Legitimate praise for Uganda’s response?
In October 2020, the Lancet ranked Uganda as one of the world’s top ten countries in suppressing COVID-19 (Ainebyoona, Reference Ainebyoona2020). This was based on the Lancet COVID-19 Commission Statement, published first in September 2020, which categorised countries in terms of level of suppression according to ‘the number of new cases per day per million population’ (The Lancet COVID-19 Commissioners, Task Force Chairs, Commission Secretariat, 2020). Suppression was defined as ≤5 new cases per million per day, when tests per case ≥20. Nineteen countries were categorised as achieving suppression according to these criteria, with Uganda (1.2 new cases per million per day, with 157.2 tests per case) and Rwanda (5 new cases per million per day, with 169.3 tests per case) the only two African countries to achieve such suppression. The commissioners attributed their success to effective key non-pharmaceutical interventions (NPI). ‘We note with satisfaction that many low-income countries have achieved sustained successes by deploying the NPI package to suppress the epidemic. Notable examples include… Uganda, which has extensive experience with the AIDS epidemic’ (ibid, p. 1110).
Other influential organisations took a similar view. In March 2021, Patricia Scotland, the Commonwealth Secretary General, lauded the Ugandan President for his response, stating that he had succeeded by ‘listening to science, listening to the empirical evidence, planning and helping to get the population to support the measures’ (Ajuna, Reference Ajuna2021). In August 2021, the Global Fund published a blog entitled Uganda’s Remarkable Response to COVID-19. The blog stated that: ‘Uganda achieved that feat by swiftly deploying health systems and community responses created to fight other infectious disease, including HIV, TB and malaria’ (The Global Fund, 2021).
This opinion piece challenges this narrative of success. Drawing on publicly available aggregated epidemiological data (including data reported to the World Health Organization), which has been collated by Johns Hopkins University (see: https://github.com/CSSEGISandData/COVID-19), it presents epidemic curves for the three main waves of COVID-19 in Uganda (Fig. 1, 2 and 4), a comparison with the South African Delta outbreak (Fig. 3), and COVID-19 mortality rates (Figs. 5 and 6). Data for Uganda and South Africa are analysed with reference to the different NPI’s – or Public Health and Social Measures (PHSM) that were introduced during these waves of infection. In so doing, it is argued that it is unclear how much the PHSM influenced the course of the first outbreak in Uganda. Furthermore, data from the second and third waves in Uganda (Figs. 2 and 4) suggest that government actions had little or no effect on these outbreaks. If government responses had been effective, then slowing of the outbreak or ‘flattening of the curve’ would have been observed as was seen in the South African delta outbreak (Fig. 3). Instead, the epidemiological curves suggest rapid uncontrolled spread and spontaneous resolution of outbreaks. More broadly, this opinion piece demonstrates the problems with reifying a small number of epidemiological indicators at the expense of considering how these indicators relate to socio-behavioural measures and the wider political context. Developing broader biosocial perspectives for Uganda – and elsewhere – would enable more accurate assessments of COVID-19 public health policy and practice.
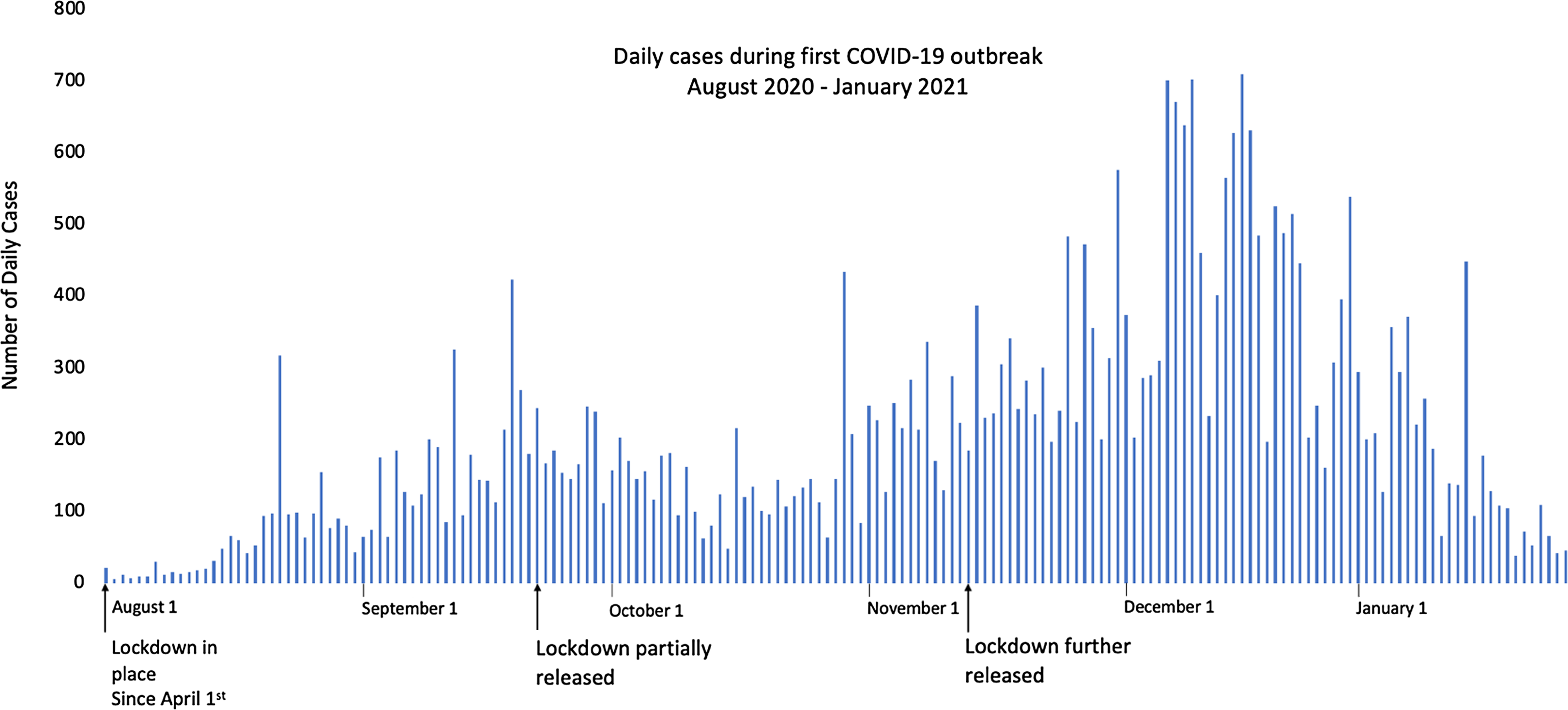
Figure 1. Daily cases of COVID-19 from August 2020 to January 2021.

Figure 2. Daily cases during the Ugandan Delta outbreak (Wave 2) May to August 2021.
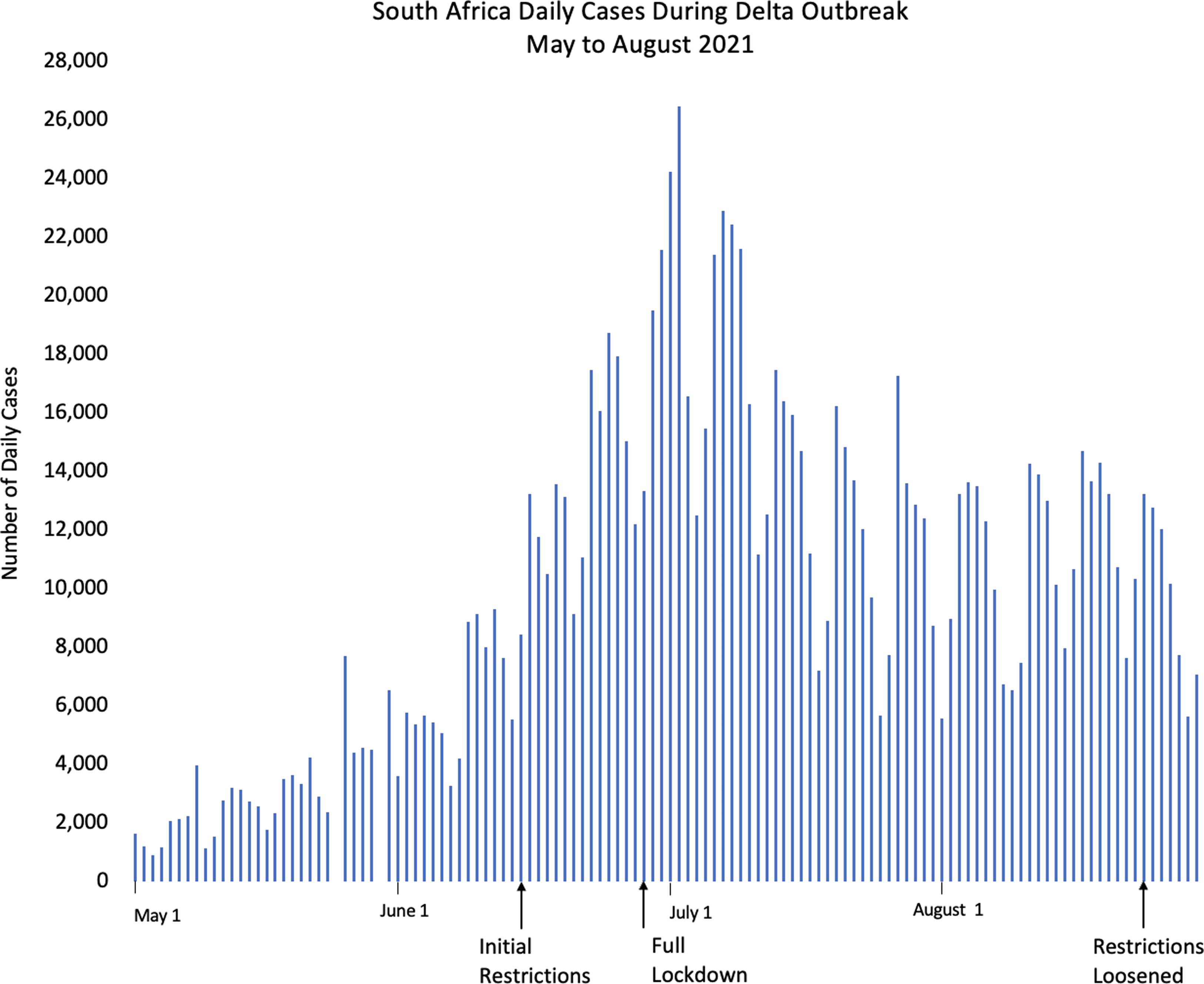
Figure 3. Daily cases during South African Delta outbreak from May to August 2021.

Figure 4. Daily cases during Omicron outbreak in Uganda, December 2021 – February 2022.
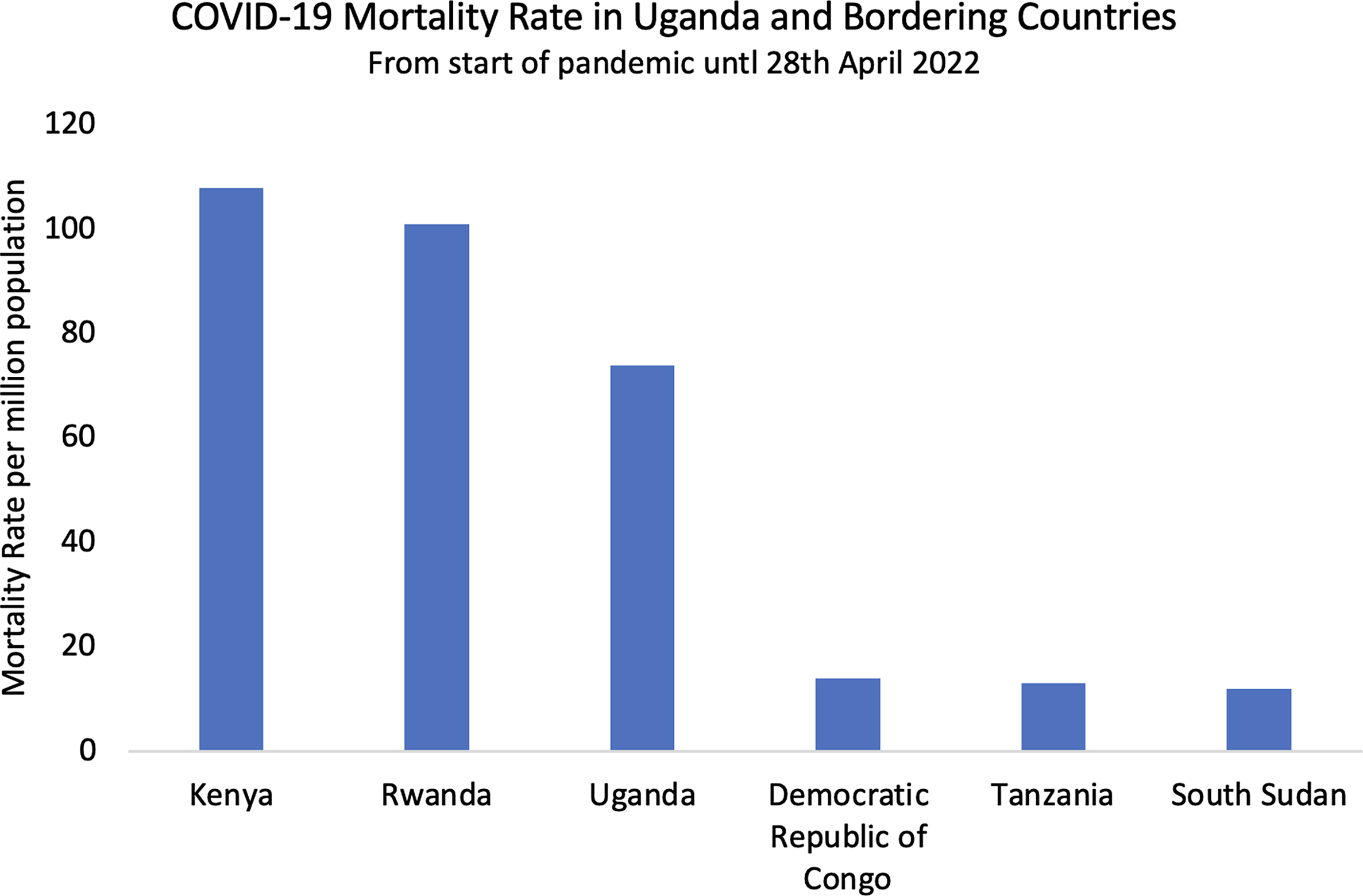
Figure 5. COVID-19 mortality rate in Uganda and bordering countries.
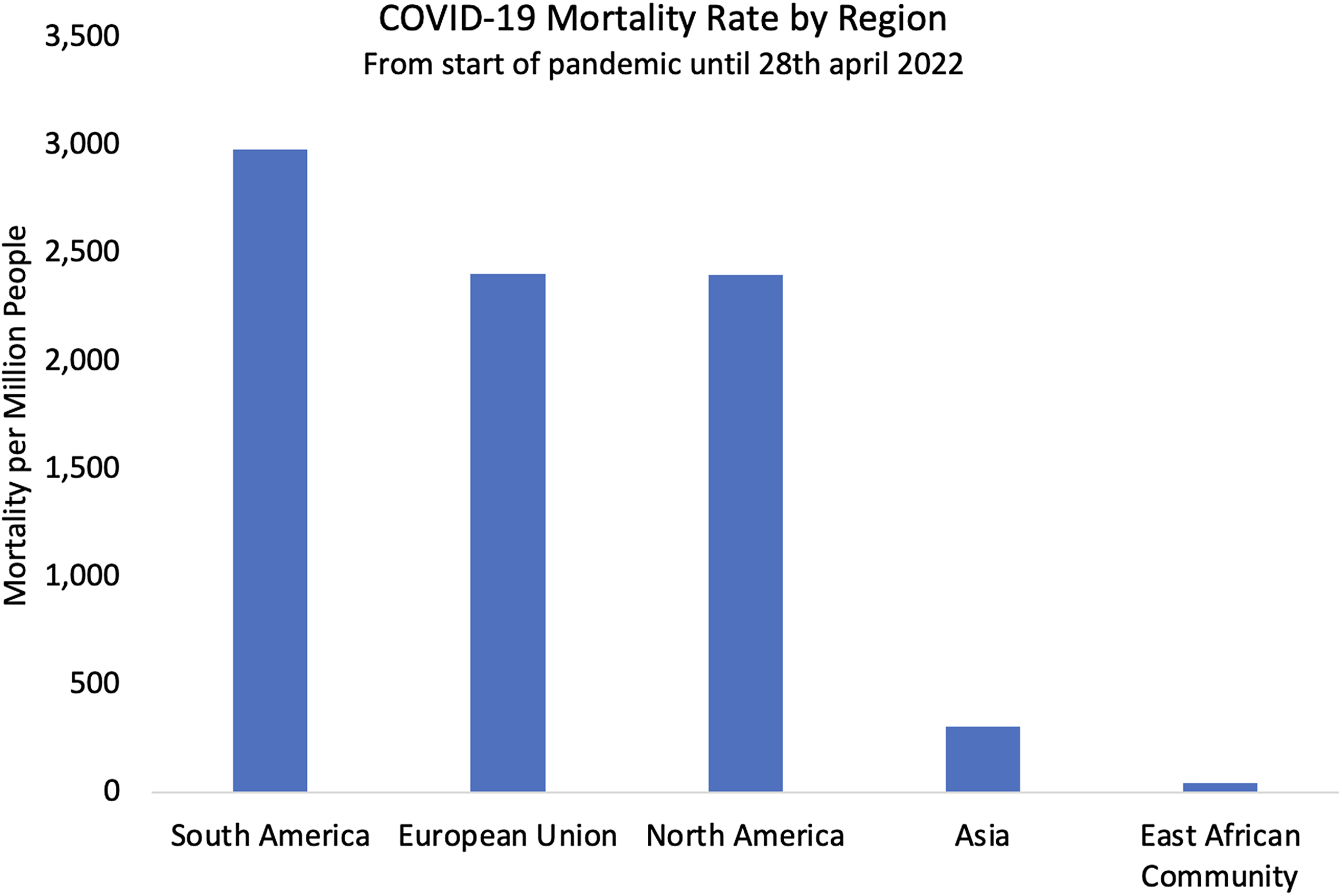
Figure 6. COVID-19 mortality rate in five regions of the world.
The initial COVID-19 outbreak in Uganda, March 2020 to January 2021
Uganda instigated its first COVID-19 measures on 20th March 2020, the day before the first case was confirmed in the country. From April 1st 2020, the harshest lockdown of the entire two-year period was implemented. With the help of the armed forces, no private or public transport was allowed, schools and places of worship were closed, and trade was forbidden unless it involved the sale or purchase of food. Air and land borders were also closed, although trucks carrying goods were permitted to cross if the driver had tested negative for COVID-19. Some of these restrictions were lifted on the 2nd June 2020, and others were removed on 21st September 2020. However, schools remained essentially closed (apart from a proportion of classes approaching exams), along with bars and nightclubs. Political rallies continued to be forbidden (Parker et al., Reference Parker, MacGregor and Akello2020).
Uganda’s strict and early restrictions may have delayed the onset of the epidemic to later in 2020 (Fig. 1), with cases only rising to the highest point in December, after lockdown measures were released in September. However, it is not straightforward interpreting these data. In March 2020, the Ugandan Viral Research Institute (which is based in Entebbe) was the only place in the country able to provide PCR testing. Testing capacity gradually increased during 2020 and by the end of the year testing was available in most major urban centres. These higher testing rates may also have contributed to higher case numbers, as monthly tests increased from an average of just 1277 tests a day earlier in April, to three times that number later that year – peaking at 3646 in December (John Hopkins University, 2022). From this point onwards, however, the number of COVID-19 PCR tests done in Uganda was relatively consistent, supporting the premise that although reported numbers of cases are unlikely to represent the ‘true’ numbers of cases, overall trends are more reliable with consistent testing rates. These data are, therefore, suitable for analysis.
The Delta outbreak in Uganda, May to August 2021
The Delta wave in Uganda progressed rapidly from the early stages of a rolling average of 100 cases a day on 18th May 2021, to the outbreak peak on June 12th 2021 (Fig. 2). Less than a month passed from the start of the outbreak to the peak. In just three months between June and August 2021, the Ugandan government reported 2,328 coronavirus deaths, over half of the overall Ugandan death toll.
The epidemiological curve of COVID-19 cases during the Delta outbreak (Fig. 2) steeply rises and falls, with no correlation between government policies of lockdowns, restrictions, or the subsequent loosening of restrictions and changes in case numbers. Initial restrictions were instituted only on June 7th, over 2 weeks after the start of the outbreak. During these ‘light’ restrictions, people moved freely on motorcycles and public transport within districts, and most markets and shops were open. An outside observer may not even have realised there were restrictions in place. In Gulu, northern Uganda, for example, where two of the authors (NL and SM) were living and working as medical practitioners at the time, it was often hard to discern whether any restrictions were actually in place. For people living close to international borders, the situation was different. In Kasese and Pakwach districts, both of which border the Democratic Republic of Congo (DRC), the Ugandan armed forces reduced movement from urban conurbations to rural areas, but the imposition of enforcement measures inadvertently created new modes of mutuality to subvert or actively resist the regulations, including the establishment of new forms of cross-border movement (Parker et al., Reference Parker, Baluku and Ozunga2022). In other words, ethnographic research carried out during this time suggested that these ‘light’ restrictions were unlikely to have had an impact on transmission. It is also noteworthy that the peak of the outbreak had already passed before a more extreme lockdown was instituted on the 18th of June, with all public transport banned and retail shops closed. This contrasts with the South African Delta outbreak during the same period (Fig. 3), where 2 months passed between the start of the outbreak and the peak, and flattening of the curve was observed after restrictions were imposed in mid-June (South African Government, 2022).
The Omicron outbreak in Uganda, December 2021 to January 2022
The Omicron outbreak followed a similar but even more rapid course than Delta in Uganda, with the peak occurring only 2 weeks after the start of the outbreak (Fig. 4). Although vaccines for COVID-19 had been introduced in Uganda during 2021, most Ugandans had not yet been vaccinated by the time of this wave (Mathieu et al., Reference Mathieu, Ritchie and Ortiz-Ospina2021). Unlike the first two waves, the Ugandan government made minimal effort to control the outbreak and imposed no new restrictions. Schools were re-opened fully for the first time in 20 months on January 10th 2022, while the outbreak continued unabated. The outbreak peaked quickly, with a steep rise and fall in the epidemic curve in under 2 months.
Alternative conclusions
Uganda’s COVID-19 outcomes reflected a general trend across East, West, and Central sub-Saharan Africa. Despite stark initial warnings from the WHO and others, these regions were almost universally spared from overwhelmed hospitals and high mortality, despite weak tertiary care infrastructure in many countries (United Nations, 2021; Bwire et al., Reference Bwire, Ario and Eyu2022). Neighbouring countries had similarly favourable outcomes to Uganda with low mortality between 12 and 108 deaths per million (Fig. 5). These rates were among the lowest in the world (Fig. 6) and were consistently low across the region despite countries adopting a wide range of COVID-19 management approaches. Kenya had fewer and less extreme nationwide lockdowns than Uganda, while the Tanzanian government denied the existence of COVID-19 until April 2021 and no lockdowns were implemented (Buguzi, Reference Buguzi2021). Although Tanzania, DRC, and South Sudan generated poor quality data due to low testing capacity (Garang et al., Reference Garang Aluk Dinyo, Ahmadi, Okereke, Yasir Essar and Eliseo Lucero-Prisno2020, Juma et al., Reference Juma, Mushabaa, Salam, Ahmadi and Lucero-Prisno2020; Buguzi, Reference Buguzi2021), Kenya, Rwanda, and Uganda may be more reliably compared as they have comparable coronavirus testing rates, life expectancy, and age demographics (John Hopkins University 2022; World Bank Group 2022). No observers within any of these countries reported overwhelming COVID-19-related mortality. Given these countries’ different approaches to the pandemic, it is likely that PHSM were less influential than claimed, and other explanations may carry more weight.
Alternative explanations for these low mortality rates include sub-Saharan countries having young populations and low rates of co-morbidities such as diabetes and obesity (Adams et al., Reference Adams, MacKenzie and Amegah2021), and high rates of physical activity (Wachira et al., Reference Wachira, Arena and Sallis2022). There may also be pre-existing immunity from previous infections, such as other coronaviruses causing the common cold (Nordling, Reference Nordling2020; Ashworth et al., Reference Ashworth, Mathie and Scott2023). Malaria might also protect people through stimulation of the innate immune system (Habibzadeh, Reference Habibzadeh2023). Environmental factors such as climate have also been postulated (Njenga et al., Reference Njenga, Dawa and Nanyingi2020). In addition, there may be other yet unknown factors that led to the region not suffering the same morbidity, mortality, and public health crises that much of the rest of the world endured.
If Uganda’s COVID-19 response was as effective as the international praise has suggested, then better outcomes than neighbouring countries would have been expected, and epidemic curves would have risen and fallen more gradually, as was observed in South Africa where PHSM measures may have been more effective. Rather, epidemiological evidence suggests that Uganda’s favourable outcomes were likely due to these alternative explanations, rather than PHSM.
Given that factors other than PHSM were likely to be responsible for the good COVID-19 outcomes in countries such as Uganda, alternative methods of measuring ‘success’ in pandemics should be explored. Countries could be compared within smaller regions where countries have similar demographic profiles rather than on a global scale. Countries could also be assessed by process measures as well as direct outcomes. These measures could include logical timing of lockdowns and releases, and whether planned PHSM were effectively implemented on a national scale.
Whatever measures are used to gauge public health successes and failures in the future, the dominant narrative of Uganda’s COVID-19 success story needs to be reconsidered, especially when the use of lockdowns had such detrimental effects on the lives of many people already living in precarity. Scrutiny could usefully be given to broader historical, political, and social issues shaping the collection and interpretation of data, including the clustering of cases and the generation of ‘at risk’ populations (Storer et al., Reference Storer, Dawson and Fergus2022), and the tendency to overlook widely reported, troubling events on the ground. As Parker et al. (Reference Parker, Baluku and Ozunga2022) have pointed out, the militarised response to COVID-19 in Uganda was sometimes violent and strengthened the position of President Museveni and his political party, the National Resistance Movement. The tendency to uncritically reify partial epidemiological data – and set aside the socio-political contexts in which enforcement measures occurred – vitiates against developing more sophisticated and nuanced understandings of public health and unwittingly lends itself to a trend towards more authoritarian forms of governance.
Acknowledgements
We would like to thank Josh Parker Allen and Dr Emmanuel Ochola for their constructive comments.
Financial support
SM is funded by the Economic and Social Research Council (ESRC) for her doctoral studies (ES/P000592/1) and received a small grant from the Parkes Foundation for fieldwork, and support from LSE through the Arts and Humanities Research Council (AHRC) Safety of Strangers grant (AH/T007524/1).MP’s research on Epidemic Preparedness and Response is funded by a Wellcome Trust Collaborative Award (212536/Z/18/Z) and the UK Economic and Social Research Council (ES/P008038/1).
Competing interests
The authors have no conflicts of interest to declare.








