Optical microscope images of machined structures on an Fe65.5Cr4Mo4Ga4P12C5B5.5 bulk metallic glass surface in 1 M CF3COOH solution with 200 ns pulses of 7 V amplitude with a 30 μm W tool electrode. [R. Sueptitz, K. Tschulik, C. Becker, M. Stoica, M. Uhlemann, J. Eckert, and A. Gebert: Micro-patterning of Fe-based bulk metallic glass surfaces by pulsed electrochemical micro-machining (ECMM). p. 3033].
Articles
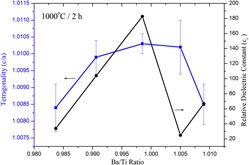
Effects of Ba/Ti ratio on tetragonality, Curie temperature, and dielectric properties of solid-state-reacted BaTiO3 powder
-
- Published online by Cambridge University Press:
- 03 October 2012, pp. 2937-2942
-
- Article
- Export citation
Phase transitions, relaxor behavior, and electrical properties in (1−x)(Bi0.5Na0.5)TiO3–x(K0.5Na0.5)NbO3 lead-free piezoceramics
-
- Published online by Cambridge University Press:
- 17 October 2012, pp. 2943-2955
-
- Article
- Export citation
Structural stability of layered n-LaFeO3–Bi4Ti3O12, BiFeO3–Bi4Ti3O12, and SrTiO3–Bi4Ti3O12 thin films
-
- Published online by Cambridge University Press:
- 29 October 2012, pp. 2956-2964
-
- Article
- Export citation
Influence of Mn incorporation on structural, optical emission and polarization switching aspect of PbO-free nanoscale PbTiO3 systems
-
- Published online by Cambridge University Press:
- 30 October 2012, pp. 2965-2972
-
- Article
- Export citation
EPR and optical properties of KY(WO4)2:Gd3+ powders
-
- Published online by Cambridge University Press:
- 12 November 2012, pp. 2973-2981
-
- Article
- Export citation
Dye-sensitized solar cells based on ZnO nanoneedle/TiO2 nanoparticle composite photoelectrodes with controllable weight ratio
-
- Published online by Cambridge University Press:
- 30 October 2012, pp. 2982-2987
-
- Article
- Export citation
Hydrothermal synthesis, phase evolution, and optical properties of Eu3+-doped KF–YF3 system materials
-
- Published online by Cambridge University Press:
- 17 October 2012, pp. 2988-2995
-
- Article
- Export citation
Impact of low viscosity ionic liquid on PMMA–PVC–LiTFSI polymer electrolytes based on AC -impedance, dielectric behavior, and HATR–FTIR characteristics
-
- Published online by Cambridge University Press:
- 30 October 2012, pp. 2996-3004
-
- Article
- Export citation
Growth of one-dimensional doped polypyrrole nanofibers on glass substrate
-
- Published online by Cambridge University Press:
- 26 November 2012, pp. 3005-3012
-
- Article
- Export citation
Electric field analysis of spinneret design for needleless electrospinning of nanofibers
-
- Published online by Cambridge University Press:
- 30 October 2012, pp. 3013-3019
-
- Article
- Export citation
Evaluation of the catalytic activity of oxide nanoparticles synthesized by the polymeric precursor method on biodiesel production
-
- Published online by Cambridge University Press:
- 13 November 2012, pp. 3020-3026
-
- Article
- Export citation
Compensation effect of boron and nitrogen codoping on the hardness and electrical resistivity of diamond-like carbon films prepared by magnetron sputtering deposition
-
- Published online by Cambridge University Press:
- 31 October 2012, pp. 3027-3032
-
- Article
- Export citation
Micropatterning of Fe-based bulk metallic glass surfaces by pulsed electrochemical micromachining
-
- Published online by Cambridge University Press:
- 31 October 2012, pp. 3033-3040
-
- Article
- Export citation
Varied linear phason strain and its induced domain structure in quasicrystalline precipitates of Zr–Al–Ni–Cu–Nb bulk metallic glass matrix composites
-
- Published online by Cambridge University Press:
- 12 November 2012, pp. 3041-3048
-
- Article
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 27 issue 23 Cover and Front matter
-
- Published online by Cambridge University Press:
- 26 November 2012, pp. f1-f4
-
- Article
-
- You have access
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 23 Cover and Back matter
-
- Published online by Cambridge University Press:
- 26 November 2012, pp. b1-b4
-
- Article
-
- You have access
- Export citation