Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Wiedenmann, Daniel
Keller, Jörg
and
Zaitsev, Anatoly N.
2010.
Melilite-group minerals at Oldoinyo Lengai, Tanzania.
Lithos,
Vol. 118,
Issue. 1-2,
p.
112.
Mattsson, Hannes B.
and
Reusser, Eric
2010.
Mineralogical and geochemical characterization of ashes from an early phase of the explosive September 2007 eruption of Oldoinyo Lengai (Tanzania).
Journal of African Earth Sciences,
Vol. 58,
Issue. 5,
p.
752.
Keller, Jörg
Klaudius, Jurgis
Kervyn, Matthieu
Ernst, Gerald G. J.
and
Mattsson, Hannes B.
2010.
Fundamental changes in the activity of the natrocarbonatite volcano Oldoinyo Lengai, Tanzania.
Bulletin of Volcanology,
Vol. 72,
Issue. 8,
p.
893.
Neukirchen, Florian
Finkenbein, Thomas
and
Keller, Jörg
2010.
The Lava sequence of the East African Rift escarpment in the Oldoinyo Lengai – Lake Natron sector, Tanzania.
Journal of African Earth Sciences,
Vol. 58,
Issue. 5,
p.
734.
Zaitsev, A. N.
Williams, C. T.
Britvin, S. N.
Kuznetsova, I. V.
Spratt, J.
Petrov, S. V.
and
Keller, J.
2010.
Kerimasite, Ca3Zr2(Fe23+Si)O12, a new garnet from carbonatites of Kerimasi volcano and surrounding explosion craters, northern Tanzania.
Mineralogical Magazine,
Vol. 74,
Issue. 5,
p.
803.
HAMADA, Maki
2011.
Sr-Na-bearing åkermanite and nepheline in nephelinite from Nagahama, Hamada, Shimane Prefecture, Southwest Japan.
Journal of Mineralogical and Petrological Sciences,
Vol. 106,
Issue. 4,
p.
187.
Andersen, Tom
Elburg, Marlina
and
Erambert, Muriel
2012.
Petrology of combeite- and götzenite-bearing nephelinite at Nyiragongo, Virunga Volcanic Province in the East African Rift.
Lithos,
Vol. 152,
Issue. ,
p.
105.
Zaitsev, A.N.
Marks, M.A.W.
Wenzel, T.
Spratt, J.
Sharygin, V.V.
Strekopytov, S.
and
Markl, G.
2012.
Mineralogy, geochemistry and petrology of the phonolitic to nephelinitic Sadiman volcano, Crater Highlands, Tanzania.
Lithos,
Vol. 152,
Issue. ,
p.
66.
Le Pioufle, Audrey
and
Canil, Dante
2012.
Iron in monticellite as an oxygen barometer for kimberlite magmas.
Contributions to Mineralogy and Petrology,
Vol. 163,
Issue. 6,
p.
1033.
Bosshard-Stadlin, Sonja A.
Mattsson, Hannes B.
and
Keller, Jörg
2014.
Magma mixing and forced exsolution of CO2 during the explosive 2007–2008 eruption of Oldoinyo Lengai (Tanzania).
Journal of Volcanology and Geothermal Research,
Vol. 285,
Issue. ,
p.
229.
Sekisova, V.S.
Sharygin, V.V.
Zaitsev, A.N.
and
Strekopytov, S.
2015.
Liquid immiscibility during crystallization of forsterite–phlogopite ijolites at Oldoinyo Lengai Volcano, Tanzania: study of melt inclusions.
Russian Geology and Geophysics,
Vol. 56,
Issue. 12,
p.
1717.
Santos, Rafael
Van Audenaerde, Aldo
Chiang, Yi
Iacobescu, Remus
Knops, Pol
and
Van Gerven, Tom
2015.
Nickel Extraction from Olivine: Effect of Carbonation Pre-Treatment.
Metals,
Vol. 5,
Issue. 3,
p.
1620.
Zaitsev, Anatoly N.
Spratt, John
Sharygin, Victor V.
Wenzel, Thomas
Zaitseva, Olga A.
and
Markl, Gregor
2015.
Mineralogy of the Laetolil Footprint Tuff: A comparison with possible volcanic sources from the Crater Highlands and Gregory Rift.
Journal of African Earth Sciences,
Vol. 111,
Issue. ,
p.
214.
Perova, E. N.
and
Zaitsev, A. N.
2017.
Thermodynamic Analysis of Secondary Minerals Stability in Altered Carbonatites of the Oldoinyo Lengai Volcano, Northern Tanzania.
Geology of Ore Deposits,
Vol. 59,
Issue. 7,
p.
584.
Ogorodova, Lyubov P.
Gritsenko, Yuliya D.
Vigasina, Marina F.
Bychkov, Andrey Y.
Ksenofontov, Dmitry A.
and
Melchakova, Lyubov V.
2018.
Thermodynamic properties of natural melilites.
American Mineralogist,
Vol. 103,
Issue. 12,
p.
1945.
Hawthorne, Frank C.
Uvarova, Yulia A.
and
Sokolova, Elena
2019.
A structure hierarchy for silicate minerals: sheet silicates.
Mineralogical Magazine,
Vol. 83,
Issue. 1,
p.
3.
Zaitsev, Anatoly N.
McHenry, Lindsay
Savchenok, Anton I.
Strekopytov, Stanislav
Spratt, John
Humphreys-Williams, Emma
Sharygin, Victor V.
Bogomolov, Evgeny S.
Chakhmouradian, Anton R.
Zaitseva, Olga A.
Arzamastsev, Andrei A.
Reguir, Ekaterina P.
Leach, Larissa
Leach, Michael
and
Mwankunda, Joshua
2019.
Stratigraphy, mineralogy and geochemistry of the Upper Laetolil tuffs including a new tuff 7 site with footprints of Australopithecus afarensis, Laetoli, Tanzania.
Journal of African Earth Sciences,
Vol. 158,
Issue. ,
p.
103561.
Minissale, Silvia
Zanetti, Alberto
Tedesco, Dario
Morra, Vincenzo
and
Melluso, Leone
2019.
The petrology and geochemistry of Nyiragongo lavas of 2002, 2016, 1977 and 2017 AD, and the trace element partitioning between melilitite glass and melilite, nepheline, leucite, clinopyroxene, apatite, olivine and Fe-Ti oxides: a unique scenario.
Lithos,
Vol. 332-333,
Issue. ,
p.
296.
Alam, Qadeer
Schollbach, Katrin
van Hoek, Corrie
van der Laan, Sieger
de Wolf, Tom
and
Brouwers, H.J.H.
2019.
In-depth mineralogical quantification of MSWI bottom ash phases and their association with potentially toxic elements.
Waste Management,
Vol. 87,
Issue. ,
p.
1.
Krzątała, Arkadiusz
Krüger, Biljana
Galuskina, Irina
Vapnik, Yevgeny
and
Galuskin, Evgeny
2020.
Walstromite, BaCa2(Si3O9), from Rankinite Paralava within Gehlenite Hornfels of the Hatrurim Basin, Negev Desert, Israel.
Minerals,
Vol. 10,
Issue. 5,
p.
407.
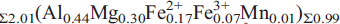 (Si1.99Al0.01O7). Alumoåkermanite is tetragonal, space group P421m with a = 7.7661(4) Å, c = 5.0297(4) Å, V = 303.4(1) Å3 and Z = 2. The five strongest powder-diffraction lines [d in Å, (I/Io), hkl] are: 3.712, (13), (111); 3.075, (25), (201); 2.859, (100), (211); 2.456, (32), (311); 1.757, (19), (312). Single-crystal structure refinement (R1 = 0.018) revealed structure topology typical of the melilite-group minerals, i.e. tetrahedral [(Al,Mg)(Si2O7)] sheets interleaved with layers of (CaNa) cations. The name reflects the chemical composition of the mineral.
(Si1.99Al0.01O7). Alumoåkermanite is tetragonal, space group P421m with a = 7.7661(4) Å, c = 5.0297(4) Å, V = 303.4(1) Å3 and Z = 2. The five strongest powder-diffraction lines [d in Å, (I/Io), hkl] are: 3.712, (13), (111); 3.075, (25), (201); 2.859, (100), (211); 2.456, (32), (311); 1.757, (19), (312). Single-crystal structure refinement (R1 = 0.018) revealed structure topology typical of the melilite-group minerals, i.e. tetrahedral [(Al,Mg)(Si2O7)] sheets interleaved with layers of (CaNa) cations. The name reflects the chemical composition of the mineral.