Osteoporosis is a skeletal disorder of reduced bone mass and disruption of bone architecture with a consequent increase in bone fragility and fracture risk(Reference Lock, Lecouturier and Mason1). The decline of gonadal function following menopause and ageing plays a key role in the pathogenesis of osteoporosis. With a worldwide increase in ageing populations, osteoporosis has become a more serious global health problem. Hormone replacement therapy was a routine method to alleviate physical symptoms and to prevent the clinical consequence of postmenopausal osteoporosis(Reference Notelovitz2). However, recent studies suggest that hormone replacement therapy is associated with an increased risk of developing breast, ovarian and endometrial cancers(Reference Davison and Davis3–Reference Rodriguez, Calle and Coates6). This has led to increased interest in identifying natural oestrogen analogues that possess osteoprotective effects without any side effects on reproductive tissues. A class of plant-derived substances, the so-called ‘phyto-oestrogens’, has been reported to possess structures similar to mammalian oestrogens and display both oestrogenic and anti-estrogenic effects(Reference Albertazzi and Purdie7–Reference Brzezinski and Debi8). Isoflavones, lignans and cumestans are the major classes of phyto-oestrogen(Reference Albertazzi and Purdie7). Numerous studies on isoflavones in preventing the loss of bone mineral density (BMD) have been reported(Reference Fanti, Monier-Faugere and Geng9, Reference Scheiber, Liu and Subbiah10), while those that focus on the bone-protective effects of lignans and cumestans are few.
Sambucus williamsii HANCE (SWH), one species of Sambucus distributed in various regions of China, Korea and Japan, has been used as a folk medicine to treat bone and joint diseases in China for thousands of years(Reference Song and Hong11). Our previous study showed that the ethanol extract of SWH had protective effects on trabecular bone and cortical bone(Reference Xie, Wu and Zhang12) and the mechanisms included stimulation of osteoblast differentiation, inhibition of osteoclastogenesis as well as osteoclast actions(Reference Zhang, Li and Wan13). Moreover, lignans(Reference Yang, Wong and Wang14), phenolic acids(Reference Yang, Wong and Wang15), triterpenoids(Reference Yang, Wang and Wong16) and some other compounds had been isolated from the ethanol extract of SWH. However, it is unclear which of the ingredients in SWH are responsible for its bone-protective actions.
In the present study, both in vivo and in vitro models were employed to identify the active fractions and ingredients in SWH that account for its bone-protective actions. The bioactivities of fractions prepared from the ethanol extract of SWH using a macroporous adsorption resin column were determined. The HPLC fingerprint of the bioactive fraction was established and the major components were characterised.
Materials and methods
Plant materials and isolation
The stems and branches of SWH were collected in Shenyang, Liaoning Province in the northeast of China in April 2007 and authenticated according to a method listed in the Chinese Bencao(Reference Song17) with the help of Professor Zerong Jiang (Shenyang Pharmaceutical University in Shenyang, China). The identity of SWH was confirmed by analysing its major ingredients pentacylic triterpenoid, phenol acid and derivatives and protein. A voucher specimen (HHXSWGZ-2007) was deposited in the Herbarium of the Institute of Traditional Chinese Medicine and Natural Products (Jinan University, Guangzhou, China).
Dry stems and branches of SWH (50 kg) were refluxed with 500 litres of 60 % (v/v) ethanol three times, for 2 h each time. After filtration, the filtrate was concentrated under reduced pressure and then freeze-dried to obtain a total extract of 1·25 kg powder. As shown in Table 1, SWH (1250 g) was applied to a D-101 macroporous adsorptive resin column (diameter 8·0 cm × height 96 cm), eluted with water and ethanol in gradient, to give SWA (water eluate; 712·5 g), SWB (30 % ethanol eluate; 160·0 g) and SWC (50 and 95 % ethanol eluates; 236·0 g). The 50 and 95 % ethanol eluates were combined to give SWC after HPLC analysis because both fractions contain similar constituents and because the yield of the 95 % ethanol eluate was too low. The HPLC fingerprint of SWC was established using the Agilent series 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA): a reversed-phase (RP)-HPLC column (Welch XB-C18, 4·60 × 250 mm; Welch Material, Shanghai, China) with a gradient of methanol–water (0·1 % acetic acid) as the mobile phase (0–9 min, 25 %; 9–39 min, 36 %; 39–54 min, 50 %; 54–59 min, 100 %), at a flow rate of 0·8 ml/min. Detection wavelength was at 280 nm and column temperature was at 35°C. The major peaks of the fingerprint were characterised with the compounds isolated from SWC using the same HPLC conditions.
Table 1 The fractions of Sambucus williamsii HANCE* and their yield
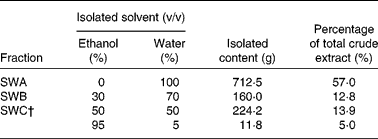
* The ethanol extract of Sambucus williamsii HANCE (1250 g) was applied to a D-101 macroporous adsorptive resin column (diameter 8·0 cm × height 96 cm), and eluted with water and ethanol in gradient to give its fractions.
† The 50 % ethanol eluate and 95 % ethanol eluate were combined together to give SWC.
Animal study
A total of fifty-six virgin C57BL/6J specific-pathogen-free (SPF) female mice, aged 12 weeks, were purchased from Laboratory Animal Services Centre (Chinese University of Hong Kong, Hong Kong, China) and housed in cages under a 12 h light–12 h dark cycle at 20 ± 1°C. During the study, all animals were allowed free access to distilled deionised water, and OVX mice were pair-fed a phyto-oestrogen-free diet (D00031602; Research Diets, Inc., New Brunswick, NJ, USA) (Table 2) based on the average daily food consumption of the sham control group.
Table 2 Formulations and estimated nutrient composition of experimental diets
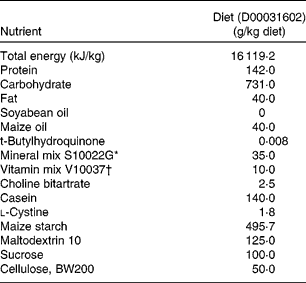
* The mineral mix composition was as follows (amount in 35 g): 5·0 g Ca, 2·0 g P, 0·5 g Mg, 3·6 g K, 0·3 g S, 1·0 g Na, 1·6 g Cl, 6·0 mg Cu, 0·2 mg I, 45 mg Fe, 10·5 mg Mn, 0·2 mg Se and 30·0 mg Zn.
† The vitamin mixture composition was as follows (amount in 10 g): 2·2 mg vitamin A (all-trans-retinyl palmitate), 25 μg cholecalciferol, 33·75 mg vitamin E (all-rac-α-tocopheryl acetate), 0·75 mg phylloquinone, 0·2 mg biotin, 25 μg cyanocobalamin, 2 mg folic acid, 30 mg nicotinic acid, 16 mg calcium pantothenate, 7 mg pyridoxine-HCl, 6 mg riboflavin, 6 mg thiamin HCl.
The mice were either sham-operated (Sham, n 8) or ovariectomised (OVX, n 48). The treatment dose of each fraction was deduced from the yield. The OVX mice were randomly divided into six groups: vehicle-treated group (OVX, n 8); 17β-oestradiol administration group (E2; n 8; 3·2 μg/g body weight per d); SWH (ethanol extract group; n 8; 1·0 mg/g body weight per d); SWA (water eluate group; n 8; 0·570 mg/g body weight per d), SWB (30 % ethanol eluate group; n 8; 0·128 mg/g body weight per d); SWC (50 and 95 % ethanol eluates group; n 8; 0·189 mg/g body weight per d). The solutions of SWH and its fractions were freshly prepared as SWH (100 mg/ml), SWA (57 mg/ml), SWB (12·8 mg/ml) and SWC (18·9 mg/ml), and were given daily to mice. A phyto-oestrogen-free diet was applied in the present study to eliminate interactions between diet and fractions of SWH that had been reported in our previous study(Reference Zhang, Li and Wan13). Drug treatment started 2 weeks after the surgery and was administrated orally through a gastric tube for 3 months. At the end of the treatment, the mice were killed, blood was withdrawn from the abdominal aorta and serum was prepared. From each animal, the uterus was collected, weighed and then stored at − 80°C. The left femur and tibia were dissected and cleaned of all soft tissue, then wrapped in gauze saturated with PBS buffer, sealed in Eppendorf tubes and stored at − 20°C for further analysis. The animal study protocols were approved by the Animal Ethics Committee of The Hong Kong Polytechnic University.
Micro-computed tomography analysis of bone properties
The distal metaphysis of femur and the proximal metaphysis of tibia were scanned with a high-resolution micro vivaCT 40 system (Scanco Medical, Bassersdorf, Switzerland). The distal and proximal site was defined as 2·0 mm away from the femur end and tibia head. After images were captured, twenty out of 100 slices were chosen and established as the volume of interest. Trabecular bone was separated from cortical bone by free drawing regions using the software provided with the scanner. Then three-dimensional (3D) modelling was generated and morphological date of the sample was evaluated using a script-based 3D volume analysis tool IPL (Image Processing Language; Scanco Medical; http://www.scanco.ch/systems-solutions/software.html). 3D parameters for trabecular bone were obtained as follows: (1) bone volume/tissue volume (BV/TV); (2) trabecular bone number (Tb.N); (3) trabecular bone separation (Tb.Sp); (4) BMD.
Three-point bending test
The left tibias were cleaned to remove their surrounding soft tissue before the test. A three-point bending machine (model H10KM; Hounsfield Test Equipment Limited, Redhill, Surrey, UK) was used to determine the mechanical strength on the mid-shaft of the left tibia(Reference Pang, Wang and Mok18). The two supporting points were fixed 4 mm apart with a single central loading point. A load was applied on the mid-shaft with a deformation rate of 2·0 mm/min and a load–deformation curve was plotted simultaneously until the specimen was broken. Bending stiffness was calculated as the slope of the linear part of the loading–deformation curve.
Cell culture
UMR 106 cells were cultured in Dulbecco's modified Eagle's medium with 10 % fetal bovine serum, which was pre-supplemented with penicillin (100 units/ml) and streptomycin (100 mg/ml). At 80–90 % confluence, the cells were seeded in a ninety-six-well microtitre plate with 4000 cells/well. After incubation for 48 h, the culture medium was changed to phenol red-free Dulbecco's modified Eagle's medium with 1 % charcoal-stripped fetal bovine serum for 24 h. The cells were then treated with SWH fractions at 0·1, 1·0, 10 or 100 μg/ml for 24 or 48 h. The cells were cultured at 37°C in a humidified atmosphere of 95 % air and 5 % CO2.
Cell proliferation assay
The proliferation effects of the fractions of SWH on the UMR 106 cell line were determined by the MTS colorimetric assay. After treating with SWH fractions (0·1 to 100 μg/ml) or vehicle for 24 h or 48 h, the medium was removed, followed by the addition of 0·2 mg/ml 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega Corp., Madison, WI, USA) and 1 mg/ml phenazine methosulfate (PMS; Sigma-Aldrich, St Louis, MO, USA) reagent. The absorbance was detected on a microplate spectrophotometer (model 680; Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm after 2 h of incubation at 37°C.
Alkaline phosphatase activity assay
Alkaline phosphatase activity of the treated cells was determined by the hydrolysis of p-nitrophenylphosphate (Promega Corp.) to p-nitrophenol to assess the differentiation activity of UMR 106 cells. The treated cells were rinsed with PBS twice before 100 μl p-nitrophenylphosphate were added to them. Absorbance of the tested samples was measured at 405 nm. All results are expressed as a ratio normalised to the corresponding total protein content (optical density at 405 nm/μg BSA).
Statistical analysis
The data from these experiments are reported as mean values with their standard errors for each group. All statistical analyses were performed using PRISM version 5.0 (GraphPad, San Diego, CA, USA). Inter-group differences were analysed by one-way ANOVA, and followed by Tukey's multiple-comparison test as a post hoc test to compare the group mean if overall P < 0·05. Differences of P < 0·05 were considered statistically significant.
Results
Body weight, uterine weight and serum biochemical makers
The effects of SWH fractions on body weight, uterus index and serum biochemical makers are summarised in Supplementary Appendix 1 (available online at http://www.journals.cambridge.org/bjn). As expected, body weight increased significantly (P < 0·05) and uterus index decreased significantly (P < 0·05) in OVX mice as a result of oestrogen deficiency. Treatment of OVX mice with SWH or SWC extracts significantly suppressed oestrogen deficiency-induced body-weight gain (v. OVX group, both P < 0·001). Uterus indexes were significantly increased in OVX mice in response to treatment with E2 or SWH. However, uterus indexes of the mice treated with the fractions of SWH did not alter as compared with those treated with vehicle. Serum Ca level and P levels were not altered by OVX or fractions of SWH.
Micro-computed tomography analysis of left femur and tibia
As shown in Table 3, ovariectomy significantly reduced BMD at the distal metaphysis of femur (P < 0·05 v. sham) and the proximal metaphysis of tibia (P < 0·05 v. sham) in mice. Treatment of OVX mice with E2 prevented the decrease in BMD at both sites (P < 0·001 v. OVX). Similarly, treatment of OVX mice with SWH or SWC significantly restored BMD at the femur and tibia. Trabecular bone microstructure, as indicated by parameters such as BV/TV, Tb.N and Tb.Sp at the distal metaphysis of femur and at the proximal metaphysis of tibia deteriorated significantly in mice upon ovariectomy (Table 3; P < 0·05 in all parameters at both sites). The damage of bone microstructure induced by ovariectomy in mice at the femur were reversed significantly by treatment with SWH (P < 0·01 in BV/TV), SWA (P < 0·05 in BV/TV) or SWC (P < 0·01 in BV/TV, P < 0·01 in Tb.N and P < 0·05 in Tb.Sp) and at the tibia by treatment with SWH (P < 0·001 in BV/TV, P < 0·05 in Tb.N and P < 0·01 in Tb.Sp) or SWC (P < 0·001 in BV/TV, P < 0·05 in Tb.N and P < 0·01 in Tb.Sp). Among all the fractions of SWH, the bone-protective effect of SWC was the same as or even higher than that of the total ethanol extract SWH. There were no differences of the trabecular bone parameters between Sham and the fraction-treated groups. The administration of SWH fractions restored the bone loss induced by ovariectomy to the normal level and maintained the microarchitecture of trabecular bone.
Table 3 Effects of Sambucus williamsii HANCE fractions on bone parameters as measured by micro-computed tomography at the distal metaphysis of femur and proximal metaphysis of tibia in ovariectomised mice
(Mean values with their standard errors)

BMD, bone mineral density; BV/TV, bone volume/tissue volume; Tb.N, trabecular bone number; Tb.Sp, trabecular bone separation; Sham, sham-operated, vehicle-treated; OVX, ovariectomised, vehicle-treated; E2, 17β-oestradiol (3·2 μg/g body weight per d); SWH, Sambucus williamsii HANCE ethanol extract (1·0 mg/g body weight per d); SWA, SWH water eluate (0·570 mg/g body weight per d), SWB, SWH 30 % ethanol eluate (0·128 mg/g body weight per d); SWC, SWH 50 and 95 % ethanol eluates (0·189 mg/g body weight per d).
Mean value was significantly different from that of the OVX group: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean value was significantly different from that of the Sham group: †P < 0·05, ††P < 0·01.
‡ Mice were subjected to the treatments for 12 weeks.
Biomechanical measurement of left tibia
Table 4 shows the effects of SWH fractions on the biomechanical properties of the left tibia in OVX mice. Three-point bending experiments indicated that treatment of OVX mice with E2 significantly increased the ultimate load (P < 0·001), breaking force (P < 0·05) as well as stiffness (P < 0·05) of the tibia mid-shaft. Ultimate load of the left tibia mid-shaft were significantly increased in OVX mice in response to treatment with different SWH fractions, namely SWA (P < 0·05), SWB (P < 0·05) and SWC (P < 0·01). Breaking forces of the left tibia mid-shaft were increased significantly in OVX mice in response to treatment with SWC (P < 0·01) while treatment with SWH did not show such effects on the breaking force. Stiffness of the tibia was only increased in OVX mice in response to treatment with SWA (P < 0·05). Treatment of OVX mice with SWH fractions suppressed the OVX-induced reduction of ultimate load and stiffness in the tibia, resulting in an increase back to the Sham level. However, E2 and all fractions of SWH had no significant effects on changes of energy of breaking.
Table 4 Effects of Sambucus williamsii HANCE fractions on biomechanical properties of the left tibia in ovariectomised mice
(Mean values with their standard errors)
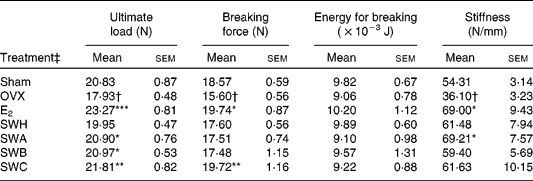
Sham, sham-operated, vehicle-treated; OVX, ovariectomised, vehicle-treated; E2, 17β-oestradiol (3·2 μg/g body weight per d); SWH, Sambucus williamsii HANCE ethanol extract (1·0 mg/g body weight per d); SWA, SWH water eluate (0·570 mg/g body weight per d), SWB, SWH 30 % ethanol eluate (0·128 mg/g body weight per d); SWC, SWH 50 and 95 % ethanol eluates (0·189 mg/g body weight per d).
Mean value was significantly different from that of the OVX group: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean value was significantly different from that of the Sham group: †P < 0·05.
‡ Mice were subjected to the treatment for 12 weeks.
Rat osteoblast-like UMR 106 cell proliferation and differentiation assay
Fig. 1(a) shows that treatment of rat osteoblast-like UMR 106 cells with SWH at 1 μg/ml for 24 and 48 h significantly increased cell proliferation (P ≤ 0·001). The stimulatory effects of SWA, SWB and SWC (Fig. 1(b), (c), (d)) on UMR 106 cell proliferation were more potent than the effects of SWH (Fig. 1(a)). Treatment of UMR 106 cells with 0·1 to 100 μg/ml of SWA (Fig. 1(b)) or SWC (Fig. 1(d)) for 24 or 48 h significantly increased cell proliferation at all concentrations. SWB at 10 or 100 μg/ml (Fig. 1(c)) significantly increased UMR 106 cell proliferation. To determine the effects of different SWH extracts on osteoblastic cell differentiation, alkaline phosphatase activities in UMR 106 cells in response to different concentrations of SWH, SWA, SWB and SWC were measured. As observed with E2, SWH (Fig. 2(a)) and SWB (Fig. 2(c)) significantly promoted cell differentiation activity at 0·1 μg/ml (both P < 0·05), while SWA (Fig. 2(b)) had no effects on cell differentiation. SWC (Fig. 2(d)) significantly increased cell alkaline phosphatase activity at all concentrations (0·1 to 100 μg/ml), especially at 1 μg/ml (122·8 %), which was higher than the increased level upon treatment with E2 (112·4 %).

Fig. 1 Effects of (a) Sambucus williamsii HANCE (SWH), (b) Sambucus williamsii HANCE water eluate (SWA), (c) Sambucus williamsii HANCE 30 % ethanol eluate (SWB) and (d) Sambucus williamsii HANCE 50 and 95 % ethanol eluates (SWC) on cell proliferation in rat osteoblast-like UMR 106 cells. (■), 24 h; (![]() ), 48 h; OD, optical density. Results were obtained from three independent experiments. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the control (24 h): **P < 0·01, ***P < 0·001. Mean value was significantly different from that of the control (48 h): †† P < 0·01, ††† P < 0·001.
), 48 h; OD, optical density. Results were obtained from three independent experiments. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the control (24 h): **P < 0·01, ***P < 0·001. Mean value was significantly different from that of the control (48 h): †† P < 0·01, ††† P < 0·001.

Fig. 2 Effects of (a) Sambucus williamsii HANCE (SWH), (b) Sambucus williamsii HANCE water eluate (SWA), (c) Sambucus williamsii HANCE 30 % ethanol eluate (SWB) and (d) Sambucus williamsii HANCE 50 and 95 % ethanol eluates (SWC) on cell differentiation activity in rat osteoblast-like UMR 106 cells. OD, optical density; BSA, bovine serum albumin. Results were obtained from three independent experiments. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the control: *P < 0·05, **P < 0·01, ***P < 0·001.
Identification of components in Sambucus williamsii HANCE 50 and 95 % ethanol eluate
The peaks of the HPLC fingerprint of SWC (Supplementary Appendix 2; available online at http://www.journals.cambridge.org/bjn) characterised with the compounds isolated from SWC were as follows: peak 1 (16·8 min, vanillic acid); peak 2 (22·7 min, coniferyl alcohol); peak 3 (28·3 min, (+)-erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropanyl)-2-methoxy-phenoxy]-1, 3-propanediol); peak 4 (29·3 min, (+)-thero-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropanyl)-2-hydro-phenoxy]-1,3-propanediol); peak 5 (29·9 min, (+)-thero-guaiacylglycerol-β-O-4′-conifery ether); peak 6 (31·0 min, (+)-erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[-4-(3-hydroxypropanyl)-2-methoxy-phenoxy]-1,3-propanediol); peak 7 (40·4 min, (+)-lariciresinol); peak 8 (42·1 min, dehydrodiconiferylalcohol); peak 9 (42·8 min, dihydrodehydrodiconiferyl alcohol). The sum of peak areas of peaks 1 to 9 occupied 87·0 % of the total peak areas.
Discussion
The present study systematically evaluated the osteoprotective effects of different fractions of SWH in OVX mice and in rat osteoblast-like UMR 106 cells. The present results clearly demonstrated that the 50 and 95 % ethanol eluates (SWC) of the ethanol extract of SWH suppressed OVX-induced loss in bone mass and bone strength and increase in body weight. Besides, SWC did not alter the uterus weight in OVX mice. Moreover, the present study showed that SWC effectively promoted proliferation and differentiation in UMR 106 cells. In addition, the present study identified that the major components isolated from SWC were lignans, which may account for the osteoprotective effects of this fraction or even this herb.
Our recent study(Reference Zhang, Li and Wan13) reported that a 60 % ethanol extract of SWH dose-dependently increased BMD but only a high-dose treatment group showed statistically significant differences compared with OVX mice. To investigate if further fractions of SWH may lead to more potent protective effects in bone, SWH was separated with a macroporous adsorptive resin column and three fractions were obtained. The bone-protective effects of the fractions and SWH were evaluated and were compared using in vivo and in vitro results.
Trabecular bone mass, trabecular bone microstructure and cortical bone biomechanical strength are three key aspects associated with bone properties and turnover(Reference Rachoń, Vortherms and Seidlova-Wuttke19). Micro-computed tomography measurement showed that SWH improved trabecular BMD and suppressed disruption of trabecular bone microstructure of the femur and tibia in OVX mice, a result that is in accordance with our previous study(Reference Xie, Wu and Zhang12, Reference Zhang, Li and Wan13). The fractions obtained from further fractionations of SWH (i.e. SWA and SWC) did inhibit bone mineral loss induced by ovariectomy. In particular, the protective effects of SWC on trabecular bone in OVX mice were comparable with the effects of SWH. Moreover, micro-architectural parameters such as BV/TV, Tb.N and Tb.Sp of trabecular bone at the distal metaphysis of femur and the proximal metaphysis of tibia in OVX mice were significantly improved by treatment with SWC and the improvement was even higher than brought about by SWH. The results of three-point bending experiments on tibial cortical bone demonstrated that all fractions of SWH could significantly increase the ultimate load of the tibia diaphysis. Furthermore, the SWC treatment significantly increased breaking force and SWA treatment significantly increased stiffness in OVX mice. These results indicated that all fractions of SWH significantly improved the biomechanical strength of the long bone in OVX mice. The analysis of micro-computed tomography and three-point bending tests suggested that SWC may contain nearly all (87 %) of the active ingredients of SWH that account for its protective effects on bone mass and bone quality.
SWH extract inhibited bone resorption by suppressing osteoclastogenesis via modulation of the osteoprotegerin:receptor activator of NF-κB ligand (OPG:RANKL) ratio in UMR 106 cells(Reference Xie, Wu and Zhang12). To investigate the effects of different SWH fractions on osteoblastic cell function, the proliferation and differentiation of osteoblast-like UMR 106 cells were determined in the present study. The studies showed that SWH promoted cell proliferation and differentiation at 1 and 0·1 μg/ml, respectively. Fractions obtained from further fractionation of SWH stimulated osteoblastic cell proliferation and differentiation. Among the fractions, SWC showed very high potency for increasing cell proliferation and differentiation at doses from 0·1 to 100 μg/ml. Our in vitro results confirmed the conclusion of the in vivo experiment that SWC was the fraction with the highest potency on bone protection and was enriched with most of the active ingredients in SWH that account for its ability to improve bone properties.
Our previous study found that the most effective dose (1000 mg/kg) of ethanol extract of SWH after conversion from animal to human dosage was 5·5 g/d in women with a body weight of 50 kg(Reference Zhang, Li and Wan13). Similarly, the treatment dose of SWC (0·189 g/kg) applied in the present study for the improvement of bone properties will be 1·0 g/d for postmenopausal women administered orally. The present finding revealed that a lower dose of SWC will be needed to obtain effects that are similar to or even better than SWH.
Oestrogen or substances with oestrogenic potential were previously shown to be able to attenuate ovariectomy-induced weight gain(Reference Rachoń, Vortherms and Seidlova-Wuttke19–Reference Rachoń, Vortherms and Seidlova-Wuttke21). The present results showed that SWH and SWC significantly suppressed the body-weight gain induced by OVX, suggesting that SWH and SWC might also contain phyto-oestrogens. Similar to our previous study(Reference Zhang, Li and Wan13), the present study confirmed that SWH stimulated the increase of uterus weight in OVX animals. In contrast to SWH, SWC did not alter uterus weight in OVX mice, suggesting that SWC did not exert undesirable oestrogen-like effects on the reproductive system. Rachoń et al. (Reference Rachoń, Vortherms and Seidlova-Wuttke19–Reference Rachoń, Vortherms and Seidlova-Wuttke21) found that daidzein and purarin at high doses (1·0 and 3·0 g/kg, respectively) exerted uterotropic effects on OVX rats. Our studies provide an exciting result for finding a new agent for bone protection without unsatisfactory effects on the uterus.
Phenolic acids(Reference Yang, Wong and Wang15), lignans(Reference Yang, Wong and Wang14, Reference Ou, Liu and Xiao22), triterpenoids(Reference Yang, Wang and Wong16) and some other compounds had been isolated from SWH in our previous study. However, the major active ingredients that account for its bone protection in vivo are still not known. In the present study, seven lignans and two phenolic acids, which are the major components in SWC, were identified. The results indicated that lignans might be the active ingredients in the SWH fraction that account for its bone-protective effects in vivo. However, the biological activities of these isolated compounds were been studied in the present investigation. Further study will be needed to confirm if these compounds can directly stimulate the growth and differentiation of bone cells as well as exert osteoprotective effects in vivo.
In conclusion, the present study revealed that SWC was the fraction of highest potency in SWH and that it could prevent bone loss, improve trabecular bone microstructure and increase cortical bone strength in OVX mice. Most importantly, the doses of SWC used in the present study can fully restore bone loss to the pre-OVX level. The in vitro experiments suggested that SWC might increase bone formation by promoting proliferation and differentiation of osteoblast-like cells. Most importantly, the effective dose of SWC to achieve similar bone-protective effects as SWH is found to be only one fifth of that of SWH. In addition, seven lignans and two phenolic acids, which are the major peaks of the HPLC fingerprint of SWC, were identified. Further studies are needed to characterise the mechanism by which the isolated lignans and phenolic acids exert anabolic effects on bone cells, presumably osteoblasts. The present study suggests that SWC needs to be investigated as a natural alternative for the management of postmenopausal osteoporosis.
Acknowledgements
The present study was supported by the Niche area Research Grant from the Research Committee of The Hong Kong Polytechnic University (I-BB8N, GU324, GU256) and the Shenzhen-Hong Kong Innovation Circle Funding Scheme (2006). We thank the support of the State Key Laboratory of Chinese Medicine and Molecular Pharmacology, HKSAR.
The authors' responsibilities were as follows: M.-S. W. and X.-S. Y. conceived of and designed the study and obtained funding; H.-H. X., Y. D. and H.-Y. W. implemented the study; H.-H. X. acquired and analysed the data, and wrote the manuscript; M.-S. W. and X.-S. Y. critically revised the manuscript and its intellectual content.
None of the authors had a conflict of interest.








