges (Bond Elut C18 500 mg; Agilent Technologies), and washed with water and hexane. The SPM were eluted with ethyl acetate (2 ml), dried under N and reconstituted in 100 ml of 5 mm ammonium acetate (pH 8·9) and methanol (50/50; v/v) for analysis by liquid chromatography-tandem MS. The SPM were quantitated using a Thermo Scientific TSQ Quantum Ultra Triple Quadrupole LC-MS System (ThermoFisher Scientific) equipped with an electrospray ionisation source operated in negative ion mode. The detection limit for 18-HEPE, 17-HDHA, and 14-HDHA was 10 pg/ml and the detection limit for other SPM was 20 pg/ml. We extracted a larger volume of plasma for SPM analysis for samples that gave results close to the limit of detection. Instrument control and data acquisition were performed using the Xcalibur 2.0.7 software.
Statistical analysis
Data were summarised using counts, percentages, means and standard deviations as appropriate. Log transformations were applied to outcomes when the data were not approximately normal. For these variables, geometric means and standard deviations were calculated. Comparisons of characteristics of children with and without blood samples were performed using chi-square tests for dichotomous data. For continuous data, group differences were assessed by t tests for normal data and Mann–Whitney U tests for data that could not achieve normality.
Due to insufficient data for RvE2 and RvE3 at 12 years, these variables were not utilised for analysis. Group differences at birth for RvE2 and RvE3 were analysed using Tobit regression. Tobit regression considers the probability of the data being below (or above) the detection limit and uses this in conjunction with data available above the limit; hence, those with values below the limit are not excluded. The effect of n-3 fatty acids on SPM concentrations between birth and 12 years was examined using linear mixed models with maximum likelihood estimation (MLE) to produce unbiased estimates (mixed-effects maximum likelihood regression). MLE handles missing data by using observed data as well as intra-class correlation to inform the likelihood function which is then maximised to obtain an estimate of the mean at a given time point. MLE therefore retains participants if data is provided for at least one time point, thus avoiding attrition bias. Outcomes with a detection limit at birth and at 12 years (RvE1 and RvD2) were assessed using random effects tobit analysis. Where normality of the data could not be met for repeated measures (RvD1 and 17R-RvD1), quantile regression with a per-person cluster adjustment was performed. Differences in the pattern over time between intervention groups were tested by the interaction of group and time in the model. In each model, interaction of group and time was used to test for a difference between groups in the change over time. The Bonferroni–Holm stepdown process was employed to adjust P values for multiple testing.
Associations between SPM and n-3 fatty acids were investigated with three-way and two way interactions of group, year and the variable of interest. Erythrocyte fatty acids were analysed using linear mixed models with MLE.
A P value of <0·05 (two-tailed) was considered statistically significant for all analyses. All statistical analyses were performed using the Stata 13.1 statistical package (StataCorp).
Statistical power
The number of subjects was based on previous studies by the host laboratory comparing cord blood immune responses of neonates with or without later allergy( Reference Dunstan, Mori and Barden 19 ). It was estimated that the sample size of eighty-three subjects would give >90 % power to detect a 58 % difference in the SPM concentrations between groups over time at a significance level of P=0·05.
Results
Study demographics
In all, eighty three women and their healthy full-term babies completed the study at birth (forty in the n-3 fatty acid group and forty-three in the OO group) as reported previously( Reference Dunstan, Mori and Barden 19 ). Age, BMI at entry, parity, gestational age, delivery method and socio-economic status were comparable between groups( Reference Dunstan, Mori and Barden 18 ). Fig. 1 shows the flow of the participants through the trial. There were no substantial differences between the n-3 fatty acid and OO groups in those with blood samples at birth (Table 1). With the exception of birth weight, all other antenatal and birth characteristics were similar in those that provided blood at the 12-year follow-up compared with those that did not participate (online Supplementary Table S1).
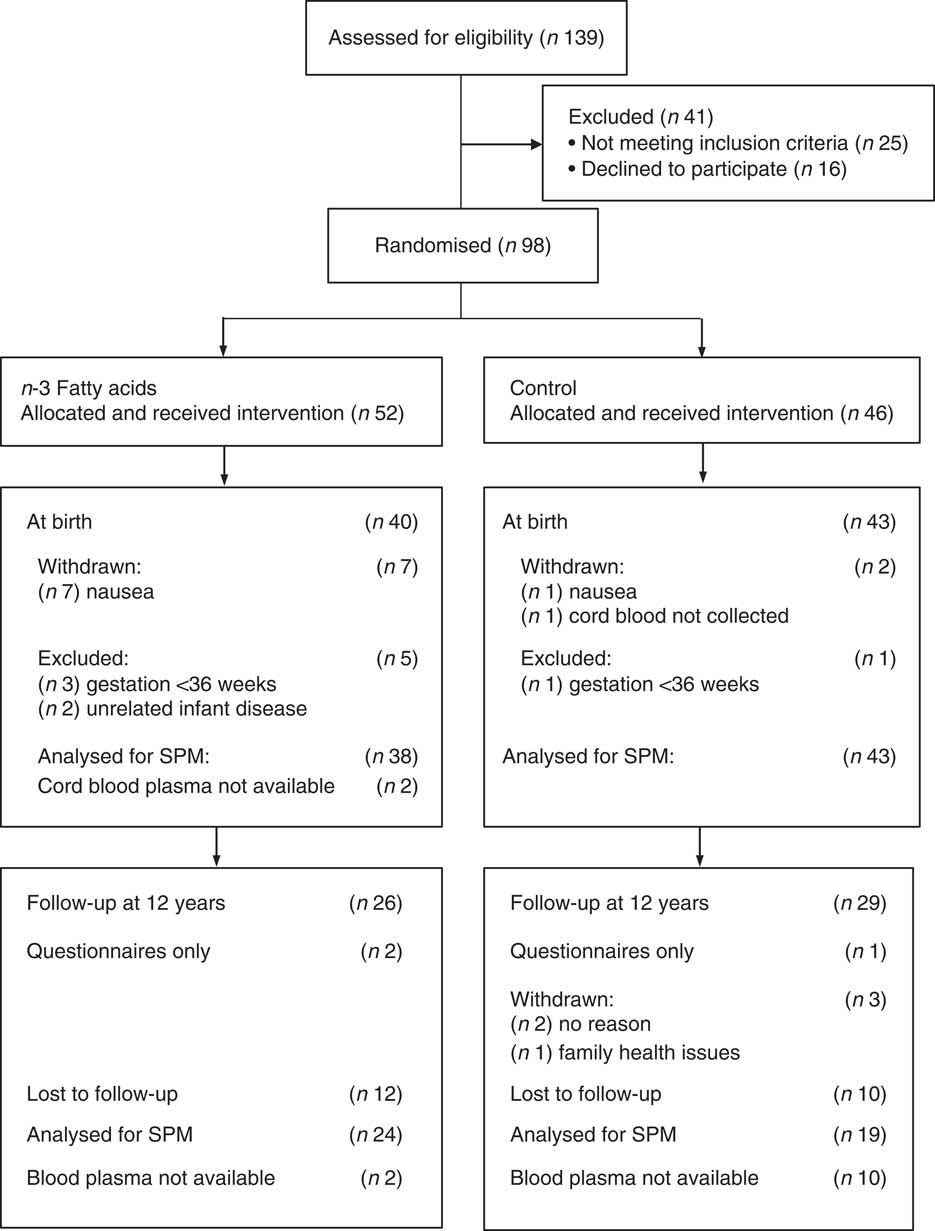
Fig. 1 The CONSORT diagram showing volunteer recruitment and randomisation.
Table 1 Characteristics of the study population (Mean values and standard deviations; numbers and percentages)
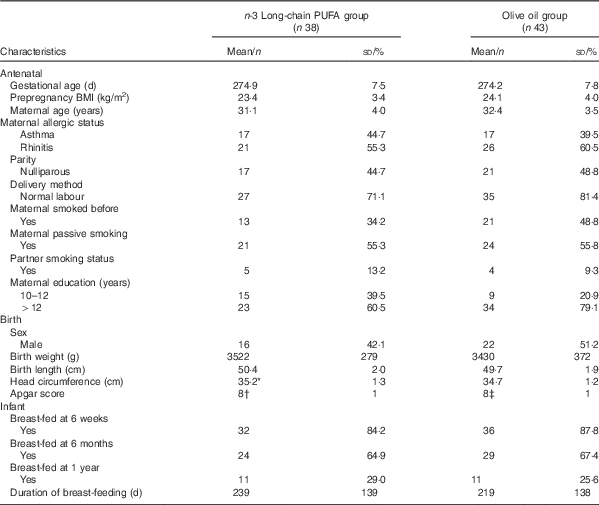
* Data available for n 37.
† Data available for n 29.
‡ Data available for n 34.
Effect of n-3 fatty acid supplementation in pregnant mothers on specialised pro-resolving mediators in childhood
At birth and at 12 years, measurable concentrations of all SPM except 10 S, 17S-dihydroxydocosahexaenoic acid (DiHDoHE), MaR-1 and PD1 in plasma were detected (Table 2). n-3 Fatty acid supplementation during pregnancy resulted in a 3-fold increase in cord blood 18-HEPE (P<0·001), a 1·5-fold increase in 17-HDHA (P=0·001), and increases of 30 % and 20 % for RvE2 (P=0·008) and RvE3 (P=0·03), respectively, compared with controls. These differences were independent of cord erythrocyte DHA and EPA concentrations. For the groups combined, cord plasma 18-HEPE was significantly positively correlated with cord erythrocyte EPA (B=151·4, P<0·001) (Fig. 2(a)). The E-series resolvins (RvE1 and 18R-RvE3), D-series resolvins (RvD1, 17R-RvD1 and RvD2) and 14-HDHA of the maresin family were not different between the groups at birth (Table 2). The differences between groups for cord blood 18-HEPE and 17-HDHA remained significant following adjustment for multiple comparisons testing.
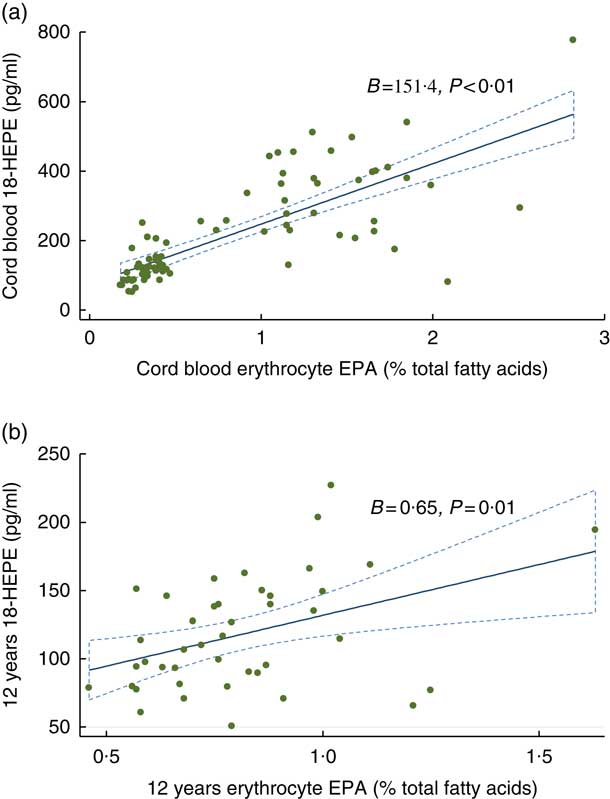
Fig. 2 Associations between 18-hydroxyeicosapentaenoic acid (18-HEPE) and erythrocyte EPA: (a) at birth; (b) at 12 years. ![]() , 95% CI.
, 95% CI.
Table 2 Effect of n-3 fatty acid supplementation from 20 weeks of gestation till delivery on specialised pro-resolving mediators (SPM) at birth and at 12 years (Geometric means and 95 % confidence intervals)
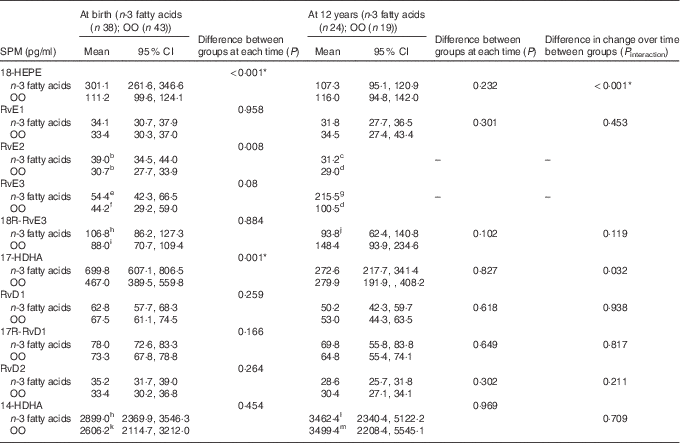
OO, olive oil; HEPE, hydroxyeicosapentaenoic acid; Rv, resolvin; HDHA, hydroxydocosahexaenoic acid.
Data available for: b n 35; c n 1; d n 8; e n 36; f n 24; g n 5; h n 37; i n 42; j n 22; k n 41; l n 21; m n 14.
* P values remained significant following adjustment for multiple testing.
At 12 years SPM were not different in the offspring of women supplemented with n-3 fatty acids compared with the offspring of women who took OO during pregnancy (Table 2). The change in the concentrations of 17-HDHA (P interaction=0·032) (Fig. 3(a)) and 18-HEPE (P interaction<0·001) (Fig. 3(b)) from birth to 12 years was greater in the n-3 fatty acid group. However, following adjustment for multiple comparisons testing, the change in the concentrations of 17-HDHA over time was no longer significant. For the groups combined, 18-HEPE was significantly positively correlated with erythrocyte EPA at 12 years (B=0·65, P=0·01) (Fig. 2(b)).
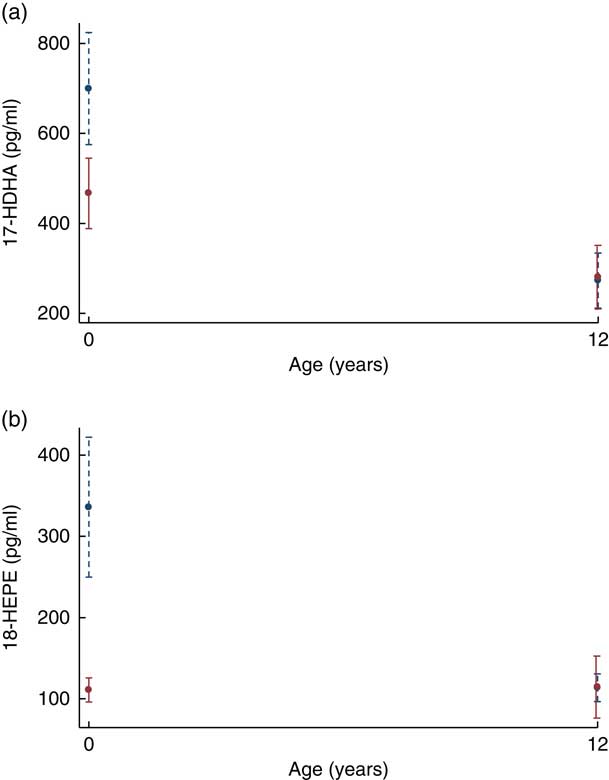
Fig. 3 (a) Plasma resolvin 17-hydroxydocosahexaenoic acid (17-HDHA) concentrations from birth to 12 years. (b) Plasma resolvin 18-hydroxyeicosapentaenoic acid (18-HEPE) concentrations from birth to 12 years. ![]() , n-3 Fatty acids;
, n-3 Fatty acids; ![]() , OO. Values are predictive margins with 95 % confidence intervals.
, OO. Values are predictive margins with 95 % confidence intervals.
Prevalence of allergic outcomes at 12 months and resolvins at birth
Table 3 shows the mean SPM concentrations for the allergic and non-allergic participants at 12 months in the n-3 fatty acid and OO groups, respectively. No formal analysis was performed due to the minimal power to detect interaction effects.
Table 3 Specialised pro-resolving mediators (SPM) concentrations and the allergic and non-allergic participants by group (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))
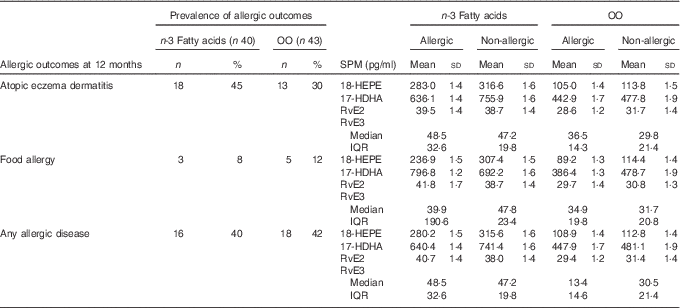
OO, olive oil; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; Rv, resolvin.
Erythrocyte fatty acid analysis from birth to 12 years
Erythrocyte EPA, DHA, linoleic acid and arachidonic acid (AA) concentrations were significantly different between the n-3 fatty acid and OO groups at birth (in cord blood plasma) (Table 4). However, these differences were not apparent at 12 years (Table 4). The change in the concentrations of EPA (P interaction<0·001) and DHA (P interaction<0·001) from birth to 12 years was greater in the n-3 fatty acid group, which remained significant following adjustment for multiple comparisons testing (Table 4). The change in the concentrations of AA was also significantly different from birth to 12 years before and after adjustments for multiple comparisons testing (P interaction<0·001).
Table 4 Comparison of groups for erythrocyte fatty acid concentrations at birth and 12 years (Mean values, geometric means and standard deviations)
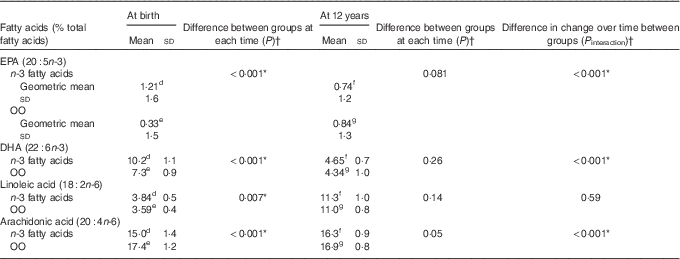
Data available for: d n 39, e n 43, f n 24, g n 19.
* P values remained significant following adjustment for multiple testing.
† P values obtained using linear mixed models with maximum likelihood estimation.
Discussion
Our study has shown for the first time that supplementing pregnant mothers from 20 weeks’ gestation till delivery with 3·7 g/d of n-3 fatty acids significantly increased several cord blood SPM that have potent biological activity (18-HEPE and 17-HDHA). These differences were not evident in the blood of the offspring at 12 years. We also identified RvE1, RvE2 and RvE3 as well as RvD1, 17R-RvD1 and RvD2 for the first time in human cord blood.
The increase in 18-HEPE and 17-HDHA with n-3 fatty acids in our study is important because these SPM are known to promote resolution of inflammation. 18-HEPE and 17-HDHA have been shown to suppress liver tumour formation( Reference Weylandt, Krause and Gomolka 34 ). 18-HEPE has been shown to inhibit neutrophil infiltration in zymosan induced peritonitis( Reference Isobe, Arita and Matsueda 35 ) and inhibit macrophage-mediated pro-inflammatory activation of cardiac fibroblasts( Reference Endo, Sano and Isobe 36 ). 17-HDHA has also been shown to protect against liver injury( Reference González-Périz, Planagumà and Gronert 37 ) and modulate macrophage function to alleviate experimental colitis( Reference Chiu, Gomolka and Dierkes 38 ).
We have previously reported in the same study cohort that placental 18-HEPE and 17-HDHA were significantly increased by approximately 3-fold and 2-fold, respectively, in the n-3 fatty acid supplemented group relative to the controls( Reference Keelan, Mas and D’Vaz 16 ). Herein we observed a consistent increase in cord blood 18-HEPE and 17-HDHA with n-3 fatty acid supplementation and these findings are supported by the fact that both mothers and fetus had significant increase in the proportions of EPA and DHA in the erythrocytes( Reference Dunstan, Mori and Barden 18 ). There were consistent coherent relationships between the patterns of SPM production and variations in membrane fatty acid composition. Specifically, cord EPA status was consistently associated with the higher production of 18-HEPE at birth, and this association was also observed between erythrocyte EPA and 18-HEPE at 12 years. This finding is similar to human studies that show that dietary intake of EPA significantly increased plasma concentration of 18-HEPE( Reference Endo, Sano and Isobe 36 ). Our current findings are supported by erythrocyte fatty acid status together with previous reports from the same study that n-3 fatty acid supplementation inhibited pro-inflammatory leukotrienes in cord blood-derived neutrophils( Reference Prescott, Barden and Mori 39 ), confirming the anti-inflammatory and pro-resolving actions of n-3 fatty acids( Reference Mori and Beilin 40 ).
Our findings are in accordance with previous reports from our group that showed markedly higher concentrations of the resolvin precursors, 18-HEPE and 17-HDHA, in plasma and serum of healthy adults and in patients with chronic kidney disease supplemented with n-3 fatty acids( Reference Mas, Croft and Zahra 5 , Reference Barden, Mas and Croft 33 , Reference Mas, Barden and Burke 41 ). These results also concur with a preliminary report by Mozurkewich et al. ( Reference Mozurkewich, Clinton and Chilimigras 42 , Reference Mozurkewich, Greenwood and Romero 43 ) who showed that 17-HDHA significantly increased in maternal and cord blood samples from EPA- or DHA- rich fish oil supplemented participants compared with a soya oil control. In contrast, Mas et al. ( Reference Mas, Croft and Zahra 5 , Reference Mas, Barden and Burke 41 ) reported an increase in RvD1, RvD2 and 17R-RvD1 in healthy adults following 3 weeks of n-3 fatty acid supplementation (35 % EPA and 25 % DHA), whereas Barden et al. ( Reference Barden, Mas and Croft 33 ) showed that supplementing n-3 fatty acids (2·4 g/d) for 7 d in healthy adults increased RvE1. Another study supplementing n-3 fatty acids (3·4 g/d) for 8 weeks in chronic kidney disease patients reported an increase in RvD1( Reference Mas, Barden and Burke 41 ). It is possible that the steps in further biosynthesis from the intermediates (precursors) to E-series and D-series resolvins may be tightly regulated and may not be subject to increased levels on a relatively short-term (20 weeks) supplementation as used in this study. Our findings highlight that dietary n-3 fatty acid supplementation in pregnancy can directly influence the pattern of biologically relevant SPM production in the offspring likely through placental transfer. Increased SPM concentrations in cord blood may be consequent on parturition which is characterised by a pro-inflammatory environment to promote the contraction of the uterus, delivering of the baby and rejection of the placenta( Reference Mor, Cardenas and Abrahams 44 ). Although SPM concentrations were not different between the groups at 12 years, we showed they are still present and may have relevant immunomodulating functions.
While the sample size was insufficient to investigate associations with allergic outcomes in the present data set, future studies may show the importance of SPM in humans in reducing and clearing infections. Pre-clinical models of allergic lung inflammation have shown that RvD1 and RvE1 reduced the consequences of allergic responses by decreasing eosinophil recruitment, type 2 cytokine production and airway hyper-responsiveness, and enhancing allergen phagocytosis and clearance( Reference Levy, Kohli and Gotlinger 45 – Reference Rogerio, Haworth and Croze 48 ).
Despite the relatively high concentrations of 14-HDHA of the maresin SPM family in both groups, MaR-1 was not detected in this sample. These findings are in accordance with those of Barden et al. ( Reference Barden, Mas and Croft 33 ) in healthy adults supplemented with n-3 fatty acids. We did not detect 10 S,17S-DiHDoHE or MaR-1 in cord blood following n-3 fatty acid supplementation, possibly suggesting a preferential synthesis of D-series resolvins from DHA during pregnancy. The non-detection of PD1 could be due to this SPM being converted by leucocytes to an n-22 monohydroxylated metabolite of PD1, which retains biological function with macrophages( Reference Connor, SanGiovanni and Lofqvist 49 , Reference Tungen, Aursnes and Vik 50 ).
A major strength of our study is the comprehensive antenatal and offspring data on the participants and their follow-up to 12 years of age. Samples for analysis of SPM at 12 years were recently collected whereas cord plasma samples had been stored long-term at −80°C before analysis, which could be a limitation of this study. Although statistical analyses employed MLE that accounts for missing data, we acknowledge the high attrition rate at 12 years could be a further limitation. The length of the dietary intervention may be another important factor as the impact of n-3 fatty acid consumption may require a longer and ongoing intervention period in order to detect a difference in SPM at 12 years of age.
Despite this, this is the first report of 18-HEPE in cord blood plasma following maternal supplementation with n-3 fatty acids. Our results show that n-3 fatty acids in pregnancy do in fact raise cord SPM concentrations. Although the study was not powered to detect any associations with allergic outcomes, SPM at birth may be relevant to the alleviation of low-grade inflammatory status associated with pregnancy which may be beneficial to the fetus. These findings add significantly to the very limited literature reporting SPM in humans, particularly in children. However, n-3 fatty acid supplementation in pregnancy on SPM are not long-lasting beyond the end of supplementation. Further studies are required to explore the biological effects of SPM in cord blood to ascertain their significance in mediating the beneficial effects of n-3 fatty acid supplementation at birth and their pharmacological applications in relation to longer-term modification of health and disease in children.
Acknowledgements
The authors thank the staff and participants who helped in this study. The authors particularly thank Carlie Dunford for her assistance with recruitment.
This work was supported by the National Health and Medical Research Council of Australia (NH&MRC) (grant no. APP1010495). The NH&MRC had no role in the design, analysis or writing of this article.
T. A. M., S. L. P., L. J. B. and R.-C. H.: designed and supervised the study; V. H. L. S.: conducted the study and had primary responsibility for the final content; V. H. L. S. and S. B.: analysed the data; V. H. L. S., E. M., S. L. P., L. J. B., A. E. B., T. A. M. and R.-C. H.: wrote the manuscript. The study sponsors were not involved in the design, implementation, analysis, presentation or interpretation of results.










