Introduction
Frailty was described for the first time more than 30 years ago, without there being a consensus regarding its definition (Rockwood et al. Reference Rockwood, Hogan and MacKnight2000). It is often described as a state of vulnerability related to an inadequate resolution of homeostasis after a stressful event and is the consequence of the cumulative decline of many physiological systems over time (Chin A Paw et al. Reference Chin A Paw, Dekker and Feskens1999; Clegg et al. Reference Clegg, Young and Iliffe2013; Koller and Rockwood Reference Koller and Rockwood2013). It can also be described in a more general way to include cognitive decline and the psychosocial dimension (Espinoza and Walston Reference Espinoza and Walston2005; Ferrucci et al. Reference Ferrucci, Guralnik and Studenski2004; Rockwood et al. Reference Rockwood, Hogan and MacKnight2000).
Palliative care is an approach that aims at improving the quality of life of people and their families when confronted with a life-threatening illness by preventing and relieving suffering through the early identification, correct assessment, and treatment of pain and other issues, whether physical, psychosocial, or spiritual (World Health Organisation n.d.). Palliative care supports people to live fully, providing education around illness and discussing preferences of treatment at the end of life (Kavalieratos et al. Reference Kavalieratos, Corbelli and Zhang2016; World Health Organisation n.d.). Today, there is sufficient evidence to support the introduction of palliative care early in the course of all life-limiting diseases, including for patients suffering from non-oncological diseases (Brumley et al. Reference Brumley, Enguidanos and Jamison2007; Greer et al. Reference Greer, Jackson and Meier2013; Hauptman and Havranek Reference Hauptman and Havranek2005; Parikh et al. Reference Parikh, Kirch and Smith2013; Salins et al. Reference Salins, Ramanjulu and Patra2016; Temel et al. Reference Temel, Greer and El-Jawahri2017, Reference Temel, Greer and Muzikansky2010).
Frailty is not considered as a disease per se, even though it has been shown to be associated with lower quality of life in older people, and this observation persists when corrected for age, gender, and depression (Crocker et al. Reference Crocker, Brown and Clegg2019). Furthermore, this state of vulnerability is responsible for social withdrawal, progressive functional decline, reduced mobility, institutionalization, and falls while also increasing the risk of hospitalizations and death (Fried et al. Reference Fried, Tangen and Walston2001; Koller and Rockwood Reference Koller and Rockwood2013). Despite this observation, it has been shown that people with frailty are less likely to access palliative care than patients with advanced oncological diseases (Hamaker et al. Reference Hamaker, van den Bos and Rostoft2020). A recent systematic review has shown that, on a worldwide scale, 10.7% of adults of age 65 years or more were considered frail (Collard et al. Reference Collard, Boter and Schoevers2012). As the worldwide population is aging, the way in which we provide care, and more specifically palliative care, to this population is of increasing importance. Currently, the needs of older frail patients who are housebound are not well known. It is therefore important to identify those patients and their needs in order to tailor their care and limit the number of futile or harmful intervention.
The aim of our study was to identify the needs of frail, older, housebound patients in terms of palliative care, regarding symptom burden, spiritual well-being, and the burden of care on family members.
The secondary aim was to evaluate the sensitivity and specificity of the surprise question in this population.
Methods
Study design
We conducted a single-center, cross-sectional study among vulnerable and frail, housebound, older people (65 years or older). The study was approved by the Ethics Committee of the Geneva University Hospitals on the 22 May 2018 (Project-ID: 2018–00239).
Setting
The study took place in the community and included patients followed by physicians working in the Geriatric Community Unit (Unité de Gériatrie Communautaire) of the Geneva University Hospitals, Switzerland. This unit provides medical care to housebound older people for a total of approximately 550 patients per year. All geriatricians are trained during their studies to provide general palliative care and end-of-life care, for example, by using the Edmonton Symptom Assessment System (ESAS) for symptom evaluation and by prescribing and adapting opioids for pain and end-of-life care.
The recruitment and data collection period lasted 10 months, from 1 June 2018 to 1 April 2019.
Population
Eligible participants were identified by their physician during a planned medical home visit in which they were screened for frailty using the Edmonton Frail Scale (EFS). If their EFS score was ≥6/17, patients were informed about the study by their general practitioner and, if interested, asked to sign the informed consent form. All consenting participants were then referred to the study team by email. Patients were excluded if they suffered from severe cognitive impairment, preventing them from answering the questionnaires and/or suffered from a rapidly progressive disease. Patients’ ability to participate was at the general practitioner’s discretion. There was no specific cutoff based on the Mini Mental State (MMS) examination as we did not want to exclude participants who, although suffering from cognitive decline, would be able to answer the questionnaires. Patients who were unable to consent to the study and did not have a family member acting as a caregiver who could have given consent for the patient were also excluded.
Outcomes
Basic data such as age, sex, and marital status was collected at the time of the interview. The MMS examination, if performed in the last 6 months, was retrieved from the patient’s electronic medical file (Arevalo-Rodriguez et al. Reference Arevalo-Rodriguez, Smailagic and Roqué-Figuls2021).
Although there are many frailty measurement tools, most of these are impractical and inapplicable to primary care screening of people in the community as they require multidimensional clinical data, time, and training (Faller et al. Reference Faller, Pereira and de Souza2019; Welsh et al. Reference Welsh, Gordon and Gladman2014). In our study, we chose to use the EFS, a valid and reliable tool, feasible for routine use by non-geriatricians (Rolfson et al. Reference Rolfson, Majumdar and Tsuyuki2006). As the tool had not yet been validated in French, the EFS was translated using a forward-backward methodology prior to the beginning of the study. Two bilingual translators, native English speakers with French as their second language, independently translated the original tool from English to French. A unified version was produced after agreement of the 2 translators. Five other translators, French-speaking clinicians who spoke English as their second language, translated the unified version from French to English. Finally, the research team reviewed the different versions and modified when appropriate. All disagreements were resolved by discussion. The final French version was then appraised for face validity by the 5 French-speaking translators and remodified if necessary.
The ESAS was completed during the home visit. The ESAS is a 9-item self-reported symptom intensity tool designed for palliative care patients (Bruera et al. Reference Bruera, Kuehn and Miller1991). A tenth patient-specified symptom can be added to the evaluation. Each symptom is rated by the patient on a scale from 0 to 10, 0 being none or best possible and 10 worst possible. This tool has been previously translated and validated in French and used for symptom assessment in patients with cognitive impairment (Murray et al. Reference Murray, Sachs and Stocking2012; Pautex et al. Reference Pautex, Vayne-Bossert and Bernard2017).
The Functional Assessment of Chronic Illness Therapy – Spiritual Well-Being scale (FACIT-sp) was also completed during the home visit. The FACIT-sp is a validated 12-item questionnaire that measures spiritual well-being in patients with cancer and other chronic illnesses (Bredle et al. Reference Bredle, Salsman and Debb2011; Monod et al. Reference Monod, Lécureux and Rochat2015). It is part of the larger FACIT questionnaire that assesses multidimensional health-related quality of life that was used in our study and has been translated and validated in many languages including French (Agli et al. Reference Agli, Bailly and Ferrand2017; Bredle et al. Reference Bredle, Salsman and Debb2011).
The surprise question – “Would I be surprised if this patient died in the next 12 months?” – was answered by the research team member having conducted the interview. The surprise question was developed more than 10 years ago and has been used in different settings to identify patients at high risk of death who might benefit from palliative care (Downar et al. Reference Downar, Goldman and Pinto2017; Lai et al. Reference Lai, Cheng and Chang2020; Moss et al. Reference Moss, Ganjoo and Sharma2008).
Finally, caregiver burden was evaluated in caregivers, when present, using the Mini-Zarit. This tool is the validated short version of the Zarit Burden Interview and has been used to identify caregiver burden and main caregiver collapse in routine medical care and has been translated into French (Dauphinot et al. Reference Dauphinot, Ravier and Novais2016; Gort et al. Reference Gort, Mingot and Gomez2007). This scale was completed with caregivers either during the patient’s home visit or later on, by telephone, if not present during the home visit.
Mortality data was retrieved from patients’ electronic data files a year after inclusion in the study.
A research nurse called eligible participants to agree on a meeting that took place at the patients’ homes. The home-based interview was conducted by an experienced clinical research nurse or by a physician with several years of palliative care training not involved in daily clinical work and lasted approximately 1 hour. Both clinical research nurses involved in the study had a Certificate of Advanced Studies in palliative care.
Analysis
Patients’ characteristics were analyzed using descriptive statistics. ESAS scores and FACIT domains were compared between vulnerable (EFS 6–7) and frail (EFS ≥ 8/17) patients using the Mann–Whitney U test. Mini-Zarit scores were compared between the same 2 groups using Fisher’s exact test or the Mann–Whitney U test as appropriate.
Kaplan–Meier’s method was employed to estimate survival since the evaluation visit. Based on preexisting literature, the following factors associated with death were used in a univariate Cox regression: the surprise question, gender, age, and frailty score. Differences with a p-value <0.05 were considered statistically significant. All analyses were conducted by an independent statistician using STATA IC 16.
Results
The recruitment period lasted 10 months during which time all patients had benefited from at least one home visit from their physician. A total of 581 patients were screened for eligibility by their general practitioner. Only 232 patients were deemed eligible by their physicians, who mainly excluded patients because of cognitive impairment. Participants were considered unable to reach if they did not respond after 3 phone calls on 3 different days, at 3 different times. A total of 42 patients declined to participate in the study, although they had initially agreed to. One patient was included in the study as he signed the informed consent sheet and completed the EFS but was excluded from further analysis as he did not wish to complete the ESAS or the FACIT-Sp (Figure 1). There were no statistically significant differences regarding age (81.1 ± 7.9 vs. 81.5 ± 8.6, p = 0.741) and female sex (56.9% vs. 61.2%, p = 0.536) between participants included and excluded from the study.

Fig. 1. Study flowchart.
The description of patients included in the study is summarized in Table 1. Most participants were female (56.9%), and mean age (SD) was 81.1 (±7.9). Patients were mostly widowed (41.7%) or married (25%). Mean Karnofsky score (SD) at inclusion was 53.1 (±8.0), which meant most participants required considerable assistance and frequent medical care. MMS score at entrance was only present for 16 patients with a mean of 23.7 (±3.6) on 30. Forty-three participants (59.7%) declared having a caregiver. EFS at entrance identified patients as being mostly vulnerable (37%) and mildly frail (31.5%). Overall, the most prevalent symptoms on the Edmonton Symptom Assessment Scale (score ± SD) were as follows: other symptoms (5.63 ± 2.63), the most often quoted being dry mouth, followed by impaired feeling of well-being (3.22 ± 2.44) and tiredness (3.19 ± 3.11). Pain was also an important complaint (2.82 ± 2.95), as was depression (2.12 ± 2.88) (Figure 2). The ESAS scores were higher in frail patients (mild, moderate, and severe) as opposed to vulnerable patients for tiredness (mean ± SD: 3.88 ± 3.16 vs. 2.07 ± 2.73, p = 0.016), feeling of well-being (3.73 ± 2.21 vs. 2.39 ± 2.60 p = 0.0132), drowsiness (2.45 ± 2.37 vs. 1.15 ± 1.41, p = 0.0196), and loss of appetite (2.20 ± 2.82 vs. 0.70 ± 1.64, p = 0.0124) (Table 2). There was no difference in the FACIT-Sp total score or in the subscales of the FACIT-G between the vulnerable and frail groups. Of the 32 caregivers who participated in the study, most were spouse (45%) or daughter (27.5%) of the participant, with a mean age (SD) of 70.6 (±13.6). The study team was unable to reach 3 caregivers. The overall burden (SD) on the caregiver measured by the Mini-Zarit was low (2.12 ± 1.49). Furthermore, there was no difference between the caregiver burden of mild, moderate, and severely frail patients as opposed to vulnerable patients (Table 3).
Table 1. Description of patients included in the study (n = 72)
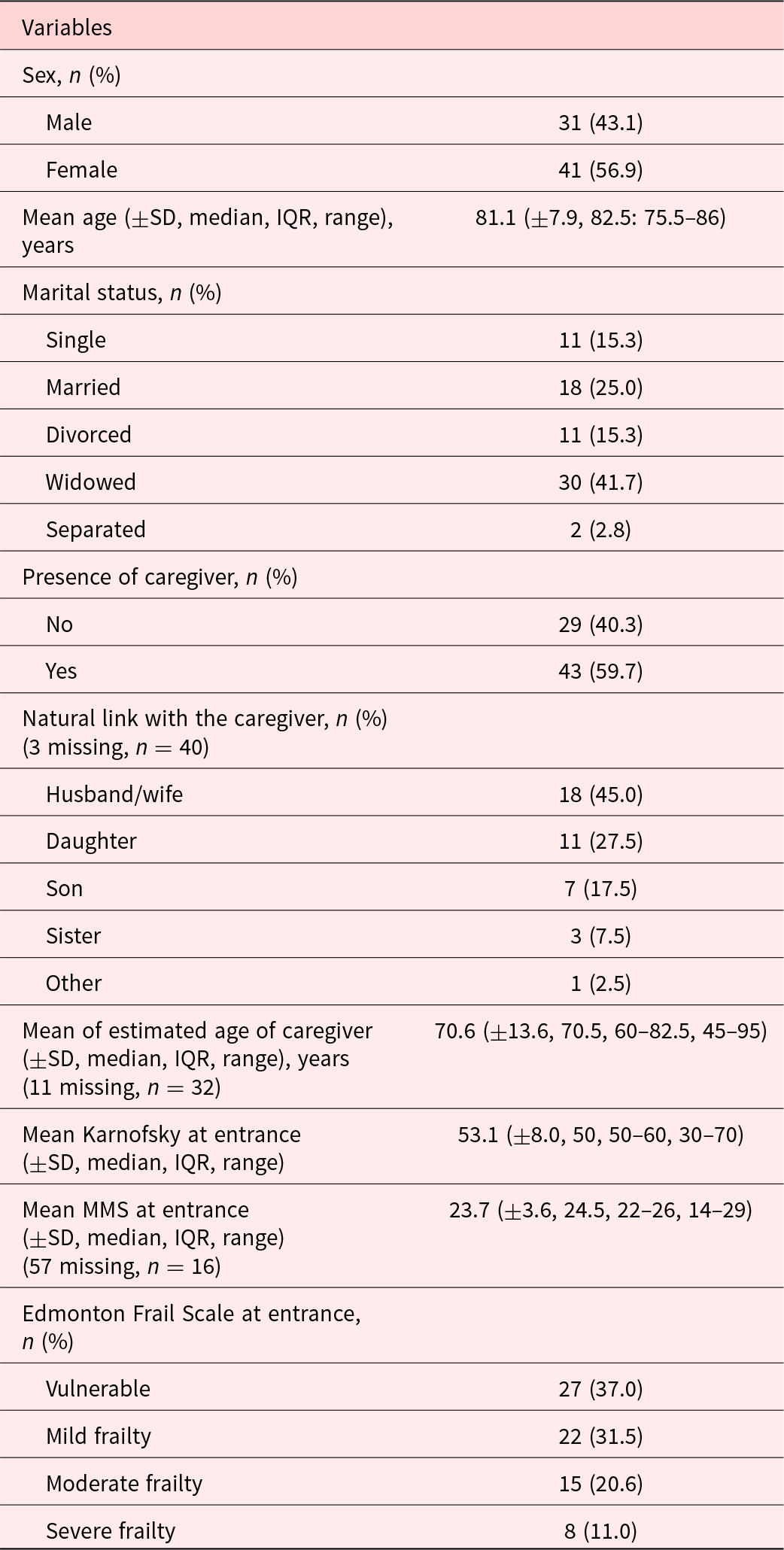

Fig. 2. Overall Edmonton Symptom Assessment Scale: mean (95% CI) by symptom.
Table 2. Description of ESAS by symptom, overall and in the 2 groups of frailty patients
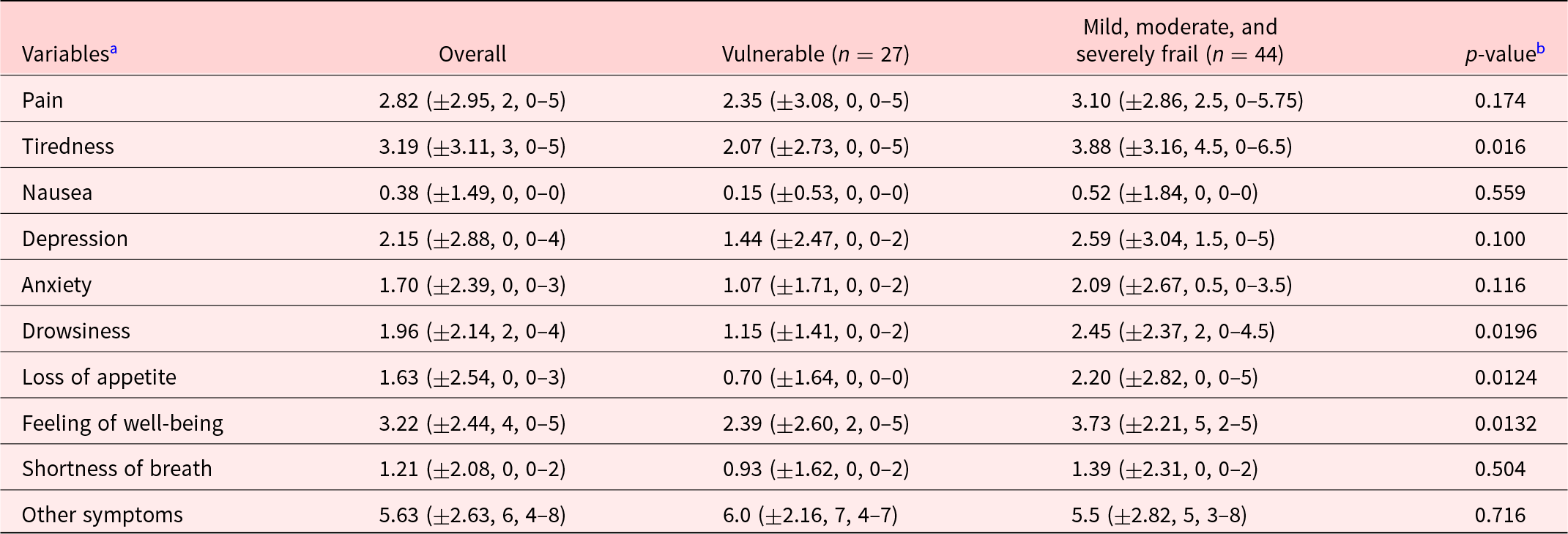
a Variables are described by their mean ± standard deviation, median, interquartile range.
b Mann–Whitney nonparametric test.
Table 3. Description of Mini-Zarit by question, overall, and in the 2 groups of frailty patients
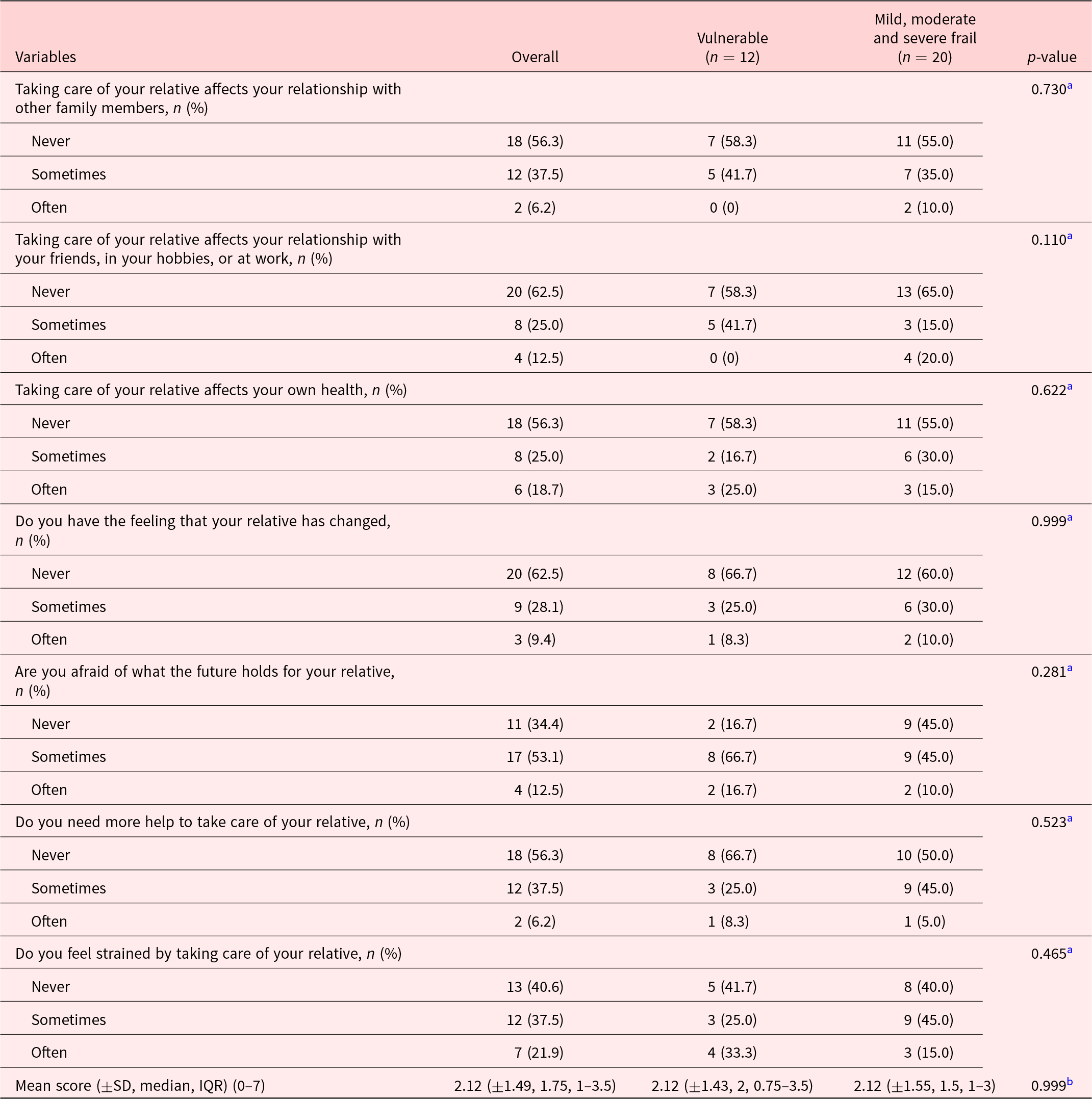
a Fisher’s exact test.
b Mann–Whitney test.
Out of 73, 10 patients died within the first year. The Kaplan–Meier survival estimate within 1 year of study entry showed a higher death rate among the severely frail as opposed to patients considered vulnerable (25% vs. 11.1%). However, the small number of patients in each group renders any further analysis hazardous.
To identify potential variables associated with death within 1 year, we conducted an exploratory univariate Cox regression analysis looking at the surprise question, sex, age, and frailty scores. However, the proportionality of hazards was not verified for the surprise question, the EFS, or age. Female gender had a hazard ratio (95% CI) of 3.21 (0.68–15.10), p = 0.141.
The surprise question’s sensitivity was low in our study as clinicians answered “No I would not be surprised” in only 50% (95% CI:18.7–81.3) of patients who died within the first year after study inclusion. Specificity was also very low with 33.9% (95% CI: 22.3–47.0%). However, the negative predictive value of the surprise question was good. When the answer to the surprise question was “yes, I would be surprised if the patient died in the next year” in 80.9% (60.6–93.4%) of the cases, the patient survived beyond a year.
Discussion
Although interventions have shown to improve levels of frailty such as physical activity, nutrition, and memory training, most of them are not accessible to elderly housebound patients (Puts et al. Reference Puts, Toubasi and Andrew2017). Therefore, finding ways to work around frailty to provide the best possible care for these patients is crucial.
Currently, there are few studies highlighting the symptoms of housebound, frail patients. Nevertheless, the identification and management of symptoms is important as higher ESAS symptom burden has been associated with an increase in emergency department visits in the following 7 days and shorter survival (Barbera et al. Reference Barbera, Atzema and Sutradhar2013; Mercadante et al. Reference Mercadante, Valle and Porzio2013; Zeng et al. Reference Zeng, Zhang and Culleton2011).
In this study, we were able to show that this population suffers from a high symptom burden, even though patients were followed by geriatricians with good knowledge of symptom assessment and management. Furthermore, symptom burden increased with the level of frailty. Indeed, frail, older, housebound patients suffer more from tiredness, drowsiness, impaired well-being, and loss of appetite than non-frail patients. These symptoms are similar to those identified in a previous study on the symptom burden of chronically ill housebound patients, with the most commonly reported symptoms being loss of appetite, impaired well-being, tiredness, and pain (Wajnberg et al. Reference Wajnberg, Ornstein and Zhang2013). This further highlights the similarity between chronically ill and frail patients. Cancer patients have been shown to have similar symptoms in a large retrospective outpatient cohort study, with the highest rating symptoms being tiredness, lack of appetite, and impaired well-being (Bubis et al. Reference Bubis, Davis and Canaj2020).
The symptoms reported by frail housebound patients are mostly unspecific. They differ, however, from other groups of patients, for example, patients with dementia who complained mainly of pain, depression, cognitive deficit, and anxiety (Murray et al. Reference Murray, Sachs and Stocking2012). Although the symptoms highlighted in this study are challenging to treat, they should be taken into account when providing care and designing interventions for frail older people in the community.
Spiritual well-being has been shown to significantly correlate with higher levels of emotional, functional, and physical well-being and better quality of life (Rego et al. Reference Rego, Gonçalves and Moutinho2020). It has also been associated with less decisional conflict, decreased uncertainty, and a feeling of being more supported and informed, which makes it an important aspect of general palliative evaluation (Rego et al. Reference Rego, Gonçalves and Moutinho2020). In our study, we found no difference in the spiritual well-being of vulnerable versus frail patients. However, although our sample size was small, overall mean (SD) score for the spiritual scale subgroup (FACIT-Sp) was considerably lower than the scores found in other populations at 23.58 (±7.30) on a scale of 0–48 (Monod et al. Reference Monod, Lécureux and Rochat2015; Munoz et al. Reference Munoz, Salsman and Stein2015). Indeed, in a study conducted among cancer survivors, the FACIT-Sp score (SD) was 37.35 (±8.65) (Munoz et al. Reference Munoz, Salsman and Stein2015). In another study, conducted in palliative care patients, the FACIT-Sp score (SD) was 31.9 (±8.6) (O’Callaghan et al. Reference O’Callaghan, Georgousopoulou and Seah2020). Whether frailty is a factor that negatively impacts spiritual well-being is still to be determined. Systematically assessing and considering patients’ spiritual needs, existential and/or religious, and offering support when needed via chaplains may be a way to improve general well-being in frail, older, housebound patients (Yoon et al. Reference Yoon, Suh and Kim2018).
As in many studies, the main carers were spouses and daughters (Maeda et al. Reference Maeda, Miyashita and Yamagishi2016; Vigna et al. Reference Vigna, de Castro and Fumis2020). In our study, carers did not seem to be burdened by the care they provided. This is possibly biased as nearly all patients included in this study benefited from homecare services. Implementing new interventions for frail housebound patients should consider involving carers, keeping in mind that most of them might have other family duties to work around (Ullrich et al. Reference Ullrich, Eicken and Coym2021).
Developing an adapted palliative intervention based on our results still presents challenges. Assignment criteria remained unclear, and optimal timing is still to be determined. Indeed, defining the appropriate timing for the introduction of palliative care for older patients with non-oncological diseases is challenging, mainly due to the unpredictable trajectory of non-malignant diseases and the lack of simple validated prognostic models (Coventry et al. Reference Coventry, Grande and Richards2005). Indeed, the surprise question was of poor prognostic value in our population. This has also been shown in a recent meta-analysis where the surprise question performed poorly as a predictive tool for death, particularly in non-cancer illnesses (Downar et al. Reference Downar, Goldman and Pinto2017). A model for a short-term integrated palliative and supportive care for frail older people in the community is currently being developed, but its effectiveness remains to be assessed (Bone et al. Reference Bone, Morgan and Maddocks2016).
Although conducted in the context of a single high-income country, the strength of this study lies in the fact that it describes a genuine population that does not have access to the usual health-care network. This study further contributes to the evidence on the subject by highlighting the palliative care and spiritual needs of this population and will hopefully help to develop tailored interventions to improve the quality of life of frail, older, housebound patients.
Conclusion
Frailty is not simply absent or present; it varies with time and is dependent on the evolution of cognitive, somatic, functional, and psychosocial domains. Older, fragile, housebound patients have specific needs that increase with the level of frailty and should guide future palliative care provision. Further studies are needed in order to assess how best to provide palliative care for frail housebound patients and whether this is effective in improving general quality of life and well-being.
Acknowledgments
The authors would like to thank Tagyane Lima Menezes for her help with extracting mortality data from patient’s files. The authors would also like to thank all the patients and caregivers who accepted to participate in the study as well as their physicians for helping us identify eligible participants.
Conflicts of interest
None declared.








