Introduction
Clostridium difficile (C. difficile) infection (CDI) is one of leading causes of mortality in the developed world [Reference Daneman1] and estimated to be responsible for 10–20% of antibiotic-associated diarrhoea cases including all cases of pseudomembranous colitis [Reference Kelly, Pothoulakis and LaMont2], resulting in an estimated medical cost of €3 billion per annum among the EU states [Reference Kuijper3] and $1.5 billion per annum in the USA [Reference Leffler and Lamont4]. It has been accepted that this organism spreads nosocomially and causes outbreaks of CDI in various clinical settings [Reference Kelly, Pothoulakis and LaMont2]. Infected or colonised patients and contaminated environments constituted potential sources of C. difficile [Reference Gerding5] in hospital settings. Asymptomatic carriers shed spores into the environment to a lesser extent than CDI patients [Reference Guerrero6], but because C. difficile colonisation is fivefold to 10-fold more common than symptomatic infection [Reference Polage, Solnick and Cohen7], they serve as an important reservoir for nosocomial transmission. Nearly 30 years ago, Clabots et al. found that most episodes of nosocomial acquisition of CDI in a study ward were epidemiologically linked to transmission from asymptomatic new admissions [Reference Clabots8]. This link was more recently supported by a Canadian study, in which the isolation of C. difficile-colonised patients was accompanied by a significantly reduced incidence of hospital-acquired CDI in recent years [Reference Longtin9]. It was also reported that asymptomatic colonisation increases the risk of subsequent clinical disease [Reference Zacharioudakis10]. Approximately 5–15% of patients newly admitted to hospitals carry C. difficile in their faeces [Reference Zacharioudakis10, Reference Leekha11], and it has been reported that one-sixth to one-third of the carriers may develop symptoms [Reference Hung12].
However, there have been no reports of associations between noted involvement of asymptomatic carriage in the transmission of toxigenic C. difficile in healthcare facilities and corresponding practice to block the transmission [Reference Donskey, Kundrapu and Deshpande13], as they have not been a focus of CDI control measures [Reference Curry14]. It is noteworthy that there was a different impression that asymptomatic colonisation with C. difficile was associated with a decreased risk of diarrhoea [Reference Truong15].
The carriage and transmission frequencies of C. difficile have been studied in an elderly population living in long-term care facilities (LTCFs) [Reference Rivera and Woods16], as well as in healthy adults aged up to 65 years (median age, 22 years) in Japan [Reference Ozaki17], many of whom were college students. To the best of our knowledge, there have been no studies of carriage among patients with a specific disease. For example, there are no reports on liver cirrhotic patients, who are more prone to acquire CDI as a result of disturbed microbiota in the gut, increased hospital visits and antibiotic usage. In this study, we investigated the asymptomatic carriage and genotype of C. difficile in these patients using stool specimens collected from admission to discharge. We determined the frequency of asymptomatic toxigenic C. difficile carriage at admission and at different time points after hospitalisation. We also investigated whether the strains isolated from these patients represented current endemic C. difficile genotypes in China.
Materials and methods
Study design
During a 6-month period in 2015 (1 May to 31 October), we conducted a cohort study of all patients who provided consent in the Infectious Disease Department of the First Affiliated Hospital, Zhejiang University. On average, there were 168 admissions per ward per month, including 34 admissions that stayed for more than 48 h. Cirrhosis was diagnosed based on previous liver biopsy results, clinical evidence of previous decompensation and laboratory tests, endoscopy and radiological imaging of portal hypertension and/or liver nodularity. Patients were excluded if they: had hepatic carcinoma or other malignancies, had diarrhoea on admission, were discharged within 2 days, were transferred from other wards to the participating wards and were readmitted during this period. Stool samples were collected within 48 h of admission and then weekly during hospitalisation until discharge or diagnosis with CDI.
Written informed consent was obtained from each patient. The study protocols were approved by the Ethical Committee of the First Affiliated Hospital, Zhejiang University School of Medicine.
Definitions
Asymptomatic carriage was defined as positivity for toxigenic C. difficile without symptoms of diarrhoea at the time of stool collection [Reference Kelly18] or in the first 48 h of admission. If identified in a negative patient after 48 h, nosocomial acquisition was considered. CDI was defined by diarrhoea episodes (⩾3 unformed stools in 24 h) occurring at least 48 h after admission, diagnosis with the two-stage algorithms, and negative results for other diarrhoea-causing pathogens including Salmonella spp., Shigella spp., Vibrio spp., Staphylococcus aureus and Escherichia coli.
When the patient was documented with C. difficile diarrhoea, caregivers and others were advised to exercise contact precautions, including using gloves, wearing gowns and using chlorhexidine for hand hygiene.
Isolation of C. difficile from faecal samples and diagnosis of CDI
The stool samples, including admission and follow-up during hospitalisation, were cultured on cycloserine-cefoxitin-fructose agar in an atmosphere composed of 80% N2, 10% H2 and 10% CO2 at 37 °C for 48 h. A maximum of five colonies was picked from each C. difficile-positive plate. Colonies with typical morphology and odour were confirmed as C. difficile by matrix-assisted laser desorption-ionisation mass spectrometry (MALDI-TOF MS, Flexcontrol3.3-microflex).
The two-stage algorithms were used to confirm the microbiological evidence of toxin-producing C. difficile in stools. Only unformed or watery stool samples were detected for C. difficile toxins (Bristol Stool Chart types 5–7) [Reference Lewis and Heaton19]. The protocol of the two-stage algorithms, including glutamate dehydrogenase and toxin A and B detection by enzyme immunoassay (EIA) (Vidas; bio-Mérieux, Marcy-l'Etoile, France), was described previously [Reference Shin20].
DNA extraction and detection of toxigenic genes
The isolates of C. difficile were grown on blood agar incubated anaerobically for 48 h. Genomic DNA from the two isolates was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). TcdA and tcdB genes were detected by PCR as described previously [Reference Kato21]. Both binary toxin genes, cdtA and cdtB, were detected as described by Stubbs et al. [Reference Stubbs22].
Multi-locus sequence typing
Multi-locus sequence typing (MLST) was performed on all toxigenic strains according to the previously described protocols [Reference Griffiths23]. The sequence type (ST) was determined according to a combination of alleles identified by comparing the obtained sequences with sequences available in the C. difficile MLST database available at: http://pubmlst.org/cdifficile/.
Results
A total of 805 patients were admitted during the study period, and 279 of them were excluded. Included in the evaluation were 1056 faeces samples from 526 patients, of which 104 (19.8%) were positive for toxigenic C. difficile upon admission (Fig. 1). Follow-up was performed on these 104 patients weekly until they were discharged or diagnosed with CDI. Among these 104 patients, 44.2% (46/104) of the patients had at least one toxigenic C. difficile-positive as least one time during their hospitalisation and the remainder had only positive at admission. Sixteen ST types were identified among the 104 isolates from admission samples (Table 1). The top four types were ST-54 (18.5%, 19/104), ST-35 (15.4%, 16/104), ST-3 (21.2%, 22/104) and ST-37 (14.4%, 15/104). There were 76 isolates and 17 ST types identified during the follow-up. The top four types were ST-54 (26.3%, 20/76), ST-3 (17.1%, 13/76), ST-35 (11.8%, 9/76) and ST-37 (6.6%, 5/76). ST-15, ST-109, ST-220 and ST-294 were identified on admission and became negative afterwards, while new STs including ST-2, ST-26, ST-48, ST-55 and ST-129 emerged. Continuous colonisation with the same STs was detected in 28 patients (26.9%), while different STs were identified in 18 patients (17.3%) over the course of hospitalisation. Ten STs detected on admission were changed during hospitalisation. ST-35 and ST-37 were most frequently changed to other STs (25% and 26.7%, respectively). Among the 46 patients who remained positive after admission, the percentages who were positive for two, three, four, or more isolations were 29.8% (31/104), 5.8% (6/104) and 8.7% (9/104), respectively. No C. difficile NAP1/BI/027/ST1 or 078 isolates were identified in this study, and no specimens contained two or more different toxin types.
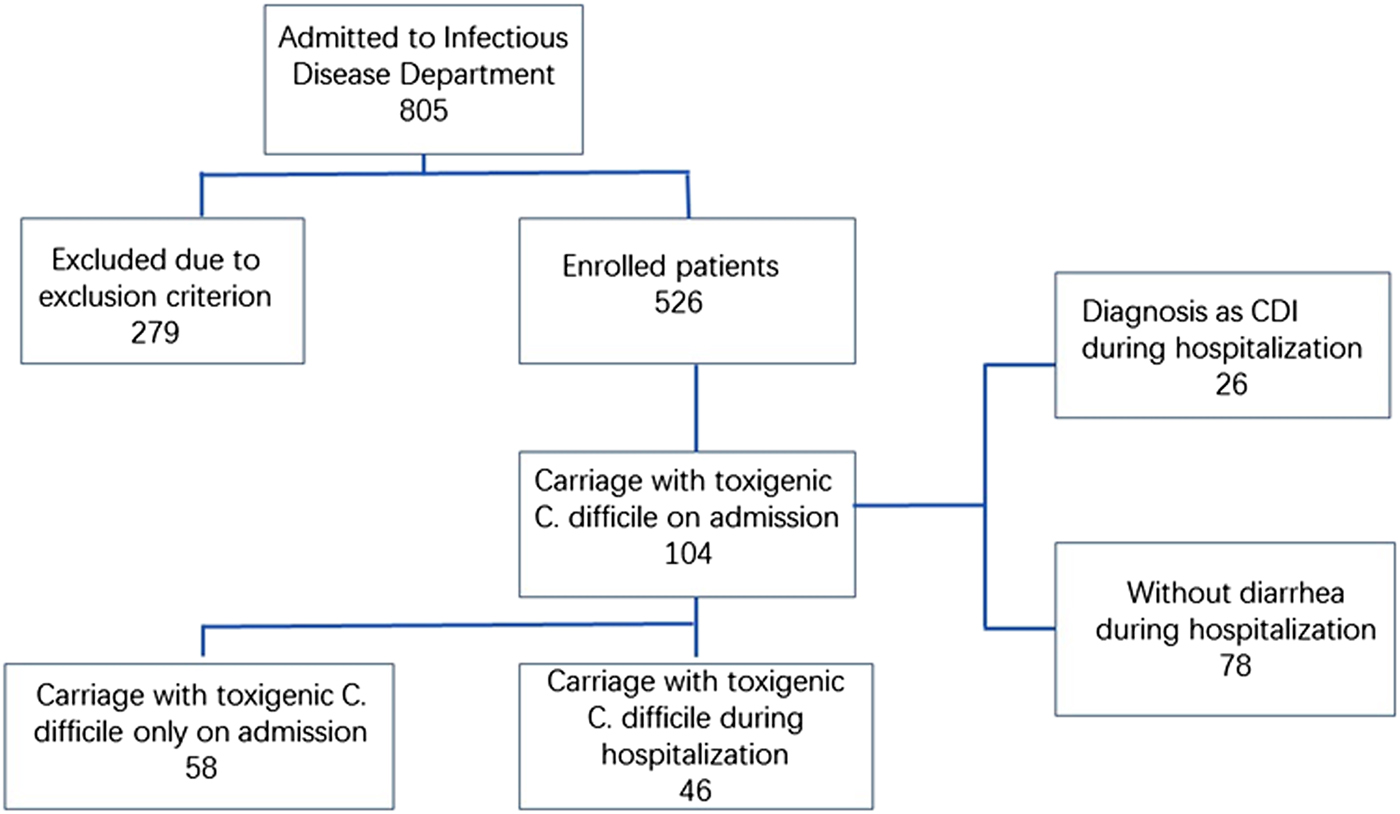
Fig. 1. Flow chart of patient recruitment and selection.
Table 1. Positive isolations and sequence types from 104 patients with C. difficile colonisation and/or infection
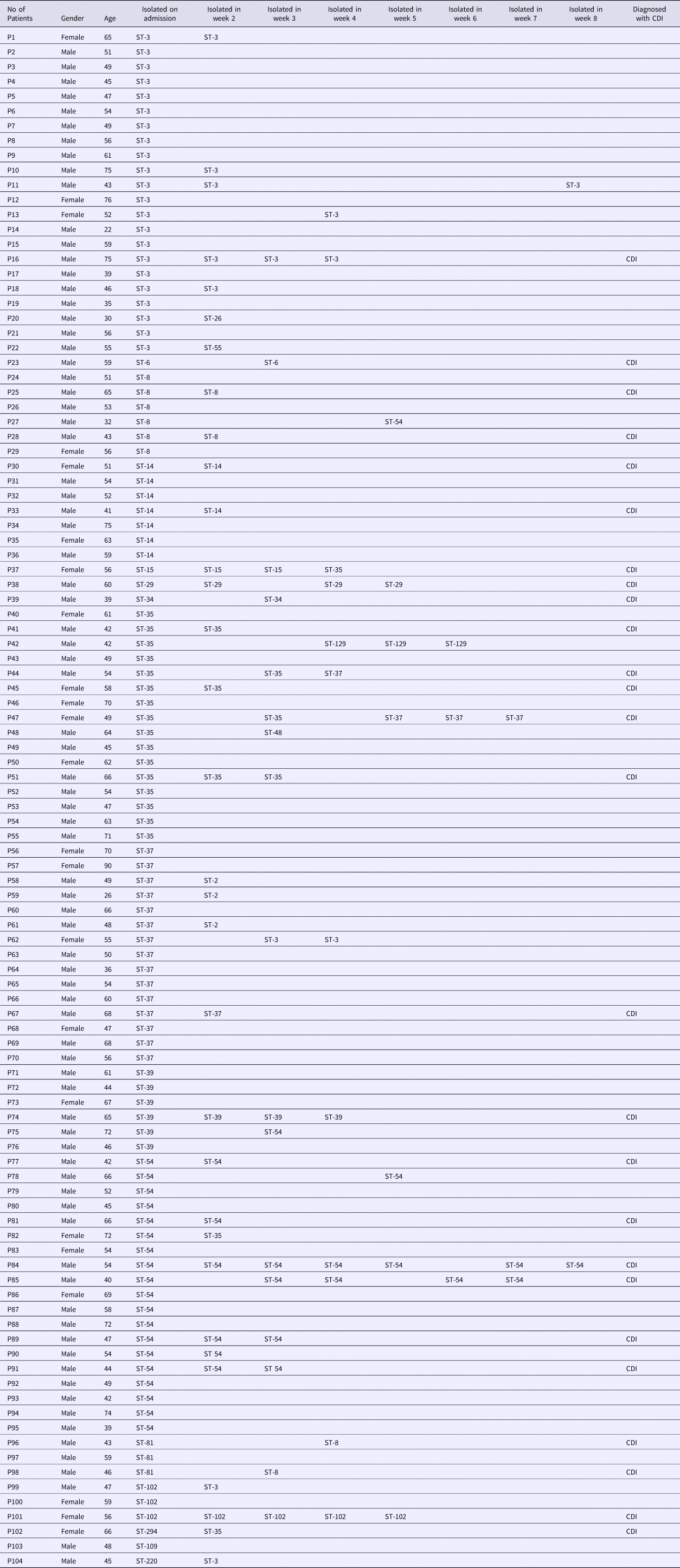
CDI occurred in 25% (26/104) of patients during their hospital stay. The percentages of patients who had two, three and four positive isolations before diagnosis with CDI were 53.8% (14/26), 15.4% (4/26) and 15.4% (4/26), respectively. Three patients remained positive for five isolations and one for seven isolations. These four patients were diagnosed with CDI in the last sample. The time between detection of colonised C. difficile and CDI diagnosis was 1–2 weeks.
Discussion
One uniqueness in this study is the availability of first stool samples taken at or near admission and then the collection of weekly samples afterwards, which allowed us to study C. difficile colonisation and infection kinetics. Toxigenic C. difficile was detected in 19.8% of hepatic cirrhosis patients on admission. Different colonisation rates have been reported by different studies [Reference Zacharioudakis10], and several explanations are possible. (1) Different detection methods were employed. Nucleic acid amplification tests were the most sensitive in some studies [Reference Gateau24], while toxin EIAs were suboptimal in sensitivity. In this study, stool sample cultures, which are more sensitive than swab cultures, were performed [Reference Bassis25]. (2) Various risk factors are associated with asymptomatic C. difficile carriage including age, admission from another healthcare facility, overnight hospitalisation within the prior 90 days and exposure to antibiotics in the 90 days prior to admission [Reference Kong26]. Our cirrhosis patients may have had these risk factors for toxigenic C. difficile carriage, leading to a higher carriage rate compared with those previously reported [Reference Loo27].
ST-54, ST-35, ST-3 and ST-37 were the most prevalent among admission samples, and these STs were reported as the most common STs causing C. difficile infection in China [Reference Chen28–Reference Wang30]. In this study, 25% (26/104) of these patients developed CDI during their hospital stay, and ST-54 and ST-35 were the dominant types identified in these CDI patients [Reference Yan31]. These results were consistent with those of a report that the asymptomatic C. difficile carriage rate is similar to the symptomatic positivity rate, implying that CDI in the majority of symptomatic patients was likely derived from C. difficile colonisation [Reference Truong15], which represents a significant infection risk. All these reports support the hypothesis that the admission of asymptomatic C. difficile colonised patients contributes to sustained C. difficile transmission within a ward [Reference Lanzas32].
In this study, nearly 60% of the patients were positive only in admission samples, suggesting a transient colonisation. As reported in a 6-month follow-up of 18 colonised healthy students, 10 (56%) of them lost the colonisation [Reference Ozaki17]. Only 46 patients (40%) were positive for multiple isolations in this cohort, comparing eight (44%) of 18 colonised more than once, of whom three (38%) harboured the same strain previously reported [Reference Ozaki17]. However, there were no reports of colonisation during the follow-up of the hospitalised population. There were patients who showed continuous colonisation of toxigenic C. difficile after admission, and thus, hospitalisation may be a risk factor for colonisation in this study. There were three patients who were C. difficile-positive three or more times, and the C. difficile isolates from each of the three patients were different from the first isolates, but for all isolates, the same isolates were detected in at least two consecutive occasions. These findings suggest that there is a marked variation in the duration of the colonised state and the environment may have been contaminated. In an investigation of repeated exposure from the environment or other colonised individuals, C. difficile strains with the same STs were isolated from 28 patients (26.9%) during their hospital stay. These results suggest that cross-transmission of C. difficile may be relatively common among colonised individuals, or C. difficile may spread from a common source in the work environment.
Our previous study and other reports have shown that carriers of C. difficile are significantly more likely to develop diarrhoea during hospitalisation than non-carriers [Reference Yan31, Reference Kagan33]. Asymptomatic carriage identified at admission was a decisive risk factor for symptomatic infection during hospital stay, accounting for about 25% of the patients (26 of 104) who developed CDI during the study. Thus, screening for toxigenic C. difficile carriage at admission could predict the likelihood of a later symptomatic CDI, as described in previous studies [Reference Zacharioudakis10, Reference Curry14].
Another important finding was with an increasing number of positive isolations, the risk for CDI increased. In this study, of 15 patients who were positive for multiple isolations, 12 developed CDI. This study showed that different types of C. difficile influenced the infection. It is important to screen the colonisation of toxigenic C. difficile when patients were admitted, especially for those patients who have a higher risk for infection.
On average, it takes 5 days for colonised patients to develop symptoms [Reference Lanzas32, Reference Yakob34]. In this cohort, symptom development took 1–2 weeks, except in one patient who was diagnosed with CDI after 2 weeks. This was similar to other reports [Reference Loo27, Reference Shim35].
We note some limitations in this study. First, we tried to collect samples from patients within 2 days after admission. However, some patients were excluded because they had no stools within 2 days, reducing the number of patients with bias for the colonisation rate. Second, we did not take into consideration the therapy, especially antibiotic treatment during hospital stay, which is expected to have an impact on the colonisation of C. difficile. Third, we did not re-examine the patients who were negative for C. difficile at admission, and these patients may colonise C. difficile during their hospitalisation. Finally, we cannot be certain that the individuals who were colonised with toxigenic C. difficile on admission to the LTCF acquired colonisation during their hospital stay; it was possible that they carried C. difficile at the time of admission to the hospital.
Conclusions
Toxigenic C. difficile was detected in 104 (19.8%) of 526 hepatic cirrhosis patients on admission. Moreover, the carriage status changed in a portion of patients, whereas 55.8% (58//104) of patients lost the colonisation of toxigenic C. difficile during their hospital stay. Among the remaining 48 patients who remained positive for toxigenic C. difficile, 25% (26/104) were diagnosed with CDI during hospital stay. This study highlights the importance of identifying asymptomatic C. difficile carriers among hepatic cirrhosis patients on admission.
Author ORCIDs
Lanjuan Li, 0000-0001-6945-0593.
Acknowledgements
This study was partially funded by grants from the National Basic Research Program of China (973 program, No. 2015CB554201).




