Introduction
Large carnivores are of conservation concern globally. More than 75% of the remaining large carnivore species have declining population trajectories (Chapron et al., Reference Chapron, Kaczensky, Linnell, von Arx, Huber and Andrén2014; Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014; Eklund et al., Reference Eklund, López-Bao, Tourani, Chapron and Frank2017). Furthermore, the majority of these species are categorized as threatened (Vulnerable, Endangered or Critically Endangered) on the IUCN Red List, with some experiencing > 90% range contraction over the last century (Ripple et al., Reference Ripple, Chapron, López-Bao, Durant, Macdonald and Lindsey2016; Wolf & Ripple, Reference Wolf and Ripple2017). Widespread concerns relating to carnivore conservation are reflected in the literature; publication of peer-reviewed research has increased exponentially in the last 3 decades (Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; Montgomery et al., Reference Montgomery, Elliot, Hayward, Gray, Millspaugh and Riley2018a,Reference Montgomery, Hoffmann, Tans and Kissuib; Lozano et al., Reference Lozano, Olszańska, Morales-Reyes, Castro, Malo and Moleón2019). This literature has identified a number of drivers of carnivore population declines, including habitat loss, prey depletion, disease and climate change (Inskip & Zimmermann, Reference Inskip and Zimmermann2009; Estes et al., Reference Estes, Terborgh, Brashares, Power, Berger and Bond2011; Ripple et al., Reference Ripple, Chapron, López-Bao, Durant, Macdonald and Lindsey2016; Wolf & Ripple, Reference Wolf and Ripple2017). However, retaliation for livestock depredation is consistently cited as one of the primary threats to carnivore population persistence (Inskip & Zimmermann, Reference Inskip and Zimmermann2009; Tumenta et al., Reference Tumenta, de Iongh, Funston and Udo de Haes2013; Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014; Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; van Eeden et al., Reference van Eeden, Crowther, Dickman, Macdonald, Ripple, Ritchie and Newsome2018a,Reference van Eeden, Eklund, Miller, López-Bao, Chapron and Cejtinb).
As funds for conservation work are limited, each conservation project needs to use resources efficiently, to maximize positive on-the-ground impacts (Balmford et al., Reference Balmford, Gaston, Blyth, James and Kapos2003; Brambilla et al., Reference Brambilla, Gustin and Celada2013; Eklund et al., Reference Eklund, López-Bao, Tourani, Chapron and Frank2017). To do so, research must be interpretable by conservation practitioners and policy makers (Balmford et al., Reference Balmford, Gaston, Blyth, James and Kapos2003; Knight et al., Reference Knight, Cowling, Rouget, Balmford, Lombard and Campbell2008; Bennett et al., Reference Bennett, Maloney and Possingham2015; Ripple et al., Reference Ripple, Chapron, López-Bao, Durant, Macdonald and Lindsey2016). However, even after extensive calls for improvement, significant gaps between research and conservation implementation remain (Knight et al., Reference Knight, Cowling, Rouget, Balmford, Lombard and Campbell2008; Eklund et al., Reference Eklund, López-Bao, Tourani, Chapron and Frank2017; Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; Montgomery et al., Reference Montgomery, Elliot, Hayward, Gray, Millspaugh and Riley2018a,Reference Montgomery, Hoffmann, Tans and Kissuib; Gray et al., Reference Gray, Booher, Elliott, Kramer, Waller and Millspaugh2019). Factors contributing to this research–implementation gap include limited interdisciplinarity within research teams, scale discordance, and limited actionability of research (Montgomery et al., Reference Montgomery, Elliot, Hayward, Gray, Millspaugh and Riley2018a,Reference Montgomery, Hoffmann, Tans and Kissuib; Gray et al., Reference Gray, Booher, Elliott, Kramer, Waller and Millspaugh2019). Another factor that may be influential in this context is taxonomic bias.
Taxonomic bias is prevalent throughout conservation research, and describes a tendency for research effort, funding, and public interest to focus on a small subset of species (Clark & May, Reference Clark and May2002; Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Stroud et al., Reference Stroud, Rehm, Ladd, Olivas and Feeley2014; Di Marco et al., Reference Di Marco, Chapman, Althor, Kearney and Watson2017; Donaldson et al., Reference Donaldson, Burnett, Braun, Suski, Hinch, Cooke and Kerr2017; Troudet et al., Reference Troudet, Grandcolas, Blin, Vignes-Lebbe and Legendre2017; Tensen, Reference Tensen2018). This bias is primarily driven by human social factors, including perceptions of species charisma, and the value of those species for society and as subjects of conservation funding (Bonnet et al., Reference Bonnet, Shine and Lourdais2002; Donaldson et al., Reference Donaldson, Burnett, Braun, Suski, Hinch, Cooke and Kerr2017; Rosenthal et al., Reference Rosenthal, Gertler, Hamilton, Prasad and Andrade2017). This uneven distribution of research and funding among taxa can result in mismatches between research effort, the resulting knowledge base, and conservation needs (Bonnet et al., Reference Bonnet, Shine and Lourdais2002; Linklater, Reference Linklater2003; Fazey et al., Reference Fazey, Fischer and Lindenmayer2005; Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Wilson et al., Reference Wilson, Proches, Braschler, Dixon and Richardson2007; Hortal et al., Reference Hortal, De Bello, Diniz-Filho, Lewinsohn, Lobo and Ladle2015; Rosenthal et al., Reference Rosenthal, Gertler, Hamilton, Prasad and Andrade2017). These biases are not only influential between taxonomic orders but also within them, and may have important consequences for the research–implementation gap (Anon., 2007; Knight et al., Reference Knight, Cowling, Rouget, Balmford, Lombard and Campbell2008; Martín-López et al., Reference Martín-López, Montes, Ramírez and Benayas2009; Trimble & van Aarde, Reference Trimble and van Aarde2012; Fleming & Bateman, Reference Fleming and Bateman2016). To mitigate these effects, regular assessments of taxonomic bias have been recommended (Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Wilson et al., Reference Wilson, Proches, Braschler, Dixon and Richardson2007; Di Marco et al., Reference Di Marco, Chapman, Althor, Kearney and Watson2017). Although previous studies have explored taxonomic bias in other conservation fields, its effect on the research–implementation gap has not previously been evaluated for the literature on the depredation of livestock by carnivores.
Here, we used livestock depredation by large carnivores in sub-Saharan Africa as a case study to assess whether taxonomic bias is evident in human–carnivore conflict research. We conducted a literature review and compared the central carnivore species of each study to those identified as being most responsible for livestock depredation. We then examined the ways in which misalignment among these factors could contribute to the research–implementation gap affecting human–carnivore conflict mitigation. We explore the role of species charisma in catalysing research effort and conservation funding, and discuss the implications of our study for interventions and policies that could promote human–carnivore coexistence.
Methods
The term human–carnivore conflict obscures the nuanced experiences inherent in interactions between people and carnivores (Dickman, Reference Dickman2010; Redpath et al., Reference Redpath, Young, Evely, Adams, Sutherland and Whitehouse2013; Redpath, Reference Redpath2015; Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; Lozano et al., Reference Lozano, Olszańska, Morales-Reyes, Castro, Malo and Moleón2019). We acknowledge that in assessing livestock depredation by carnivores, our study does not allow a broader perspective on both positive and negative human–wildlife interactions. However, we focused our review on livestock depredation as it is often a primary driver of agonistic interactions between people and carnivores and thus is a threat to carnivore conservation (Inskip & Zimmermann, Reference Inskip and Zimmermann2009; Tumenta et al., Reference Tumenta, de Iongh, Funston and Udo de Haes2013; Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014). Furthermore, minimizing depredation is a common aim of efforts to improve human–carnivore coexistence (Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; van Eeden et al., Reference van Eeden, Crowther, Dickman, Macdonald, Ripple, Ritchie and Newsome2018a,Reference van Eeden, Eklund, Miller, López-Bao, Chapron and Cejtinb). We chose to highlight sub-Saharan Africa because it is a hotspot for livestock depredation by carnivores, and carnivore biodiversity (Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014; Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; Lozano et al., Reference Lozano, Olszańska, Morales-Reyes, Castro, Malo and Moleón2019).
We completed our review in June 2019, using four bibliographic databases: Web of Science Core Collection (Clarivate Analytics, Philadelphia, USA), Scopus (Elsevier, Amsterdam, The Netherlands), Wildlife and Ecology Studies Worldwide (EBSCO, Baltimore, USA) and Google Scholar (Google, Mountain View, USA). We conducted our review in English, as it is the predominant publication language for studies on livestock depredation by carnivores (Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018; van Eeden et al., Reference van Eeden, Crowther, Dickman, Macdonald, Ripple, Ritchie and Newsome2018a,Reference van Eeden, Eklund, Miller, López-Bao, Chapron and Cejtinb). Using an iterative search process, we searched each database a total of three times. We first included the term ‘human carnivore livestock’, adding ‘conflict’ in the secondary search and ‘depredation’ in the tertiary. As the database used by Google Scholar is not limited to scientific publications, we used additional specificity in our search on that platform. Specifically, we started our search of Google Scholar using ‘human carnivore conflict’ as a bound phrase (i.e. with the search term enclosed in quotations, so that the search results only include the exact phrase) and added ‘livestock’ and ‘depredation’ in the secondary and tertiary searches, respectively. We excluded any studies that were not published in a peer-reviewed journal, those that were outside the geographical extent of sub-Saharan Africa, and those that were not directly relevant to our assessment (e.g. carnivore predation of wild prey, carnivore attacks on people, or human attitudes towards conservation actions). For each study we recorded: (1) the location of the field site, (2) the central (i.e. focal) carnivore species, and (3) the carnivore species responsible for the majority of livestock depredation. For studies that did not provide exact geographical coordinates, we approximated the field site location based on site maps and study area descriptions. We selected the centroid for those that included multiple field sites.
Central species
We identified the central carnivore species of each study using lexical analysis with MAXQDA Analytics Pro 20.0.8 (Kuckartz & Radiker, Reference Kuckartz and Radiker2019). We conducted lexical searches among all studies to record the number of times that depredating carnivore species were mentioned. Our search terms included ‘African lion’ Panthera leo, ‘spotted hyaena’ Crocuta crocuta, ‘African wild dog’ Lycaon pictus, ‘leopard’ Panthera pardus, ‘Ethiopian wolf’ Canis simensis, ‘cheetah’ Acinonyx jubatus, ‘jackal’ Canis mesomelas, ‘brown hyaena’ Parahyaena brunnea, ‘African wolf’ Canis lupaster, ‘caracal’ Caracal caracal and ‘striped hyaena’ Hyaena hyaena. We used only the common name of each species as a search term, included the alternate spelling of hyaena (i.e. hyena) for all three hyaena species, and specified each search term to be a character string instead of a bound phrase. We also included words from the lemma list without case sensitivity. With these search settings, MAXQDA returned a hit for any combination of the search terms and any word forms (Kuckartz & Radiker, Reference Kuckartz and Radiker2019). For example, ‘lion’ returned a hit for the exact match, along with ‘Lion’ and ‘lions’. For each study, we recorded the number of hits for each carnivore species within all sections of the document, excluding the references and running title. We converted the number of hits by carnivore species into a per cent of the total hits in the study. We considered a carnivore species to be central if the number of hits were ≥ 25% of the total hits for that study. Thus, it was possible for a study to have multiple central species. We classified a study as having no central species if none had ≥ 25% of the total hits.
Lexical analysis is an established tool for assessing text-based media, as high frequency terms are representative of content themes and biases (Wodak & Meyer, Reference Wodak, Meyer, Wodak and Meyer2008; Bednarek & Caple, Reference Bednarek and Caple2014). Lexical analyses are replicable, quantifiable and unbiased, and thus are valuable for studies of taxonomic bias (dos Santos et al., Reference dos Santos, Correia, Malhado, Campos-Silva, Teles, Jepson and Ladle2020). However, as the application of this method is emergent in conservation, we used a secondary document analysis to verify our results, identifying the central species based upon references to carnivore species throughout the document. For example, we classified a study to be centred around the spotted hyaena if that was the primary species around which the introduction, methods and results were framed. We performed the document analysis separate from the lexical analysis, to minimize the risk of implicit coding bias from the results of the lexical analysis.
Measures of livestock depredation
Next, we determined the carnivore species responsible for the majority of livestock depredation in each study. We used the two most prevalent methods for measuring livestock depredation (Krafte Holland et al., Reference Krafte Holland, Larson and Powell2018): quantitative measures of livestock depredated by carnivores (e.g. the number of livestock killed), and perceptions of depredation risk among livestock owners (e.g. the proportion of respondents who considered a carnivore species to be the greatest threat to their livestock; Marker et al., Reference Marker, Mills and Macdonald2003; Kissui, Reference Kissui2008; Miller et al., Reference Miller, Jhala and Jena2016a,Reference Miller, Jhala and Schmitzb). We identified the carnivore species with the greatest contribution to these two conflict measures, depending on which was reported. Thus, our final database consisted of the geographical location, the central carnivore species and primary depredator for each study. We then mapped the distribution of all studies in ArcMap 10.5 (ESRI, Redlands, USA) and assessed the alignment between central species and primary livestock depredator.
Results
Our literature review returned 119 peer-reviewed publications on livestock depredation in sub-Saharan Africa published during 1997–2019. We eliminated 19 studies that did not fit the conditions of our review (e.g. did not directly examine livestock depredation or were not published in a peer-reviewed journal), so our final database comprised a total of 100 studies (Fig. 1, Supplementary Material 1). The majority of these were conducted in Eastern Africa (i.e. Ethiopia, Tanzania, Kenya; n = 51), and Southern Africa (i.e. Botswana, Namibia, South Africa, Zimbabwe; n = 43). Six were based in Western and Central Africa (i.e. Niger, Guinea, Chad, Cameroon, Benin). Seven studies did not have any central species, as identified via lexical analysis and confirmed through the document analysis. Among those with a single central species, the African lion was the most common (n = 29; Figs 2 & 3). Other single central species included the spotted hyaena (9), African wild dog (9), cheetah (7), leopard (6), black-backed jackal (5), Ethiopian wolf (4) and brown hyaena (1). The studies with at least two central species included African lions/spotted hyaenas (n = 10), African lions/leopards (3), African lions/spotted hyaenas/leopards (3), and spotted hyaenas/leopards (2). There was one study each that included Ethiopian wolves/African wolves, cheetahs/black-backed jackals, black-backed jackals/caracals, spotted hyaenas/leopards/black-backed jackals, and leopards/black-backed jackals/caracals (Fig. 2).

Fig. 1 The location of field sites featured in 100 studies of livestock depredation in sub-Saharan Africa published during 1997–2019.

Fig. 2 The central carnivore species among 100 studies on livestock depredation in sub-Saharan Africa (Fig. 1), indicating the number of studies with a single central species, and those with two or more central species, in Western and Central Africa, Southern Africa and Eastern Africa.
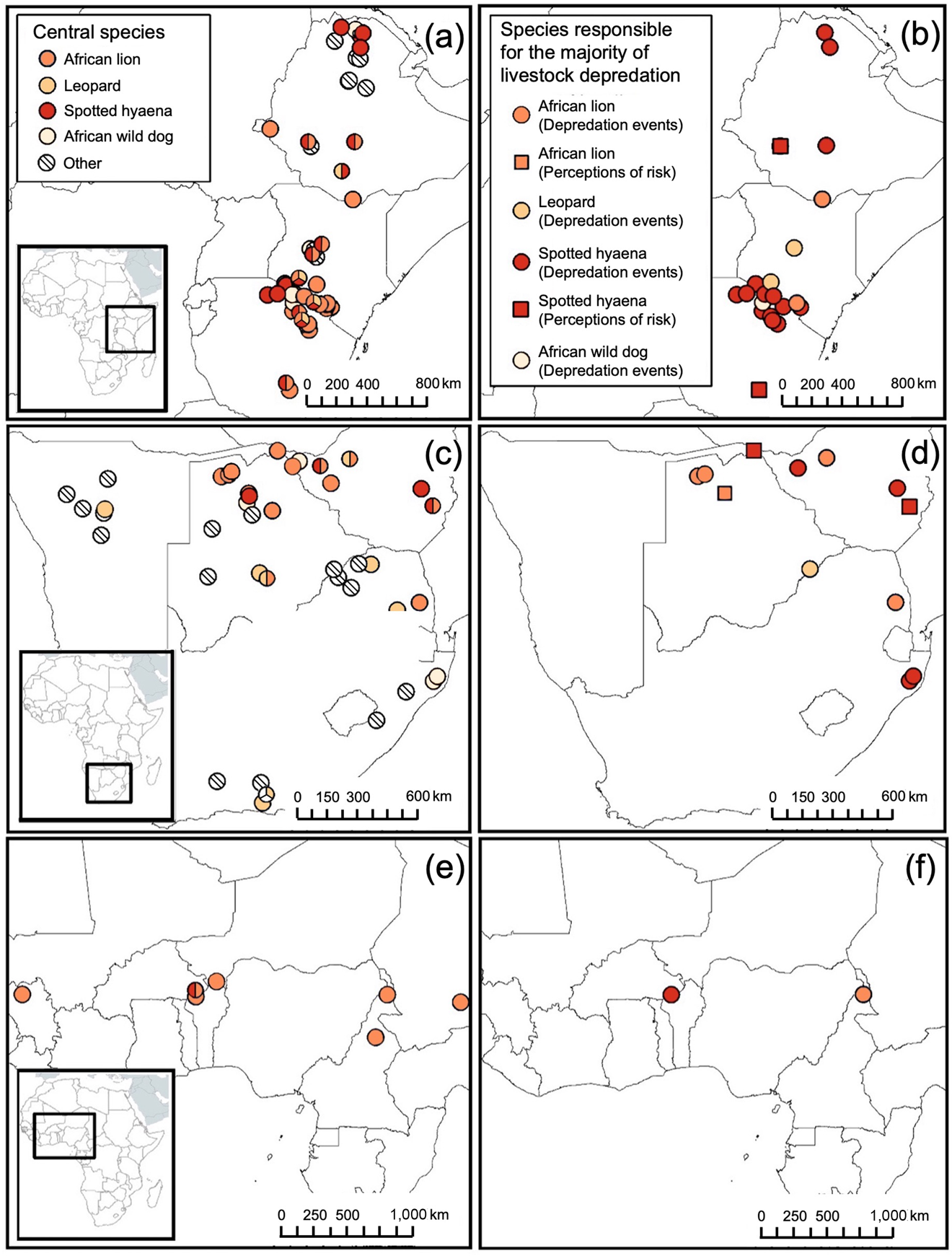
Fig. 3 The misalignment between central species (left-hand panels) and species responsible for the majority of livestock depredation (right-hand panels) for the four most common single central species reported to depredate livestock in the reviewed studies: African lion Panthera leo, leopard Panthera pardus, spotted hyaena Crocuta crocuta and African wild dog Lycaon pictus, by geographical region: (a) and (b) Eastern Africa, (c) and (d) Southern Africa, (e) and (f) Western and Central Africa.
There were 41 studies that included measures of livestock depredation. Over three-quarters (85.4%, n = 35) of these studies reported depredation events and the remainder (14.6%, n = 6) reported perceptions of depredation risk. Spotted hyaenas were the primary livestock depredator in the majority of these studies (58.5%, n = 18 and n = 4 for depredation events and perceptions of depredation risk, respectively), followed by African lions (24.4%, n = 9 and n = 2), leopards (7.3%, n = 3 and n = 0), black-backed jackals (4.9%, n = 2 and n = 0), African wild dogs (2.4%, n = 1 and n = 0), and African wolves (2.4%, n = 1 and n = 0; Figs 2 & 3). Notably, not all reported measures of livestock depredation were indicative of the magnitude of loss resulting from the depredation (e.g. monetary value of the livestock killed).
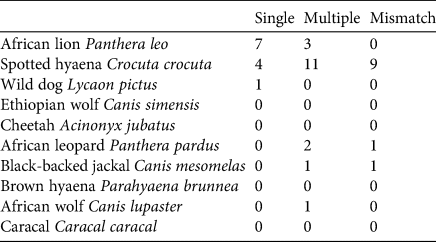
Among the four most common single central species reported to depredate livestock in the reviewed studies (African lions, spotted hyaenas, African wild dogs, and leopards), there was a mismatch between central species and primary depredator for spotted hyaenas and leopards (Table 1). Spotted hyaenas were not a central species in 37.5% of the studies (9 of 24) in which they were the primary livestock depredator. We detected such a mismatch for leopards in one study (Table 1).
Table 1 The alignment between central carnivore species and primary livestock depredator for 41 studies in sub-Saharan Africa published during 1997–2019, showing the number of studies in which the carnivore species responsible for the majority of livestock depredation was the only central species (single), one of multiple central species (multiple) or not a central species (mismatch) in the same study.
Discussion
Applied conservation research is most effective when research findings and conservation outputs are aligned (Balmford et al., Reference Balmford, Gaston, Blyth, James and Kapos2003; Stroud et al., Reference Stroud, Rehm, Ladd, Olivas and Feeley2014; Eklund et al., Reference Eklund, López-Bao, Tourani, Chapron and Frank2017). A discrepancy between these two factors may limit the applicability of research findings for policy and management practices (Balmford et al., Reference Balmford, Gaston, Blyth, James and Kapos2003; Linklater, Reference Linklater2003; Eklund et al., Reference Eklund, López-Bao, Tourani, Chapron and Frank2017; Gray et al., Reference Gray, Booher, Elliott, Kramer, Waller and Millspaugh2019). We detected a misalignment of this type within research on livestock depredation by carnivores in sub-Saharan Africa. Specifically, we found that spotted hyaenas were the central species in only a small proportion of studies despite being the most common primary livestock depredator. Similarly, in over one-third of the studies that reported spotted hyaenas as the primary livestock depredator, they were not a central species in that same study. In contrast, none of the other most common single central species reported to depredate livestock in the reviewed studies (African lion, African wild dog, leopard) showed similar rates of mismatch (Table 1). All three were more commonly central species than the primary livestock depredator. African lions, in particular, were disproportionately listed as the central species relative to their contributions as livestock depredators. They were central species in 45% of the studies but recorded as the primary livestock depredator in only 24% of the studies. These misalignments emerged in both the lexical and document analyses. These patterns are probably attributable, at least in part, to differing levels of charisma among large carnivores.
Species charisma is a relational trait, derived not from the inherent attributes of a species, but from the ways in which people respond to those attributes (Lorimer, Reference Lorimer2007; Albert et al., Reference Albert, Luque and Courchamp2018). Consequently, charisma is subjective and must be interpreted within the context of culture, experiences and values (Smith et al., Reference Smith, Veríssimo, Isaac and Jones2012; Ducarme et al., Reference Ducarme, Luque and Courchamp2013; Albert et al., Reference Albert, Luque and Courchamp2018). Charisma is often used to refer to a species' ability to rally financial support for conservation (Courchamp et al., Reference Courchamp, Angulo, Rivalan, Hall, Signoret, Bull and Meinard2006; Lorimer, Reference Lorimer2007; Macdonald et al., Reference Macdonald, Burnham, Hinks, Dickman, Malhi and Macdonald2015; Albert et al., Reference Albert, Luque and Courchamp2018). As the majority of conservation funding comes from Western societies (Albert et al., Reference Albert, Luque and Courchamp2018), charisma is most often framed in a Western context (Ducarme et al., Reference Ducarme, Luque and Courchamp2013; Courchamp et al., Reference Courchamp, Jaric, Albert, Meinard, Ripple and Chapron2018). Within this Western perspective, African lions and leopards are consistently highly ranked in lists of the most charismatic species (Smith et al., Reference Smith, Veríssimo, Isaac and Jones2012; Macdonald et al., Reference Macdonald, Burnham, Hinks, Dickman, Malhi and Macdonald2015; Albert et al., Reference Albert, Luque and Courchamp2018; Davies et al., Reference Davies, Cowley, Bennie, Leyshon, Inger and Carter2018). African wild dogs are also considered to be charismatic, but their overall cultural appeal is probably reduced because they are comparatively less recognizable outside their range countries (Di Minin et al., Reference Di Minin, Fraser, Slotow and MacMillan2013; Monsarrat & Kerley, Reference Monsarrat and Kerley2018). Spotted hyaenas, in contrast, tend to be perceived negatively nearly everywhere (Dickman, Reference Dickman2010; Macdonald et al., Reference Macdonald, Burnham, Hinks, Dickman, Malhi and Macdonald2015). There are notable examples of local reverence, respect and tolerance for the species (Baynes-Rock, Reference Baynes-Rock2013). However, spotted hyaenas are commonly perceived in Western cultures as ugly, greedy, unintelligent scavengers and are almost exclusively absent from the scientific literature on charisma (Goldman et al., Reference Goldman, De Pinho and Perry2010; De Pinho et al., Reference De Pinho, Grilo, Boone, Galvin and Snodgrass2014; Mitchell et al., Reference Mitchell, Bruyere, Otieno, Bhalla and Teel2019). We infer that these narratives, and comparative lack of charisma, limit the ability of spotted hyaenas to draw financial support from Western institutions for sustained research-informed conservation.
Species such as the African lion, considered to be charismatic in the West (Albert et al., Reference Albert, Luque and Courchamp2018), also tend to be associated with complex social dynamics in their range countries (Inskip & Zimmermann, Reference Inskip and Zimmermann2009; Dickman, Reference Dickman2010; Goldman et al., Reference Goldman, De Pinho and Perry2013). These dynamics may be driven by factors such as the role of the species in traditional ceremonies, the relative socio-economic position of the local communities, or the political history of the region (Inskip & Zimmermann, Reference Inskip and Zimmermann2009; Dickman, Reference Dickman2010; Pooley et al., Reference Pooley, Barua, Beinart, Dickman, Holmes and Lorimer2017). The cultural implications of these factors influence the willingness of local people to participate in conservation actions (Pooley et al., Reference Pooley, Barua, Beinart, Dickman, Holmes and Lorimer2017; van Eeden et al., Reference van Eeden, Crowther, Dickman, Macdonald, Ripple, Ritchie and Newsome2018a,Reference van Eeden, Eklund, Miller, López-Bao, Chapron and Cejtinb). The social context surrounding depredating carnivores is also linked to the species' life history. The African lion, for instance, tends to select cattle over concurrently available smaller livestock such as sheep and goats (Holmern et al., Reference Holmern, Nyahongo and Røskaft2007; Kissui, Reference Kissui2008; Hemson et al., Reference Hemson, Maclennan, Mills, Johnson and Macdonald2009). As in many communities cattle carry higher economic and cultural value than other livestock types, preventing depredation by African lions is of particular importance in many parts of sub-Saharan Africa (Holmern et al., Reference Holmern, Nyahongo and Røskaft2007; Hemson et al., Reference Hemson, Maclennan, Mills, Johnson and Macdonald2009). Combined with high levels of charisma, this cultural context has probably resulted in species such as the African lion being prioritized as central species in human–carnivore conflict research, with less charismatic species, such as the spotted hyaena, under-emphasized. Not all reported measures of livestock depredation in the studies we reviewed were indicative of the financial or emotional impact of livestock loss, and therefore we do not contend that the African lion's prevalence in the literature is without merit. Nevertheless, we did find that taxonomic bias exists within the human–carnivore conflict literature.
This taxonomic bias has two primary consequences for the mitigation of livestock depredation, and therefore for the conservation of large carnivores in sub-Saharan Africa. Firstly, coexistence between people and large carnivores largely depends upon increasing the tolerance of local people for carnivores (Bruskotter & Wilson, Reference Bruskotter and Wilson2014; Treves & Bruskotter, Reference Treves and Bruskotter2014; Pooley et al., Reference Pooley, Barua, Beinart, Dickman, Holmes and Lorimer2017). Tolerance is informed by a complex combination of attitudes, behaviours and perceptions, all of which are informed by socio-cultural norms as well as political and economic trends (Goldman et al., Reference Goldman, De Pinho and Perry2013; Bruskotter & Wilson, Reference Bruskotter and Wilson2014; Treves & Bruskotter, Reference Treves and Bruskotter2014; Margulies & Karanth, Reference Margulies and Karanth2018; van Eeden et al., Reference van Eeden, Crowther, Dickman, Macdonald, Ripple, Ritchie and Newsome2018a,Reference van Eeden, Eklund, Miller, López-Bao, Chapron and Cejtinb). Importantly, tolerance of large carnivores is also strongly influenced by overall rates of livestock depredation (Kolowski & Holekamp, Reference Kolowski and Holekamp2006; Bruskotter & Wilson, Reference Bruskotter and Wilson2014; Treves & Bruskotter, Reference Treves and Bruskotter2014). Increased rates of livestock depredation can degrade human attitudes towards carnivores and increase the probability of retaliatory killing, even for unoffending species or individuals (Romañach et al., Reference Romañach, Lindsey and Woodroffe2007; Miller et al., Reference Miller, Jhala and Jena2016a,Reference Miller, Jhala and Schmitzb; Farhadinia et al., Reference Farhadinia, Johnson, Hunter and Macdonald2017). Spotted hyaenas, as the primary depredators of livestock across much of sub-Saharan Africa, may be eroding human tolerance of sympatric carnivore species. Yet, few studies emphasize livestock depredation by this species.
The second consequence of this bias is the restriction of the knowledge base upon which conservation efforts are built. Taxonomic biases result in a large amount of knowledge on a small number of species, limiting the development of broad theoretical insights (Clark & May, Reference Clark and May2002; Hortal et al., Reference Hortal, De Bello, Diniz-Filho, Lewinsohn, Lobo and Ladle2015; Rosenthal et al., Reference Rosenthal, Gertler, Hamilton, Prasad and Andrade2017). This is not to suggest that research centred on one carnivore species necessarily omits others during fundraising, data collection and analysis. It is possible that the studies we reviewed had comprehensive research-informed conservation programmes that equitably assessed depredation patterns of multiple carnivore species. However, our findings suggest that the resultant publications framed the issue of human–carnivore conflict around a small group of highly charismatic species. As one-third of the studies that identified spotted hyaenas as the primary depredator of livestock did not include them as a central species, it follows that conflict management recommendations derived from these studies are not emphasizing the impact of this species. Additionally, we suspect that conflict management recommendations as a whole are being framed around an understanding of livestock depredation by charismatic species, with recommendations for interventions derived from knowledge of the behavioural patterns of these species. The limited research on livestock depredation by spotted hyaenas indicates they exhibit patterns of depredation different from those of African lions and leopards (Ogada et al., Reference Ogada, Woodroffe, Oguge and Frank2003; Woodroffe et al., Reference Woodroffe, Frank, Lindsey, ole Ranah and Romañach2007; Kissui, Reference Kissui2008). Therefore, interventions built upon understandings of charismatic species may omit behaviours of more common depredators and consequently be limited in their ability to prevent livestock depredation.
The taxonomic bias that we detected in research on livestock depredation by carnivores in sub-Saharan Africa is consistent with other patterns observed in the conservation literature (Clark & May, Reference Clark and May2002; Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Troudet et al., Reference Troudet, Grandcolas, Blin, Vignes-Lebbe and Legendre2017; Tensen, Reference Tensen2018; Lozano et al., Reference Lozano, Olszańska, Morales-Reyes, Castro, Malo and Moleón2019). Conservation research tends to be biased towards vertebrates, with mammals and birds receiving a level of research effort disproportionate to their prevalence in nature and, in many cases, to their level of extinction risk (Clark & May, Reference Clark and May2002; Donaldson et al., Reference Donaldson, Burnett, Braun, Suski, Hinch, Cooke and Kerr2017; Davies et al., Reference Davies, Cowley, Bennie, Leyshon, Inger and Carter2018). These types of biases correlate with species charisma, resulting in the majority of research focusing on taxa that contain colourful, large, distinctive species (Bonnet et al., Reference Bonnet, Shine and Lourdais2002; Clark & May, Reference Clark and May2002; Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Donaldson et al., Reference Donaldson, Burnett, Braun, Suski, Hinch, Cooke and Kerr2017). For example, Brambilla et al. (Reference Brambilla, Gustin and Celada2013) found that more appealing bird species in Italy received significantly more research attention, and Fleming & Bateman (Reference Fleming and Bateman2016) reported that unattractive Australian mammals were underrepresented in the literature. The consequences of such biases have been examined in the fields of climate change mitigation (Feeley et al., Reference Feeley, Stroud and Perez2017), animal behaviour (Rosenthal et al., Reference Rosenthal, Gertler, Hamilton, Prasad and Andrade2017), species reintroductions (Seddon et al., Reference Seddon, Soorae and Launay2005) and conservation more broadly (Clark & May, Reference Clark and May2002; Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Stroud et al., Reference Stroud, Rehm, Ladd, Olivas and Feeley2014; Di Marco et al., Reference Di Marco, Chapman, Althor, Kearney and Watson2017). There is clear evidence that these biases limit the development of ecological theory and conservation management practices (Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Fleming & Bateman, Reference Fleming and Bateman2016). Thus, taxonomic bias is a potential driver of the research–implementation gap in conservation (Seddon et al., Reference Seddon, Soorae and Launay2005; Amori et al., Reference Amori, Gippoliti and Helgen2008; Martín-López et al., Reference Martín-López, Montes, Ramírez and Benayas2009; Troudet et al., Reference Troudet, Grandcolas, Blin, Vignes-Lebbe and Legendre2017).
Another important component of taxonomic bias relates to conservation funding, which tends to disproportionately support charismatic species (Stroud et al., Reference Stroud, Rehm, Ladd, Olivas and Feeley2014; Fleming & Bateman, Reference Fleming and Bateman2016; Di Marco et al., Reference Di Marco, Chapman, Althor, Kearney and Watson2017; Davies et al., Reference Davies, Cowley, Bennie, Leyshon, Inger and Carter2018; Curtin & Papworth, Reference Curtin and Papworth2020). Many of the largest conservation NGOs explicitly focus their funding efforts on charismatic species (Brockington & Scholfield, Reference Brockington and Scholfield2010a,Reference Brockington and Scholfieldb; Holmes et al., Reference Holmes, Scholfield and Brockington2012). Prioritization of funding in conservation is determined by both political agendas and social contexts (Martín-López et al., Reference Martín-López, Montes, Ramírez and Benayas2009; Stroud et al., Reference Stroud, Rehm, Ladd, Olivas and Feeley2014). Public interest in charismatic species motivates donations, which support further opportunities to study those same species (Davies et al., Reference Davies, Cowley, Bennie, Leyshon, Inger and Carter2018). Furthermore, as reviewers and researchers are implicitly biased towards articles that emphasize their own study organisms, the literature continues to highlight the same subset of charismatic species (Bonnet et al., Reference Bonnet, Shine and Lourdais2002; Wilson et al., Reference Wilson, Proches, Braschler, Dixon and Richardson2007; Martín-López et al., Reference Martín-López, Montes, Ramírez and Benayas2009; Rosenthal et al., Reference Rosenthal, Gertler, Hamilton, Prasad and Andrade2017). This bias is evident in carnivore conservation, with large felids consistently receiving more funding and research effort than other species (Davies et al., Reference Davies, Cowley, Bennie, Leyshon, Inger and Carter2018; Curtin & Papworth, Reference Curtin and Papworth2020), which is particularly notable in Africa (Di Marco et al., Reference Di Marco, Chapman, Althor, Kearney and Watson2017). For example, in 2017 the Leonardo DiCaprio Foundation announced a USD 1 million seed donation to establish the Lion Recovery Fund in collaboration with the Wildlife Conservation Network. This effort was subsequently supported by a variety of additional sponsors, including the Disney Conservation Fund. Within its first year, the fund distributed c. USD 2.4 million across 28 research and conservation projects centred on the African lion (Lion Recovery Fund Progress Report, Reference Lion Recovery Fund2018). Similarly, National Geographic's Big Cat Initiative had an open request for proposals, up to mid 2021, for research programmes examining lion conservation in 20 lion-specific priority areas. Through this initiative, up to USD 100,000 of support was awarded per project. These conservation funds are allocated across a geographical range where less charismatic spotted hyaenas co-occur and tend to be more problematic for livestock owners than African lions.
It is possible that the negative effects of taxonomic bias could be ameliorated by the flagship species concept, with conservation of co-occurring species aided by the focus of conservation attention on large, charismatic species (Andelman & Fagan, Reference Andelman and Fagan2000; Roberge & Angelstam, Reference Roberge and Angelstam2004; Smith et al., Reference Smith, Veríssimo, Isaac and Jones2012; Albert et al., Reference Albert, Luque and Courchamp2018). Flagship species tend to be large-bodied mammals that are often described as beautiful or impressive (Albert et al., Reference Albert, Luque and Courchamp2018). Conservation status can also contribute to species charisma (Martín-López et al., Reference Martín-López, Montes, Ramírez and Benayas2009; Albert et al., Reference Albert, Luque and Courchamp2018). Species at greater risk of extinction, particularly those that are charismatic, tend to motivate conservation engagement and fundraising (Courchamp et al., Reference Courchamp, Angulo, Rivalan, Hall, Signoret, Bull and Meinard2006; Smith et al., Reference Smith, Veríssimo, Isaac and Jones2012; Brambilla et al., Reference Brambilla, Gustin and Celada2013; Albert et al., Reference Albert, Luque and Courchamp2018). The spotted hyaena is categorized as Least Concern on the IUCN Red List and thus carries little power for motivating conservation engagement from the perspective of species rarity. However, the African lion and leopard are categorized as Vulnerable and the African wild dog as Endangered. As the populations of these three species continue to decline, their value as conservation flagships grows (Martín-López et al., Reference Martín-López, Montes, Ramírez and Benayas2009; Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014; Wolf & Ripple, Reference Wolf and Ripple2017). However, the extent to which the flagship species concept demonstrably supports the conservation of species other than the flagship is a source of debate (Andelman & Fagan, Reference Andelman and Fagan2000; Caro et al., Reference Caro, Engilis, Fitzherbert and Gardner2004).
Recent studies have indicated the benefits of strategic prioritization of charismatic species to further broad-scale biodiversity conservation (Smith et al., Reference Smith, Veríssimo, Isaac and Jones2012; Bennett et al., Reference Bennett, Maloney and Possingham2015; McGowan et al., Reference McGowan, Beaumont, Smith, Chauvenet, Harcourt and Atkinson2020). However, such an approach may have adverse effects on the development of practices intended to address human–carnivore conflict. We recognize that as our study focuses on large carnivores in sub-Saharan Africa, it could be considered to have its own bias. Nevertheless, we believe our critical assessment contributes to the literature on human–carnivore interactions by helping to improve the conservation impact of future research in this field. Our findings indicate that current patterns of research prioritization are resulting in a misalignment between the drivers of human–carnivore conflict and research on that topic. Consequently, conflict intervention practices founded upon that research may be limited in their ability to mitigate declines in large carnivore populations. Solutions for this global conservation challenge may be better served by alternate prioritization schemes that promote species-specific knowledge and more comprehensive understanding of the patterns of livestock depredation. We advocate increased incentivization of the study of livestock depredation by less charismatic carnivore species, including the spotted hyaena. This will facilitate the explicit examination of the effectiveness of conflict mitigation efforts.
The call for research on less charismatic species is often based upon the conservation status of those species, where their relative omission from the literature may be increasing their risk of extinction (Seddon et al., Reference Seddon, Soorae and Launay2005; Brambilla et al., Reference Brambilla, Gustin and Celada2013). Here, we provide evidence for an additional motive for addressing this bias, as the underrepresentation of spotted hyaenas is unlikely to put the species itself at risk of extinction. Instead, we show that in the case of livestock depredation, and subsequent human–carnivore conflict, this bias may be negatively impacting the conservation of other depredating species as well. As taxonomic bias is widespread in conservation, further examination is likely to reveal similar trends in other regions and fields of study. Our study suggests that increased examination of current patterns of funding and research effort is needed to bridge the existing gap between conservation priorities and conservation research.
Acknowledgements
We thank J.M. Beck for insightful comments. CFH is supported by the University Fellowship Program at Michigan State University, but this research received no specific grant from any funding agency, or commercial or not-for-profit sectors.
Author contributions
Analysis, writing: all authors.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards.






