The term ‘placebo’ is used in different contexts with different meanings (Box 1). Placebo effects have been a scientific curiosity for years, regarded as deceptive therapies and tainted by associations with quackery and dishonesty. Anthropological studies of the placebo (Thompson 2010) highlight the universality of charms and ritual to produce healing effects in all cultures, suggesting an evolutionary basis to placebo-responding.
BOX 1 Some uses of the word placebo
A psychological trick
A treatment for neurotic patients when the doctor has nothing better to offer
A device to establish the ‘true’ biochemical effect of drug treatments
A device for eliminating bias in trials
A tool in the study of mechanisms of treatment action
A catch-all for non-pharmacological effects in pharmacology RCTs
A trigger of psychosomatic reactions
A logically inconsistent, conceptually unstable artefact of RCT design
A group of psychosomatic mechanisms via which some drugs exert their effects
A range of behavioural responses to the psychosocial context
A denigratory term for psychosocial aspects of therapeutic action
A ‘straw man’ used in trials to show the superiority of the researchers' preferred treatment
A range of evolved behavioural responses subject to natural selection, allowing the organism to regulate investment in self-healing processes according to the favourability of the environment and prospects for recovery or further threat

Historical roots of placebo
The use of the Latin word placebo dates to the 14th-century psalm Placebo Domino (‘I shall please the Lord’), sung by mourners at funerals. In George Motherby's New Medical Dictionary of 1785, it was defined as ‘a common place method or medicine’, and in the Hooper's Medical Dictionary in 1811 as ‘any medicine adapted more to please than benefit the patient’ (Reference AronsonAronson 1999).
Early uses of placebo as a control condition are recorded, although not by that name, during ‘trick trials’ of exorcism rituals in the 16th century, whereby sham holy water or religious relics were used to expose false exorcisms during the Counter-Reformation (Reference Kaptchuk, Kerr and ZangerKaptchuk 2009).
Placebo in the era of the RCT
The concept radically changed after the Second World War with the development of the randomised placebo-controlled trial (RCT). The term ‘placebo effect’ entered medical literature with the American anaesthetist Henry Beecher's paper ‘The powerful placebo’ (Reference BeecherBeecher 1955). During this period, the concept of placebo came to include a wide array of treatment and non-treatment effects that occurred in the placebo arm of an RCT, including natural history of the illness, regression to the mean, and effects of routine nursing and medical care. These components inflated the apparent size of the placebo effect (Reference KaptchukKaptchuk 1998). Thus, the placebo came to be regarded as a threat to scientific medicine and, in consequence, unethical (Reference ShorterShorter 2011).
Early placebo research
Early placebo research, informed by expectancy theory, identified many variables that influence the size of placebo effects (Reference GoldacreGoldacre 2008): larger numbers of pills work better than fewer; capsules are more effective than tablets; intravenous administration is better than oral; elaborate rituals are better than simple ones; branded placebos are more effective than generic; expensive treatments work better than cheap ones. Placebo morphine is more effective than placebo propoxyphene, which is more effective than placebo aspirin. Placebo and active analgesics are more effective when presented with a well-known brand name. The colour of a placebo is influential: blue placebo pills produce depressant effects, whereas red placebos induce stimulant effects. Patients report falling asleep more quickly after taking a blue capsule than after taking an orange capsule. Red placebos seem to be more effective pain relievers than white, blue or green placebos. The magnitude of the placebo response varies as a function of the stated dose consumed (Reference KirschKirsch 2005).
In the attempt to separate placebo effects from ‘true’ treatment effects, researchers have distinguished between characteristic and incidental constituents of a treatment. However, this distinction is driven by the theory of the intervention. Only those effects designated as characteristic by the theory are considered important. In biomedical research, this leads to an exclusive focus on surgical or pharmacological effects, and research methods designed to maximise detection of these effects, while minimising psychosocial aspects of treatment (Reference Wampold, Minami and TierneyWampold 2005). For example, in the pharmacological treatment of depression, an increase in synaptic monoamine levels is considered to be a characteristic constituent of antidepressant pharmacotherapy from the perspective of the monoamine theory of depression, whereas other effects, such as the patient deriving hope from treatment, feeling validated, and feeling deserving of treatment and recovery, are considered to be incidental constituents of treatment. A similar process exists in psychotherapy research, with adherents of different therapeutic schools focusing on their preferred concepts and neglecting or minimising the contribution of processes that are not valued within their school, the so-called researcher allegiance bias (Reference Luborsky, Diguer and SeligmanLuborsky 1999). For example, in cognitive–behavioural therapy (CBT) of depression, adherence to the psychodynamic prototype was a better predictor of outcome than adherence to the CBT prototype (Reference Ablon and JonesAblon 1998), although psychodynamic processes would be considered incidental from the CBT perspective. Researcher allegiance in psychotherapy will be discussed further in our second article (Reference McQueen, Cohen and St John-SmithMcQueen 2013, this issue).
According to some studies, placebo effects are larger than pharmacological effects in the pharmacotherapy of depression (Reference McKay, Imel and WampoldMcKay 2006; Reference Kirsch, Deacon and Huedo-MedinaKirsch 2008). We return to this later in our article.
Defining placebo effects in psychotherapy is particularly problematic. Some authors (Reference WilkinsWilkins 1984; Reference KirschKirsch 2005) argue that, as psychotherapy contains no directly acting chemical ingredient, it is analogous to a pharmaceutical placebo, that psychotherapy and placebos are synonymous, that placebo psychotherapy is an oxymoron, and that ‘evaluating the efficacy of psychotherapy by controlling for non-specific or placebo factors is based on a flawed analogy and should be abandoned’ (Reference KirschKirsch 2005).
Placebo research has emerged as a way of studying the ‘healing situation’, with placebo effects the primary object of study (Reference Papakostas and DarasPapakostas 2001). In June 2011, the Royal Society devoted a themed issue of its Philosophical Transactions to ‘the placebo’, presenting current understanding of psychobiological mechanisms, anomalies in placebo research, and how to harness placebo effects in clinical practice (Reference Meissner, Kohls and CollocaMeissner 2011). Research into the psychologically mediated effects of receiving drug treatment has identified cognitive and emotional mechanisms, including expectation, social learning, emotional change (e.g. reduction in anxiety or depression from receiving care, diagnosis and treatment) and classical conditioning, any of which may, or may not, lead to physiological changes through neurologically regulated mechanisms.
Recognition of the interactions between mind and body, and elaboration of brain pathways underlying placebo effects, demonstrate that placebo effects are true psychobiological events.
Illness, disease and healing
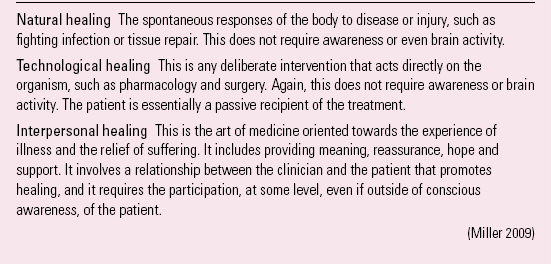
Reference Miller, Colloca and KaptchukMiller et al (2009) distinguish between disease, a biological dysfunction of the organism to be understood pathophysiologically, and illness, a lived experience of distress that includes symptomatic manifestations and is to be understood phenomenologically. They describe three types of healing (Box 2) and argue that the evidence indicates that placebo effects primarily influence illness, affecting disease only minimally if at all.
BOX 2 Three types of healing
Natural healing The spontaneous responses of the body to disease or injury, such as fighting infection or tissue repair. This does not require awareness or even brain activity.
Technological healing This is any deliberate intervention that acts directly on the organism, such as pharmacology and surgery. Again, this does not require awareness or brain activity. The patient is essentially a passive recipient of the treatment.
Interpersonal healing This is the art of medicine oriented towards the experience of illness and the relief of suffering. It includes providing meaning, reassurance, hope and support. It involves a relationship between the clinician and the patient that promotes healing, and it requires the participation, at some level, even if outside of conscious awareness, of the patient.
(Reference Miller, Colloca and KaptchukMiller 2009)
Furthermore, illness may lead to disease and vice versa. For example, negative emotions affect the sympathetic nervous system, hypothalamic–pituitary–adrenal axis and inflammatory pathways and increase the risk of cardiovascular disease (Reference Kiecolt-Glaser, Mcguire and RoblesKiecolt-Glaser 2002; Reference Brotman, Golden and WittsteinBrotman 2007), while physical disease, particularly when chronic, can cause mental illness (Reference Naylor, Galea and ParsonageNaylor 2012).
Problems of definition
Imprecise and misleading definitions of placebos and placebo effects have contributed to conceptual confusion (Reference GrunbaumGrunbaum 1981; Reference G⊘tzscheGøtzsche 1994; Reference Moerman and JonasMoerman 2002; Reference Wampold, Minami and TierneyWampold 2005; Reference NunnNunn 2009). Reference G⊘tzscheGøtzsche (1994) found shortcomings in all the definitions that he tested, and concluded that placebo cannot be defined in a logically consistent way, but that the term might be retained for pragmatic reasons. Reference NunnNunn (2009) concluded that the placebo construct does not make sense and questioned whether placebo exists, comparing it to the emperor's new clothes. Reference GrunbaumGrunbaum (1981) was able to derive a definition of placebo, but one that depended on the practitioner's beliefs about the mechanism of action.
Conceptual confusion may arise in part because different experimental settings lend themselves to different conceptions of placebo. The methodology of RCTs compares different groups, typically one or more treatment groups, a placebo group and a no-treatment group. Group means are then subtracted from each other to give measures of change attributed to the specific treatment and placebo effects. In this ‘additive model’ of treatment, placebo effects are defined as the difference between outcome in a placebo group and a no-treatment group (Fig. 1). One inference that is frequently implied, incorrectly, from RCT research is that placebo effects are a property of the treatment, independent of the individual and clinical setting.
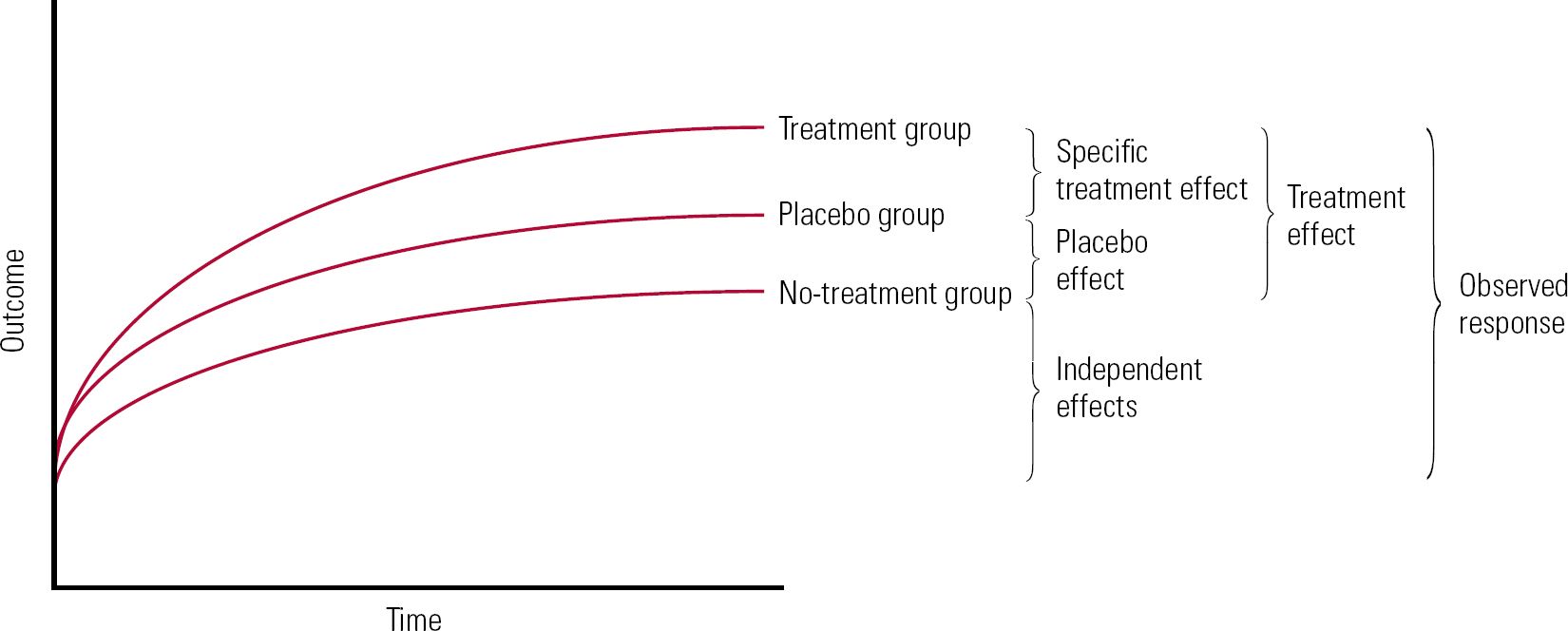
FIG 1 Additive model of treatment effects.
The RCT is designed to minimise individual differences, including individual relationship effects. However, individual meaning and relationship factors are central to placebo effects (see below). A number of writers explicitly or implicitly define placebo effects as changes resulting from symbolic communication, for example the meaning response (Reference Moerman and JonasMoerman 2002), the knowledge that therapy is being performed (Reference Benedetti, Maggi and LopianoBenedetti 2003), the healing encounter (Reference Thompson, Ritenbaugh and NichterThompson 2010) and interpersonal healing (Reference Miller, Colloca and KaptchukMiller 2009). The meaning response will be discussed in more detail in our second article (Reference McQueen, Cohen and St John-SmithMcQueen 2013, this issue).
Additive models of treatment effects
Additive models of treatment effects imply that an observed response to treatment can be broken down into a number of discrete processes that can be added together to explain the observed response.
Reference Ernst and ReschErnst & Resch (1995) give a clear exposition of an additive model of treatment effects (Fig. 2). They define a range of factors that contribute to change in trials and distinguish true treatment effects, true placebo effects, perceived placebo effects and non-specific effects. Perceived placebo effects comprise true placebo effects plus non-specific effects, i.e. effects independent of receiving a treatment. Non-specific or independent effects include natural course and variation in the disease, regression towards the mean, other time effects and unidentified parallel interventions.
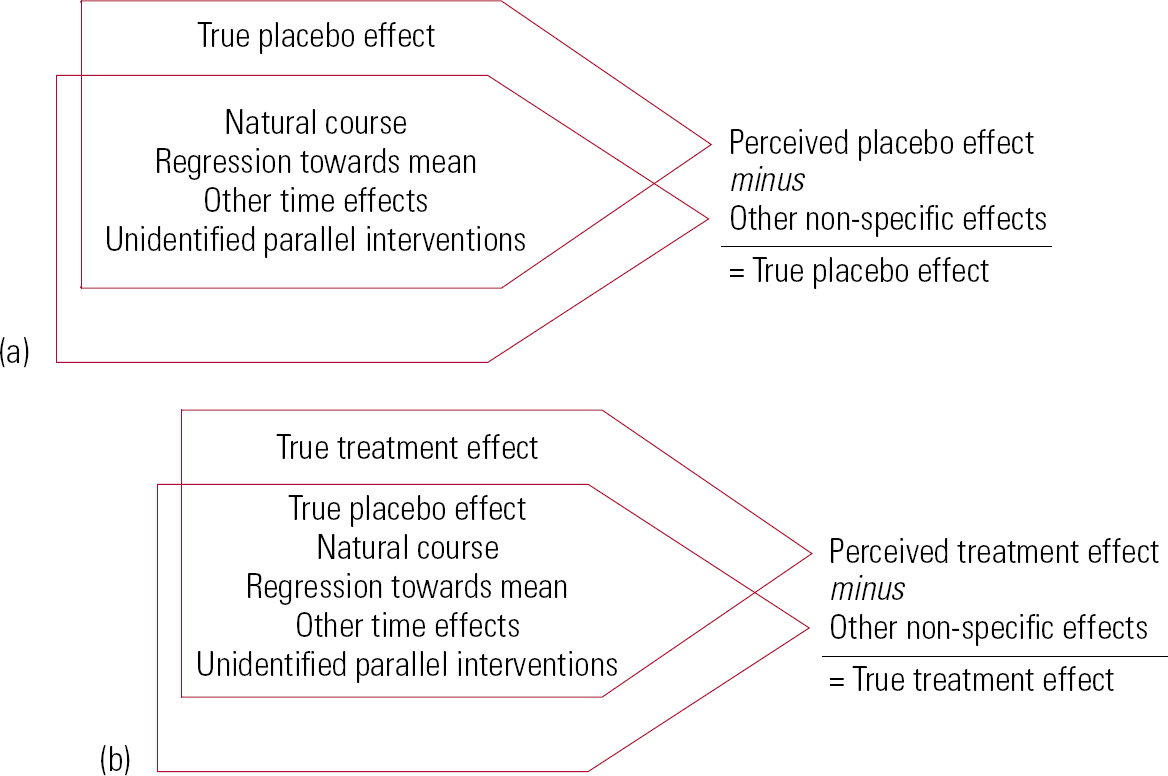
FIG 2 Reference Ernst and ReschErnst & Resch's (1995) exposition of an additive model of treatment effects. (a) Differentiation of true placebo effect from perceived placebo effect; (b) differentiation of true treatment effect from perceived treatment effect (redrawn with permission of BMJ Publishing Group).
Natural course and variation in the disease
Some diseases are self-limiting and spontaneously remit. Changes in disease severity, which are part of the natural course of the disease, may be incorrectly ascribed, by patient or clinician, to an intervention.
Regression towards the mean
Measurements of biological variables are subject to different types of variation. After measuring any variable subject to random variation, it is probable that subsequent measurements will be less extreme: this is the statistical phenomenon of regression towards the mean.
Other time effects
Treatments are usually initiated when symptoms are most frequent and/or severe and so may tend to improve with time, regardless of the intervention. Some time effects result from trial design, for example recruiting patients through financial inducements incentivises them to exaggerate their symptoms (consciously or not) so as to meet the study entry criteria (Reference McAllister-WilliamsMcAllister-Williams 2008). Reference Landin, Debrota and DevriesLandin et al (2000) have identified selective score inflation, where both trial participants and eager researchers inflate subcriterion scores in response to restrictive entry criteria in clinical trials to permit recruitment. Symptoms rapidly decrease after initiation of treatment, because the exaggeration is no longer required for people to be in the trial. These effects, if confused with placebo effects, make placebo effects appear short-lived.
Another group of time effects relate to a change in the accuracy or method of measurements taken at the start of an intervention compared with later. For example, the clinician's skill in giving the intervention increases with practice. Or the patient becomes accustomed to the clinical setting and is more relaxed, leading to a change in a measured variable such as blood pressure. Or the patient habituates to symptoms. There may be seasonal changes or changes in the patient's circumstances that are entirely independent of the intervention but that affect the measured variable. At the end of treatment, patients may report or perceive that their symptoms are improved because they wish to please their clinicians, or they may proclaim themselves well as a way of avoiding further self-exploration, the defensive mechanism of ‘flight into health’ (Reference MalanMalan 2001).
Unidentified parallel interventions
Receiving medical attention in itself may lead the patient to modify behaviour, for example changing diet, smoking patterns, alcohol consumption, exercise or other aspects of self-care. This has been called the Hawthorne effect, whereby being observed leads to changes in behaviour. The Hawthorne effect may be accounted for in trials by including a no-treatment group or controlling for relevant variables and changes in them. However, no-treatment arms may still have significant Hawthorne effects arising from recruitment and encounters with researchers.
It is difficult and rare for research to adequately control for the Hawthorne effect. It is debatable whether the Hawthorne effect should be considered an independent effect or part of the placebo effect. This is linked to whether the intervention is conceived of narrowly – based on the theory of the intervention – or from an inclusive, biopsychosocial perspective.
Identifying independent effects
Independent effects are assumed to apply equally to treatment, placebo and no-treatment arms in RCTs. They may be responsible for a considerable part of the treatment response seen in a placebo arm. To separate independent effects from placebo effects it is necessary to have a no-treatment arm in addition to a placebo arm. This is rarely the case in RCTs (Reference Miller, Colloca and KaptchukMiller 2009). In RCTs with only two arms, a treatment arm and a placebo arm, it is not possible to separate placebo and independent effects.
Validity of the additive model
Theoretical objections have been raised against the additive model of treatment effects. Reference AderAder (2000) has written: ‘we have no a priori right to assume that placebo effects and drug effects are additive. It may be more reasonable to hypothesise that they interact in complex ways that have not been studied in any systematic manner’.
Non-additive models of treatment effects
In RCTs, active placebos have greater effects than inactive placebos. This is thought to arise through changed expectancy: if the patient has side-effects, this informs them that they are taking a drug, which increases the expectation of clinical response, which in itself leads to improvement through placebo mechanisms (Reference ThomsonThomson 1982). Conversely, if the patient has no side-effects, they are more likely to infer that they are receiving placebo and consequently may have lower expectations of improvement. Thus, side-effects lead to unmasking (‘unblinding’) in trials. This inflates apparent treatment responses and underestimates placebo effect sizes (Reference Gaudiano and HerbertGaudiano 2005).
Similarly, more effective drugs may have additional placebo effects over less effective drugs because awareness that the drug is working leads to increasing expectations of benefit. This may lead to a positive feedback loop in which, as the treatment is experienced as effective, it further increases positive expectations and thereby produces additional placebo effects. Consequently, placebo effects may be even larger with effective treatments than with ineffective treatments. Therefore, in a placebo-controlled trial, if the treatment is effective, placebo effects may be greater in the treatment arm than in the placebo arm. Conversely, lack of response in the placebo group may lead to reduced placebo effects. The RCT model assumes additive placebo effects and is not powered to distinguish complex placebo–drug interactions. However, a meta-analysis by Reference Kirsch, Deacon and Huedo-MedinaKirsch et al (2008) indicates that placebo effects interact with illness severity and are smaller as the severity of depression increases. We will return to this later in our article.
Open v. hidden treatment
In recent years, it has been possible to redefine the specific effects of a treatment as effects that occur without the patient being aware that they are receiving the treatment. This is made possible by the open v. hidden (or deceptive) design, in which drugs can be administered without the patient's knowledge (i.e. through an in situ cannula). Independent manipulation of the patient's belief that treatment is being received then allows separation of pharmacological from psychosocial mechanisms (Reference Benedetti, Maggi and LopianoBenedetti 2003). This has revealed the very important contribution of verbal suggestion to the effectiveness of analgesics (Reference Price, Finniss and BenedettiPrice 2008).
Another complication comes from the demonstration that pharmacological and symbolic mechanisms interact at a physiological level. Reference Benedetti, Amanzio and MaggiBenedetti et al (1995), using pain as an outcome variable known to be highly influenced by emotional state, demonstrated that some drugs can exert their effect by acting on placebo pathways. They showed that in patients with postoperative pain, proglumide was more effective in reducing pain than placebo, which was more effective than no treatment. (Proglumide is a cholecystokinin antagonist, and cholecystokinin is an endogenous opiate antagonist. Therefore, proglumide prevents cholecystokinin from blocking opiates: put simply, it facilitates the action of opiates.) However, when proglumide was given in a hidden way it had no effect. The authors concluded that proglumide has no direct effect on pain pathways, but instead potentiates a placebo-activated endogenous opiate system, and therefore is only effective when combined with the placebo mechanisms inherent in the clinical encounter (Reference Finniss, Kaptchuk and MillerFinniss 2010). This provides evidence that placebos and drugs may act via the same neural pathways.
Can the placebo effect be measured?
Reference Meissner, Distel and MitzdorfMeissner et al (2007) examined heterogeneity among placebo trials. They found that not all physical parameters are equally responsive to placebo effects: 50% of trials measuring ‘physiological’ parameters (e.g. heart rate and blood pressure) showed significant placebo effects, compared with 6% of trials measuring biochemical parameters.
A Cochrane review of placebo interventions for all conditions analysed trials containing placebo and no-treatment groups (Reference Hróbjartsson and G⊘tzscheHróbjartsson 2010). It concluded that placebo interventions do not have important clinical effects in general. However, there was heterogeneity between trials. Meta-regression analyses showed larger placebo effects associated with physical placebo interventions (e.g. sham acupuncture), patient-involved outcomes (patient-reported outcomes and observer-reported outcomes involving patient cooperation), small trials and trials with the explicit purpose of studying placebo. Larger effects of placebo were also found in trials that did not inform patients about the possible placebo intervention (the meta-analysis compared trials in which participants were informed that the trial compared two active interventions with a control group, and trials in which participants were informed that the trial involved a placebo intervention). The effects on pain varied, even among trials with low risk of bias, from negligible to clinically important. Variations in the effect of placebo were partly explained by variations in how trials were conducted and how patients were informed. This supports the importance of expectancy, in patients and researchers, in determining the size of placebo effects. There is a potential conceptual and methodological problem in assuming that there are no placebo effects in no-treatment arms. Patients in no-treatment arms in trials may still be recruited, assessed, selected, randomised and followed up. These processes linked to trial entry all carry symbolic weight and may lead to emotional, behavioural and cognitive changes. Failure to account for these Hawthorne effects leads to underestimation of the size of placebo effects in placebo treatment arms. Ultimately, placebo effects are so dependent on the psychosocial context that it is questionable what meaning an average figure for placebo effects, i.e. a figure independent of individual context, can have.
Placebos are less effective when participants are aware that they may receive a placebo. In studies that use placebo analgesia as a control condition, effect sizes are very small (mean effect size of 0.15). However, when the purpose of the trial is explicitly to examine placebo analgesic mechanisms, the magnitude of placebo effects is much higher (mean effect size of 0.95) (Reference Vase, Riley and PriceVase 2002). Reference Lidstone, Schulzer and DinelleLidstone et al (2010) manipulated the expectation of individuals with Parkinson's disease that they were receiving placebo by changing the perceived probability of receiving a placebo, and found a direct relationship between strength of expectation of receiving active treatment and the amount of dopamine released in response to placebo. Manipulating the expectations of clinicians has also been demonstrated to influence the size of placebo effects, a subject discussed in our second article (Reference McQueen, Cohen and St John-SmithMcQueen 2013, this issue).
Thus, placebo is not a unitary thing and there is no fixed value of a placebo effect for a given therapy. Its size depends on many factors, including the individual, the situation, the patients' and clinicians' expectations, the relationship between the individual and the clinician, and the specific variable being measured. Placebo effects vary widely as a result of the clinical setting or trial design. These points will be considered in our second article.
Nocebo and iatrogenesis
The term ‘nocebo’ was first used by Reference KennedyKennedy (1961), who distinguished nocebos from placebos in terms of negative v. positive outcomes. Reference HahnHahn (1997) considers that the nocebo effect occurs because of expectation of negative outcomes, including side-effects. Research into nocebo effects is ethically complicated, in terms of both potential detrimental effects for patients and the inability to get informed consent of patients, who need to be deceived in the process of being given a nocebo. Thus, research is limited. But just as clinicians can potentially enhance and improve clinical outcomes by promoting the placebo response, they can also contribute to a nocebo response.
As hope can augment placebo responses, so its converse, disappointment or fear, can lead to worse outcomes. Disappointment may come from various sources. Clinicians who raise their patients' expectations to unreasonably high levels should expect that disappointment will follow. Clinicians who induce feelings of rejection in their patients similarly should anticipate that their treatments will be less efficacious. Nocebo responses can be provoked directly by inconsiderate behaviour (lateness, rudeness, insensitivity) and by disappointing patients' wishes to feel understood.
Placebo in clinical psychiatry: the pharmacotherapy of depression
Placebo responses have not been studied in equal depth in all psychiatric disorders, but there is an extensive literature on placebo in the pharmacotherapy of depression. Reference Kirsch and SapirsteinKirsch & Sapirstein (1998), in a meta-analysis of published antidepressant medication trials, demonstrated a change of 1.16 s.d. on measures of depression following administration of placebo antidepressants, compared with 0.37 s.d. among untreated controls. Subtracting the two suggests a 0.79 s.d. placebo effect size. They analysed active placebos (amylobarbitone, lithium, liothyronine and adinazolam) separately and showed that the change produced by active placebos (1.69 s.d.) was greater than that of inert placebos and as great as that observed in response to antidepressants (1.68 s.d. for selective serotonin reuptake inhibitors (SSRIs); 1.52 s.d. for tricyclics). Whether these are active placebos is questionable, as all would be expected to have direct effects on physical symptoms or anxiety that would affect scores on depression rating scales (e.g. activation, sedation, changes in bowel habit). Nonetheless, Kirsch & Sapirstein concluded that placebos reproduced about 75% of the improvement found in the active drug, and suggested that the remaining 25% of improvement accounted for by the active drug could be the result of an enhanced placebo response due to the side-effects of taking the active drugs (i.e. an unmasking effect) or other non-specific factors.
Kirsch et al repeated their original meta-analysis using a larger and more complete data-set (35 trials), including unpublished data obtained from the Food and Drug Administration (Reference Kirsch, Deacon and Huedo-MedinaKirsch 2008). They found publication bias, which resulted in drug–placebo differences that were even smaller than shown previously, with placebos producing 82% of the improvement found in drug groups. Only patients with a Hamilton Rating Scale for Depression (HRSD) score > 28 had an improvement of > 3 points on the scale (the minimum change considered as clinically significant by the National Institute for Health and Clinical Excellence). The team also demonstrated that placebo responses fall with increasing baseline severity of depression, whereas drug responses remain more consistent. From this they concluded that the relationship between initial illness severity and antidepressant efficacy is attributable to decreased responsiveness to placebo among severely depressed patients, rather than to increased responsiveness to medication.
It may be that placebo responses fall in the severely depressed group because of unmasking and lowered expectations resulting from the lack of clinical progress. Conversely, the severely depressed group who receive ‘active’ antidepressants may experience unmasking as a result of either side-effects or treatment response, and this increases expectations. The important implication of this is that placebo responses may be higher in the active treatment group than in the placebo group. Thus, the true ‘efficacy’ of the drug may be less than it appears to be.
Reference Quitkin, Rabkin and StewartQuitkin et al (1991) analysed patterns of symptom improvement in antidepressant and placebo treatments of depression. They found that improvement in depressive symptoms in response to placebo tends to be early, abrupt and short lived, whereas improvement in response to antidepressants tends to be slower, more gradual and more persistent.
Antidepressant trials also have high rates of withdrawal due to adverse reactions to placebo. These adverse reactions may be considered nocebo effects.
Interpretation of the data
How should psychiatrists use this information when offering antidepressants to patients? Undue optimism on the part of psychiatrists is unlikely to be helpful, as it will lead to disappointment and demoralisation. Similarly, unrealistic patient expectations should be managed. For instance, ‘This will not get rid of all of your bad feelings, but it will take the edge off the bad days’. Realistic enthusiasm for treatment should be supported. Patients who have negative expectations may experience less benefit, so coercion should be avoided if possible. With ambivalent patients it may be useful to say explicitly: ‘Antidepressants work well when people want to take them, but less well if they do not want to take them’. Care should be taken when describing side-effects as this may become a self-fulfilling prophecy (a nocebo effect)and undermine effectiveness. A balance has to be struck between informed consent, informing patients of dangerous side-effects that they need to look out for (such as increased impulsiveness and suicidality), and undermining the treatment. Picking up on clues and using open questions to routinely ask about side-effects experienced may be more helpful than planting the expectation.
Placebo and complementary and alternative medicine
Some forms of complementary and alternative medicine (CAM) have real and substantial benefits for patients. Evidence suggests that these are not mediated through the official mechanisms identified by the theory of CAM. For example, although homeopathy is effective in many disorders, it is no more so than placebo, and homeopathic remedies are confirmed by scientific testing to be pharmacologically inert.
Patients seek out CAM because they expect or hope that it will work and they are motivated to get better. Complementary and alternative medicine generally takes place in pleasant environments, and is provided by practitioners who listen to their patients and their concerns, make them feel validated, reassure and promise hope and who generally themselves believe in the effectiveness of their treatments. They may also have more time for their patients than state-salaried clinicians in the National Health Service. All of these factors maximise placebo responses (Reference Rampes, Pilkington, Norman and RyrieRampes 2009).
Reference KaptchukKaptchuk (2002) considers the placebo effect of CAM as the broad array of non-specific effects in the patient–physician relationship, including attention, compassionate care and the modulation of expectations, anxiety and self-awareness.
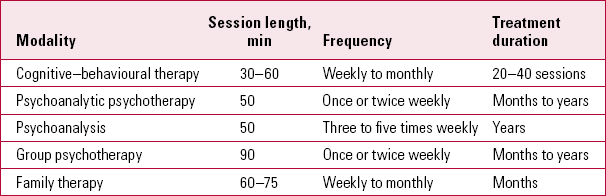
Given the importance of the clinician–patient relationship in maximising placebo responses, it is interesting to consider the time allocated to consulting in different clinical contexts (Table 1) and the duration of psychotherapeutic interventions (Table 2). The amount of contact influences interpersonal processes and the development of attachment relationships. In our experience, a typical first psychiatric assessment appointment lasts for 60–90 min and a typical follow-up 30 min. In specialist services, times may be even longer. In our second article (Reference McQueen, Cohen and St John-SmithMcQueen 2013, this issue) we will consider interpersonal processes and the role of attachment in generating treatment responses.
TABLE 1 Average consultation times
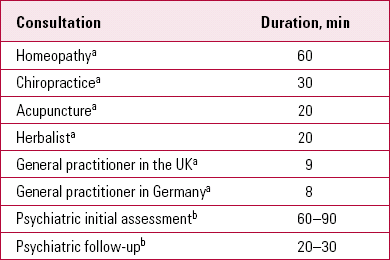
| Consultation | Duration, min |
|---|---|
| HomeopathyFootnote a | 60 |
| ChiropracticeFootnote a | 30 |
| AcupunctureFootnote a | 20 |
| HerbalistFootnote a | 20 |
| General practitioner in the UKFootnote a | 9 |
| General practitioner in GermanyFootnote a | 8 |
| Psychiatric initial assessmentFootnote b | 60–90 |
| Psychiatric follow-upFootnote b | 20–30 |
a. Data from Ernst 2011.
b. Authors' estimate.
TABLE 2 Duration of psychotherapy (typical values)
| Modality | Session length, min |
Frequency | Treatment duration |
|---|---|---|---|
| Cognitive–behavioural therapy | 30–60 | Weekly to monthly | 20–40 sessions |
| Psychoanalytic psychotherapy | 50 | Once or twice weekly | Months to years |
| Psychoanalysis | 50 | Three to five times weekly | Years |
| Group psychotherapy | 90 | Once or twice weekly | Months to years |
| Family therapy | 60–75 | Weekly to monthly | Months |
Ethical issues
Direct prescription of a placebo has traditionally implied necessary concealment of treatment and deception of the patient. This is ethically problematic, and it violates respect for patient autonomy, patient choice and informed consent to treatment. It also has the potential, if and when the true nature of the treatment is exposed, to undermine the clinician–patient relationship by undermining trust. On the other hand, another paradox of the placebo is that, although it may be unethical to use a placebo, it is also unethical to withhold something that is known to heal (Reference NewmanNewman 2008: pp. 134–139). Nonetheless, placebos of some sort are widely used, with half to two-thirds of physician respondents reporting that they regularly prescribe them (Reference Nitzan and LichtenbergNitzan 2004; Reference Tilburt, Emanuel and KaptchukTilburt 2008).
Deception, however, is not necessary for placebos to work. Reference Kaptchuk, Friedlander and KelleyKaptchuk et al (2010) performed an RCT involving individuals diagnosed with irritable bowel syndrome, with one arm of the trial receiving placebo medication, which they were told was inert but had been shown in previous studies to produce significant improvement in symptoms of their disorder. The control group received no medication, although they had the same level of contact with the same supportive clinicians. Both groups improved in their symptoms, but the placebo-treated group had significantly better clinical outcomes. This suggests that prescribing a placebo without deception, along with instructions that foster a positive but realistic expectancy, allows the healing effects of placebo to be harnessed. When asked what they understood about the placebo treatment, the placebo-treated patients had a reasonable understanding of what it consisted of. They reported that what was of particular importance to them was the one-to-one attention from, and chance to discuss their condition with, a clinician knowledgeable about it, and a sense of personal validity gained by being taken seriously. Also worth noting is that the clinical outcomes for this placebo treatment of irritable bowel syndrome were better than the outcome on placebo in placebo-controlled trials of pharmaceutical agents for the disorder. This suggests that presenting the placebo as an active treatment with a plausible explanation for how it works (despite being pharmacologically inert) generated a larger expectancy of benefit than giving either active treatment or placebo treatment in pharmaceutical RCTs, where the expectancy of obtaining benefit may have been lowered by the way that the chance of receiving an inactive placebo treatment was presented.
If open-label placebo medication can be so effective in irritable bowel syndrome, and if placebos have such large effects in depression, one might ask whether it is ethical to withhold such well-researched, evidence-based, efficacious, safe and inexpensive treatments as placebos for these conditions.
In the ritual of pill-taking, the act of a clinician handing over a physical token concretely symbolising care has an additional effect in itself, even when there is transparency about what this token is. It can be argued that the widespread practice of prescribing pharmacological treatments of limited, dubious or no clinical benefit, such as antibiotics for upper respiratory tract infections or diarrhoea, and antidepressants for mild depression, particularly in children, is prescribing drugs for their placebo effect, albeit with the possibility of real adverse effects.
How and why placebo effects occur will be considered in our second article (Reference McQueen, Cohen and St John-SmithMcQueen 2013, this issue).

MCQs
Select the single best option for each question stem
-
1 Natural healing responses have been defined to include:
-
a reassurance from carers
-
b the subjective experience of illness
-
c deliberate technical interventions
-
d the responses of the body to injury or infection
-
e conscious awareness of pathology.
-
-
2 The effects of the following cannot be regarded as true placebo effects:
-
a healing and other cultural rituals
-
b doctors wearing a white coat and stethoscope
-
c technological healing
-
d interpersonal healing
-
e investigative surgery.
-
-
3 Placebo effects usually decrease in studies with:
-
a physical treatments
-
b patient-reported outcomes
-
c small numbers of participants
-
d patients aware that they may be taking placebo
-
e subjective outcomes.
-
-
4 In Western culture, placebo responses decrease with the use of:
-
a red tablets
-
b bigger tablets
-
c branded tablets
-
d higher-dose tablets
-
e generic tablets.
-
-
5 Nocebo effects as described do not include:
-
a side-effects experienced on inactive placebo medication
-
b effects due to rejection by the clinician
-
c unrealised patient expectations
-
d inconsiderate behaviour on the part of the physician
-
e disorganised attachment.
-
MCQ answers
| 1 | d | 2 | c | 3 | d | 4 | e | 5 | e |







eLetters
No eLetters have been published for this article.