The loss of functional status and the related high level of dependence is the main cause of admission to long-term care services(Reference Quinn, McArthur and Ellis1). Disability may be intrinsic to age, but more frequently reflects other diseases. This is likely to be due to the presence of sarcopenia, which, in turn, has been shown as an independent predictor of outcome(Reference Quinn, McArthur and Ellis1, Reference Cruz-Jentoft, Baeyens and Bauer2). Malnutrition is a prominent feature of elderly patients admitted to long-term care facilities(Reference Salva, Coll-Planas and Bruce3–Reference Cereda and Pedrolli5). It is associated with adverse outcomes(Reference Salva, Coll-Planas and Bruce3–Reference Cereda, Pedrolli and Zagami6), and previous studies have found a relationship with functional status(Reference Cereda and Pedrolli5, Reference Cereda, Valzolgher and Pedrolli7–Reference Kaiser, Winning and Uter9). However, the evidence collected in institutions where patients are characterised by the highest levels of dependence is still limited. Moreover, there is no information on the relationship between nutritional risk and functional status. Nutritional screening is currently recommended in any healthcare setting(Reference Salva, Coll-Planas and Bruce3, Reference Arvanitakis, Beck and Coppens4, Reference Kondrup, Allison and Elia10). However, nutritional screening tools do not provide the same information. There is still confusion between an index of nutritional status and that of nutritional risk. The latter takes into account nutritional status-related complications, and it is worthy of mention that a tool's validity should be more consistently discussed in view of the association with outcome. The Geriatric Nutritional Risk Index (GNRI) was proposed to clinicians in 2005 for predicting the risk of nutrition-related complications in a geriatric rehabilitation care setting(Reference Cereda, Pedrolli and Zagami11, Reference Bouillanne, Morineau and Dupont12), but several studies have supported its use in institutions. The GNRI has been strongly associated with short- and long-term outcomes in this setting and in respect of this, it appears to perform better than the recommended Mini Nutritional Assessment, particularly in newly institutionalised elderly(Reference Cereda and Pedrolli5, Reference Cereda, Pedrolli and Zagami11, Reference Cereda, Pusani and Limonta13). It has also been associated with the loss of muscle function(Reference Cereda and Vanotti14, Reference Cereda and Vanotti15) assessed by handgrip strength evaluation. However, the handgrip strength assessment as a surrogate for functional status requires the participation of the patient, which may be frequently unavailable in a long-term care setting. In the present study, we investigated the association between the GNRI and functional status, as assessed by the Barthel Index (BI) of basic activities of daily living, and the relationship of these two domains with mortality in newly institutionalised elderly.
Materials and methods
Study design
The present study is part of a larger multi-centric prospective cohort study conducted in four long-term care facilities in the provinces of Como (n 1), Pavia (n 1) and Trento (n 2). Baseline data collection began in May 2002 and ended in May 2007. The recruitment procedure was performed as follows: every year, for 2 weeks, all the subjects newly admitted to the facilities, aged >65 years and agreeing to participate were assessed for eligibility. Principal exclusion criteria were terminal illness, as assessed by the responsible physician, and a diagnosis of cancer at the time of recruitment(Reference Cereda, Pedrolli and Zagami6).
The study was performed in adherence to the principles of the Declaration of Helsinki and the protocol was approved by the local Institutional Ethics Committee. Written informed consent was obtained for every patient.
Nutritional assessment
Information was collected within 72 h after admission on the following: body weight; 3-month history of unintentional weight loss (percentage of usual body weight on a continuous scale); height or knee height (for appropriate estimation)(Reference Cereda, Bertoli and Vanotti16, Reference Cereda, Limonta and Pusani17); BMI; arm muscle area (derived from the mid-arm circumference and triceps skinfold thickness)(18). Fasting venous blood samples were assessed for the evaluation of Hb, total lymphocyte count, serum albumin, transthyretin, transferrin, total cholesterol and uric acid. Thereafter, nutritional risk was graded by the GNRI tool. The score was initially derived from the following formula:
In this equation, the ideal body weight is calculated from height (either measured or estimated)(Reference Cereda, Limonta and Pusani17) according to the Lorentz formula and the weight:ideal body weight ratio is set to 1 when the patient's body weight exceeds the ideal body weight(Reference Bouillanne, Morineau and Dupont12).
Then, patients were categorised as follows: at high risk, GNRI < 92; at low risk, GNRI 92–98; at no risk, GNRI >98(Reference Cereda and Pedrolli5, Reference Cereda, Bertoli and Vanotti16). As was made previously, in the present study, we avoided distinguishing the ‘severe risk’ group (GNRI < 82) from the ‘moderate risk’ one (GNRI 82–92) because it was found that there is no additional advantage in distinguishing these two categories(Reference Cereda8). As the GNRI was designed and validated in 2005(Reference Bouillanne, Morineau and Dupont12), nutritional risk was retrospectively derived for those patients included before 2005 and prospectively calculated for those entered after its introduction.
Functional status
Functional status and disability were scored by local trained nurses using the BI, a tool based on questions covering all the aspects of self-care independence in daily living activities (transfer, walking, stairs, toilet use, dressing, feeding, bladder, bowel, grooming and bathing). A total score of 100 points indicates complete self-sufficiency, while a score of zero point means completely dependent(Reference Quinn, McArthur and Ellis1, Reference Mahoney and Barthel19). Given the low median level of functional status and the skewed distribution of data in the present study, participants were categorised into population-specific tertiles (cut-points: 5 and 25) rather than into normal BI score tertiles.
Covariates
The following information was systematically collected: age; sex; admission diagnosis; all co-morbidities. Diagnoses were coded according to the International Classification of Diseases, 10th Revision. The selection of main admission diagnosis groups to be considered in descriptive and survival analyses was based on the literature review and previous association with nutritional status and/or survival. Data were primarily retrieved from medical records (reported diagnosis) and, in some cases, ascertained through the patient's interview and physical examination. Particularly, hypertension was assessed by the measurement in triplicate of systolic and diastolic blood pressure according to standard procedures, while diabetes was defined by a fasting blood glucose ≥ 1260 mg/l on two different measurements(Reference Chobanian, Bakris and Black20, 21). The use of pertinent pharmacological therapy was also taken into account for the assessment of co-morbidities. Finally, data on the use of oral nutritional supplements (sip-feeding) during the stay were also retrieved(Reference Cereda, Pedrolli and Zagami6).
Outcome
Vital status and cause of death were ascertained by active follow-up (Como and Pavia) or by means of record linkage with centralised provincial registry (Trento). Accordingly, events were classified as being due to cardiovascular, respiratory, neoplasms or other/not reported causes by the International Classification of Diseases, 10th Revision.
Statistical analyses
Data are presented as means and standard deviations, medians and interquartile ranges (25th–75th percentile) or counts and percentages when appropriate. One-way ANOVA or Kruskal–Wallis test analysis (continuous variables according to a normal and non-normal distribution, respectively) or the χ2 test (categorical variables) were used for the comparison of more than two groups. The independent correlates of functional status were investigated by means of multivariable linear regression analysis. In this model, given the non-normal distribution of data, the BI was entered as a continuous variable on a logarithmic scale. Accordingly, data are reported as β-coefficients with their standard errors.
Risk factors for mortality were assessed with the Cox proportional hazards regression model and associations are presented as hazard ratios (HR) and 95 % CI. The selection of covariates to be included in the models was initially based on the literature review and previous findings in our study population(Reference Cereda, Pedrolli and Zagami6, Reference Cereda, Pedrolli and Zagami11, Reference Cereda, Pedrolli and Zagami22). With respect to co-morbidities, the sum of all those recorded was used in the analyses. However, in the original cohort(Reference Cereda, Pedrolli and Zagami6), diabetes was independently associated with mortality. Accordingly, it has not been included in the total count but has been investigated as an independent predictor of outcome.
In all multivariable analyses, only non-collinear variables (checked by Pearson's statistics) with a P< 0·20 by univariate analysis were included(Reference Haynes, Sackett and Guyatt23).
Statistical analyses were performed using MEDCALC® for Windows version 11.3.0.0 (MedCalc Software) with statistical significance set to a two-tailed P value of < 0·05.
Results
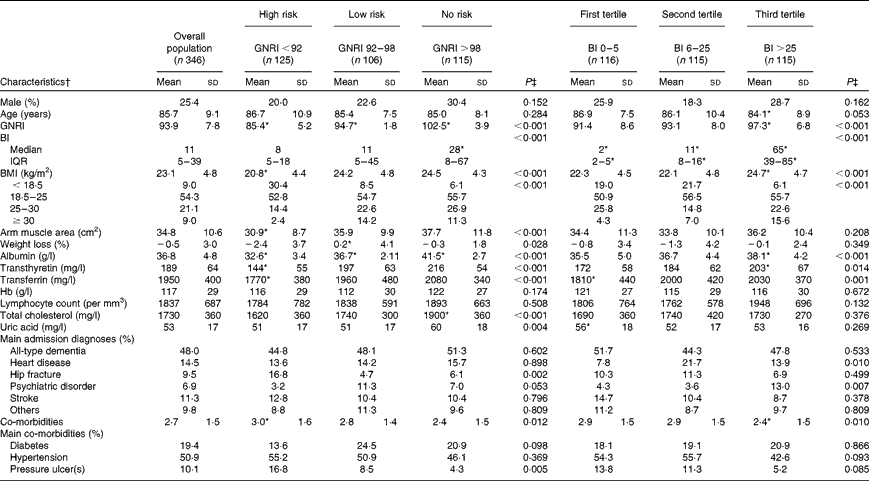
From the original cohort (n 533) assessed during the recruitment phases, 346 patients were included in the present analysis (n 14, lost to follow-up; n 173, incomplete data on the BI). The distribution and the features of the study sample according to risk categories by the GNRI and tertiles of the BI are presented in Table 1. The lack of data on the BI was due to the fact that, in the initial stages of the study, two centres did not collect information on functional status. However, at the group level, features were substantially similar to those of the original cohort in terms of age, sex, anthropometry, biochemistry and the prevalence of main admission diagnoses(Reference Cereda, Pedrolli and Zagami6). The population characteristics were also similar among the recruiting centres.
Table 1 Baseline clinical and demographic characteristics of the population by Geriatric Nutritional Risk Index (GNRI) and Barthel Index (BI) categories (Mean values and standard deviations; medians and interquartile ranges (IQR); percentages)

* Values were significantly different between the groups (P< 0·05; post hoc test).
† Percentages are calculated within the single groups.
‡ Continuous and categorical variables were compared between the groups with one-way ANOVA or the χ2 test, respectively.
During a median follow-up of 4·7 years (25th–75th percentile 3·7–6·2), 230 (66·5 %) subjects died. The median survival time was significantly reduced in patients at nutritional risk: GNRI < 92, 17·7 months (25th–75th percentile 9·4–30·1); GNRI 92–98, 28·8 months (25th–75th percentile 15·4–30·2); GNRI >98, 30·8 months (25th–75th percentile 14·2–43·0) (P for trend < 0·001; high-risk group significantly different from the others by the post hoc comparison). The survival time was also longer in the case of higher functional status: BI tertile I, 20·5 months (25th–75th percentile 9·3–31·1); BI tertile II, 24·6 months (25th–75th percentile 13·9–30·9); BI tertile III, 30·4 months (25th–75th percentile 18·4–56·1) (P for trend < 0·001; last tertile significantly different from the others by the post hoc comparison). The causes of death were grouped as follows: cardiovascular, 67·8 % (coronary artery disease or heart failure 53·2 %, stroke 40·4 % and others or unspecified 6·4 %); respiratory diseases, 25·2 % (89·3 % by pneumonia); neoplasm, 2·6 %; others, 4·4 %.
Nutritional risk and functional status
At multivariable linear regression, functional status by BI (log-transformed) was significantly associated with nutritional risk by GNRI (one-point increase in the score: β = 0·018 (se 0·004); P< 0·001). Other independent correlates were as follows: age (1-year increase: β = − 0·007 (se 0·004); P= 0·045); arm muscle area (1 cm2 increase: β =0·009 (se 0·004); P= 0·048); surrogate for skeletal muscle mass; number of co-morbidities (per additional co-morbidity: β =− 0·048 (se 0·021); P= 0·027).
Nutritional risk, functional status and mortality
In multivariable analysis, all-cause mortality (Table 2; Fig. 1) was significantly associated with nutritional risk. Other independent predictors were age, sex, number of co-morbidities and diabetes. However, the association between functional status and outcome was significant only in univariate analysis. Due to a slight collinearity (Pearson's r 0·31) between the GNRI and the BI, we also tested the possible interaction (GNRI × BI) between these two domains in outcome prediction. The association was not significant (P= 0·068).
Table 2 Predictors of all-cause mortality according to Cox proportional hazards regression models (Hazard ratios and 95 % confidence intervals)

GNRI, Geriatric Nutritional Risk Index.
* Risk of death at univariate analysis (variables presented were those having a P value < 0·20).
† Risk of death at multivariable analysis.
‡ Linear increase in risk over the categories assumed (checked with the likelihood ratio test).
§ Per additional co-morbidity (other than diabetes).

Fig. 1 Adjusted cumulative survival curves for all-cause mortality across (a) nutritional risk (Geriatric Nutritional Risk Index) categories (P< 0·001; ![]() , high risk;
, high risk; ![]() , low risk;
, low risk; ![]() , no risk) and (b) tertiles of the Barthel Index (P= 0·658;
, no risk) and (b) tertiles of the Barthel Index (P= 0·658; ![]() , first tertile;
, first tertile; ![]() , second tertile;
, second tertile; ![]() , third tertile).
, third tertile).
Similar results were found in cause-specific analysis where nutritional risk was the main predictor of cardiovascular mortality (model adjusted also for hypertension): GNRI < 92 HR 1·93 (95 % CI 1·28, 2·91), P< 0·001; GNRI 92–98 HR 1·31 (95 % CI 0·85, 1·99), P= 0·221 (linear increase over risk categories, HR 0·71, 95 % CI 0·58, 0·88, P= 0·001); BI HR 0·95 (95 % CI 0·78, 1·16), P= 0·619 (linear increase over tertiles). Other causes were not investigated due to the limited number of events.
During the follow-up, sixty-nine participants (19·9 %) received oral nutritional supplements (sip-feeding). The frequency of nutritional support among the GNRI categories and BI tertiles was GNRI < 92, 16·8 %; GNRI 92–98, 28·3 %; GNRI >98, 15·7 %; BI tertile I, 19·0 %; BI tertile II, 25·2 %; BI tertile III, 15·6 %. Risk analyses adjusted for the use of oral nutritional supplements confirmed the associations found. Nutritional support was also associated with reduced mortality risk (all-cause: HR 0·58 (95 % CI 0·34, 0·98), P= 0·045; cardiovascular: HR 0·54 (95 % CI 0·32, 1·00), P= 0·050).
Finally, fully adjusted risk models (sample size, n 259) refitted after the exclusion of participants who died in the first year of follow-up confirmed all the associations found with both all-cause (events, n 143; GNRI < 92 HR 1·98 (95 % CI 1·27, 3·09), P= 0·003); GNRI 92–98 HR 1·81 (95 % CI 1·19, 2·75), P= 0·006) and cardiovascular (events, n 95; GNRI < 92 HR 2·14 (95 % CI 1·26, 3·66), P= 0·005); GNRI 92–98 HR 1·59 (95 % CI 0·94, 2·67), P= 0·083) mortality.
Discussion
In the present study, it was found that nutritional risk, as assessed by the GNRI, is significantly associated with functional status and outcomes in newly institutionalised elderly. However, no association between functional status and mortality was observed.
The present results, which support the association between nutritional risk by the GNRI and mortality, are in agreement with the observations recently collected in a similar cohort(Reference Cereda, Pedrolli and Zagami11, Reference Cereda, Pusani and Limonta13) and with those preliminarily provided by some of the present investigators(Reference Cereda, Zagami and Vanotti24). However, compared with the findings reported few years ago, both high and low nutritional risks have been associated with poor prognosis. This difference could be reasonably ascribed to the greater sample size and the characteristics of the patients included (only newly admitted).
A limited number of studies have focused on the association between nutritional and functional status in a long-term care setting(Reference Cereda, Valzolgher and Pedrolli7, Reference Kaiser, Winning and Uter9, Reference Cereda, Pedrolli and Zagami11, Reference Cereda and Vanotti14, Reference Bahat, Saka and Tufan25). Moreover, the level of available evidence is hampered by the heterogeneity of the tools used for the assessment of both nutritional and functional domains, by the various underlying diseases and by the different sample sizes investigated. Moreover, there is still confusion between an index of nutritional status and that of nutritional risk, because the latter is more likely to account for nutritional status-related complications. Although several tools for nutritional screening are now available, recent literature supports the value of the GNRI in this patient population, due to its relationship with several parameters of nutritional status, muscle function and outcomes(Reference Cereda and Pedrolli5). Moreover, the prognostic value in newly institutionalised elderly appears superior to that of the recommended Mini Nutritional Assessment(Reference Cereda and Pedrolli5, Reference Cereda8, Reference Cereda, Pedrolli and Zagami11). Having a tool such as the GNRI in the evaluation of the patient may be an advantage because it requires minimal participation of the patient and, at the same time, it is able to both reflect functional status and predict the outcome. The strong relationship between the GNRI and the BI presented herein expands previous knowledge that was mainly related to disability as assessed by handgrip strength(Reference Cereda and Vanotti14, Reference Cereda and Vanotti15). Indeed, there is supportive evidence that the prevalence of nutritional derangements is inversely associated with the setting-related level of dependence, which, in turn, relies on functional status(Reference Cruz-Jentoft, Baeyens and Bauer2, Reference Cereda8). In a previous investigation, we have reported that the association between nutritional status and the BI was substantially explained by weight loss, muscle mass and energy intake(Reference Cereda, Valzolgher and Pedrolli7). In the present study, we did not assess energy intake, a factor that seems to improve the identification of muscle dysfunction independently of nutritional risk(Reference Cereda and Vanotti14). We have previously discussed that the prognostic value of the GNRI lies in its structure, which is mainly based on serum albumin as well as on the deviation of body weight from ideal conditions. As a nutritional marker, serum albumin is likely to account for disease severity and acute changes in nutritional balance(Reference Cereda and Pedrolli5, Reference Ballmer26). Looking at the GNRI structure, we could say that also the body weight factor is of help in describing the status and turnover of protein stores(Reference Cereda and Pedrolli5, Reference Ballmer26). Nutritional derangements are related to the underlying disease(Reference Bahat, Saka and Tufan25), which is recognised as an important determinant of functional status(Reference Quinn, McArthur and Ellis1). However, the association between the GNRI and the BI was independent of admission diagnosis. Accordingly, it could be sustained that the GNRI is likely to account for functional and metabolically active components of the body, which, in turn, have been associated with outcome(Reference Cereda and Pedrolli5, Reference Kaiser, Winning and Uter9, Reference Volpato, Romagnoni and Soattin27). However, the cause–effect relationship behind the association between nutritional and functional status is difficult to establish; however, recent data support the concept of functionality being more relevant for nutritional status(Reference Kaiser, Winning and Uter9). This issue should be the object of prospective investigations. In the present study, functional status was not an independent predictor of mortality in multivariable analysis. The result may appear in contrast with previous findings(Reference Cooper, Kuh and Hardy28). However, it should be highlighted that most of the evidence on the association with adverse outcomes has been collected in community-living elderly(Reference Cooper, Kuh and Hardy28), in particular sets of patients (e.g. stroke)(Reference Huybrechts and Caro29), or using data recorded in acute or post-acute conditions(Reference Bellelli, Magnifico and Trabucchi30–Reference Baztán, Gálvez and Socorro32). Accordingly, patients could be characterised by more heterogeneous conditions, while in the present study, about 70 % of the population had a BI score < 30 points, thus suggesting that in the presence of high levels of dependence, other factors are more likely to affect the outcomes. Nonetheless, the recent study by Kaiser et al. (Reference Kaiser, Winning and Uter9) supports the evidence that functional status is less relevant to outcomes even in the presence of lower levels of dependence.
Finally, in agreement with previous observations(Reference Cereda, Pedrolli and Zagami11), this was an interesting finding that nutritional risk is associated with cardiovascular mortality. It could be speculated that changes in inflammatory and hormonal status (glucocorticoids and catecholamines) and the dysregulation of the autonomic nervous system occurring with malnutrition and ageing both act as risk modifiers(Reference Avraham, Hao and Mendelson33–Reference Yeh and Schuster35).
Some other limitations should be considered. First, the low level of dependence and the choice to categorise participants on the basis of BI data distribution make the results strictly linked to the sample population, hampering their generalisability and the comparison with other data in the literature. The same may also be argued for the prevalence of some major co-morbidities, such as diabetes and hypertension. Second, a consistent number of patients (approximately 30 %) of the original cohort have been excluded due to the lack of BI data. However, we believe that this did not represent a source of bias because it was observed that the present study population was comparable with the original cohort and features were similar among the recruiting centres. Third, the findings could have been strengthened by the evaluation of inflammatory background, although the association between nutritional risk and functional status was independent of co-morbidities. Fourth, as frequently occurs in observational studies, the effect of several potential factors acting during the follow-up has not been taken into account, particularly the main outcome predictors have been assessed only upon admission. Accordingly, it could not be excluded that changes in functional status occurring during the follow-up could play a role in modifying the patient's outcome. On the other hand, albumin, being a marker of disease severity, is more likely to be associated with outcome. Fifth, the cognitive capacity of those people without a diagnosis of dementia has not been fully explored by the nursing home staff and residual bias in the association between functional and nutritional status may have occurred. However, the association between these two domains was the strongest in our multivariable model, and it is reasonable to argue that the results were affected only to a minimal extent. Finally, as previously discussed, the introduction of risk estimation bias could not be excluded due to the procedure of recruitment through which patients surviving complications leading to institutionalisation were more likely to be included.
In summary, nutritional risk is significantly associated with functional status and mortality (all-cause and cardiovascular) in newly institutionalised elderly. The present study supports the concept that the nutritional domain is more relevant than functional status to the outcome of newly institutionalised elderly. The potential role of nutritional support in improving the outcome is also suggested.
Acknowledgements
The study was supported by the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. E. C. received consultancy honoraria and investigator grants from Nutricia Italia and the ‘Fondazione Grigioni per il Morbo di Parkinson’. Abstract accepted as oral presentation and awarded a ‘Travel Fellowship’ at the upcoming 34th ESPEN Congress 2012, Barcelona, Spain.
All authors significantly contributed to the study, read and approved the final version of the manuscript. E. C. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. E. C., C. P. and A. V. contributed to the study concept and design. E. C., C. P., S. P., M. F., M. R. and A. Z. were involved in the acquisition of the data. E. C. and C. P. participated in the analysis and the interpretation of the data. E. C. drafted the manuscript. E. C., R. C., C. P. and M. R. critically revised the manuscript for important intellectual content.
The authors certify that there are no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter or materials discussed in the manuscript.