According to a recent position paper on nutrition and exercise, protein intake in the early recovery phase after exercise is recommended to help improve muscle protein synthesis(Reference Thomas, Erdman and Burke1). Although the protein-containing drinks after exercise are known to be useful for muscle recovery(Reference Jäger, Kerksick and Campbell2), post-exercise protein-containing ingestion has been reported to reduce energy intake compared with the carbohydrate-containing drinks(Reference Monteyne, Martin and Jackson3,Reference Rumbold, Shaw and James4) , but inconsistent results were also reported(Reference Clayton, Stensel and Watson5).
To date, three laboratory-based studies have examined the effects of protein-containing drinks ingestion on subsequent energy intake in various individuals(Reference Monteyne, Martin and Jackson3–Reference Clayton, Stensel and Watson5), with discrepancies in the results. Monteyne et al. and Rumbold et al. showed that consuming a protein-containing drink after exercise reduced energy intake compared with consuming a carbohydrate-containing drink(Reference Monteyne, Martin and Jackson3,Reference Rumbold, Shaw and James4) . Clayton et al. reported that consuming a protein-containing drink after exercise reduced energy intake compared with a placebo drink, but did not differ from the carbohydrate-containing drink(Reference Clayton, Stensel and Watson5). These studies vary in protocols including the amount of protein-containing drink ingested (i.e., 500–600 ml), the type of exercise (resistance exercise and aerobic exercise) and the type of protein-containing drink (whey protein isolate drink, skimmed milk and 6 % whey protein isolate drink). Also, only one study clearly reported the drink temperature used in the study (i.e. about 5°C)(Reference Rumbold, Shaw and James4). Although the reasons for these discrepancies among studies are unclear, the energy intake might be affected by the drink temperature. Although the effectiveness of internal thermal changes such as consuming cold drink after exercise on exercise performance and preventing dehydration has been addressed in previous studies(Reference Burdon, O’Connor and Gifford6), no studies have investigated the effects of ‘drink temperature’ after exercise on energy intake. The rate of gastric emptying and the magnitude of gastric motility (i.e. measured via cross-sectional pyloric antral area reflecting gastric distention, rate of gastric emptying and frequency of gastric contractions) are known as one of the factors of appetite regulation after exercise(Reference Horner, Schubert and Desbrow7) and are influenced by the temperatures of consumed energy-containing drinks(Reference Verhagen, Luijk and Samsom8,Reference Mishima, Amano and Takahashi9) . Indeed, Verhagen et al. showed that the frequency of gastric motility increased after a liquid meal at 37°C and 55°C compared with after a liquid meal at 4°C(Reference Verhagen, Luijk and Samsom8). Also, Mishima et al. showed that the rate of gastric emptying was faster after a liquid and solid meal at 60°C compared with after a meal at 37°C(Reference Mishima, Amano and Takahashi9). Furthermore, our previous study has demonstrated that pre-meal water ingestion at 2°C reduces subsequent energy intake compared with the water ingestion at 37°C and 60°C, and reduced energy intake after consuming cold water (i.e. at 2°C) ingestion is accompanied by a change in gastric contractions(Reference Fujihira, Hamada and Yanaoka10). Thus, the rationale behind the intention of the present study is based on these reported findings. Collectively, to our knowledge, no studies have examined the effects of different temperatures of a post-exercise protein-containing drink on gastric motility and subsequent energy intake(Reference Horner, Schubert and Desbrow7). Investigations in this area represent an important gap in current knowledge since adequate energy intake after exercise is critical in preventing low-energy availability conditions(Reference Holtzman and Ackerman11).
The purpose of this study was to investigate the effects of different temperatures of protein-containing drink after exercise on gastric motility and subsequent energy intake in healthy young men. We hypothesised that consuming a protein-containing drink at 60°C would increase gastric motility and subsequent energy intake compared with consuming a protein-containing drink at 2°C.
Experimental methods
Subjects
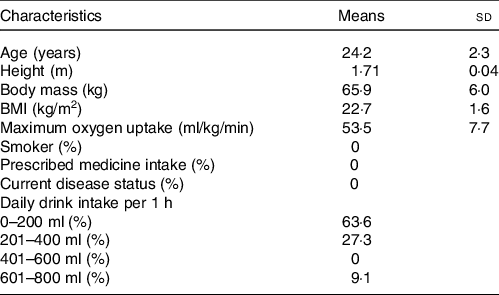
After approval from the Ethics Committee on Human Research of Waseda University (Approval number: 2019-016), twelve healthy young men gave written informed consent to participate in this study. This study was registered in advance with the University Hospital Medical Information Network Center, a system for registering clinical trials (ID: UMIN000036537). The physical and descriptive characteristics of the subjects are shown in Table 1. All subjects were non-smokers and were not taking any medicine, and their body masses had been stable for at least 3 months before the study.
Table 1. Characteristics of the subjects
(Mean values and standard deviations, n = 12)
Standardisation of diet and physical activity
In the 15 h before the first main trial, subjects were weighed and dietary intake was recorded. This dietary intake was replicated in the 15 h preceding the second and third main trials. Food diaries were analysed using software to determine energy intake and macronutrient content (Excel Eiyoukun version 5.0; Kenpakusha). In addition, the subjects were asked to remain inactive for 1 d before each main trial. Subjects wore a uniaxial accelerometer (Lifecoder-EX; Suzuken Co. Ltd) to monitor daily activity the day before each main trial. The accelerometer defined 11 activity intensity levels (0, 0·5 and 1–9) with 0 indicating the lowest intensity and 9 indicating the highest intensity. A level of 4 corresponds to an intensity of about 3 metabolic equivalents(Reference Kumahara, Schutz and Ayabe12). The total step count (steps/d) was recorded and calculated from the accelerometer using software (Lifelyzer 05 Coach; Suzuken Co. Ltd).
Study design
The subjects underwent three, 1-d laboratory-based trials in random order: (1) exercise (i.e. no preload), (2) exercise + cold drink at 2°C and (3) exercise + hot drink at 60°C. The interval between trials was at least 6 d. All subjects were asked to maintain their normal eating habits among the trials and to refrain from vigorous exercise and alcohol intake for 24 h before each trial. Before the main trials, each subject underwent the preliminary tests to determine their maximum oxygen uptake (VO2max) and maximum heart rate (HRmax). Two preliminary exercise tests were performed as follows: (1) submaximal treadmill running test and (2) maximum oxygen uptake treadmill running test. After these preliminary tests, the subjects were given at 6 d to recover from the exercise testing before the first main trial began (11 (sd 6) days – the range from 6 to 29 d).
Preliminary exercise tests
After treadmill familiarisation, the subjects performed two preliminary exercise tests on a treadmill (JOG NOW 700; Technogym). The first test involved a 16-min treadmill running test to establish the relationship between treadmill running speed and oxygen uptake. Participants completed four, 4-min incremental stages with a starting at a speed of 7 or 8 km/h and increasing by 1·5 km/h every 4 min. The treadmill set at 0 % incline throughout. After resting for 15 min, participants then completed a VO2max test. Maximum oxygen uptake was measured directly using an incremental uphill protocol at a constant speed until the subjects reached volitional exhaustion(Reference Taylor, Buskirk and Henschel13). The treadmill incline was initially set at 3·5 % and increased by 2·5 % every 3 min. Oxygen uptake, carbon dioxide production and respiratory exchange ratio were measured continuously throughout the tests using an online breath-by-breath gas analyser (Quark CPET; COSMED). Heart rate was measured continuously throughout the test using short range telemetry (Polar RCX3; Polar Electro). Ratings of perceived exertion were recorded at the end of each stage during both exercise tests using the Borg scale(Reference Borg14). Criteria used to confirm a maximum value included two or more of the following: (1) heart rate > 95% of age-predicted HRmax, (2) respiratory exchange value > 1·15, (3) a plateau in oxygen consumption and (4) ratings of perceived exertion ≥ 19.
Experimental protocol
On the day of each main trial day, subjects reported to the laboratory at 0850 after a 10-h overnight fast – subjects were allowed to drink only one glass of water no later than 2 h before each trial. Subjects ran on the treadmill for 30 min (09.00–09.30) at the running speed predicted to elicit 80% of HRmax in all trials. Running speed was adjusted by target HR. The ratings of perceived exertion was recorded at 5-min intervals during the tests using the Borg scale(Reference Borg14). Within 5 min after the running exercise (09.30–09.35), subjects consumed 300 ml of protein-containing drink (60·0 % energy as protein, 12·6% energy as fat and 11·6 % energy as carbohydrate; 0·34 MJ) at 2°C or 60°C in the exercise + cold drink and exercise + hot drink trials, respectively. The protein-containing drink contained 300 ml of water mixed with 21 g of protein powder composed of 2·9 g of carbohydrate, 15·0 g of protein and 1·2 g of fat (SAVAS whey protein 100; Meiji Co. Ltd). Temperature of protein-containing drink was measured using an electric thermometer (testo 106; Testo K.K.). A volume of 300 ml was chosen for the protein-containing drink because this volume was shown to delay gastric emptying in the previous study(Reference Kashima, Harada and Miyamoto15). In the exercise trial, subjects sat on a chair in a fixed position for the same amount of time as they consumed the designated drink (i.e. 5 min). Subjects then sat on a chair in a fixed position (i.e. the angle between the upper and lower part of the body was approximately 120°) in the laboratory for 30 min (09.35–10.05) in all trials. The subjects were then asked to consume the test meal from 10.05 and were instructed to eat as much as they feel satisfied until 11.05 in all trials. The 30-min interval between ingesting the protein-containing drink and subsequent meal was chosen because a previous study that has examined the effects of drink temperatures at 4°C, 37°C and 50°C on gastric motility over 60 min reported greater pyloric motility was observed in the 50°C drink than the 2°C drink in the first 30 min after consumption of the drinks(Reference Sun, Penagini and Hebbard16). A 2D ultrasound scan was performed to assess the change in the cross-sectional pyloric antral area and gastric contractions at 09.00, 09.35, 09.45, 09.55 and 10.05. Venous blood samples were collected by venepuncture at 09.00, 09.35, 10.05 and 10.35 with subjects placed in a seated position to analyse plasma glucose, plasma insulin, plasma acylated ghrelin, plasma peptide YY (PYY) and serum NEFA concentrations. Subjects also completed a 100-mm visual analogue scale using a paper-based questionnaire(Reference Flint, Raben and Blundell17), which assessed the subjective perceptions of appetite and feelings of stomach condition at 09.00, 09.35, 10.05 and 10.35.
Subjective appetite perceptions and energy intake
A prior written survey ensured the acceptability of the test meal; cereal (i.e. 9·7 % energy as protein, 20·5 % energy as fat and 69·8 % energy as carbohydrate) and milk (i.e., 19·8 % energy as protein, 51·1 % energy as fat and 29·1 % energy as carbohydrate) were selected as the test meal at 10.05. Subjects were instructed to eat using a small bowl until they felt comfortably full and satisfied, and that additional food was available if desired(Reference Ueda, Yoshikawa and Katsura18). The test meal was prepared in excess of expected consumption on the table so that the subjects do not need to ask experimenters for serving additional food and was served in a feeding booth in the laboratory to remove outside distractions. During the trials, the subjects and experimenters were instructed to refrain from talking about the food. Drinking water was prohibited while the subjects were consuming the test meal. The upper limit of meal intake time was set at 1 h, referring to a previous study that examined the effects of pre-meal water intake on energy intake(Reference Fujihira, Hamada and Yanaoka10), and mean time to consume the test meal in the exercise, exercise + cold drink and exercise + hot drink trials was 25·5 (sd 6·5), 23·1 (sd 4·5) and 15·5 (sd 4·3) min, respectively. The total amount of food intake (g) was ascertained by examining the weighted difference in the test meal remaining than that initially presented. The total energy intake from the test meal was calculated using manufacture-reported values. Subjects completed visual analogue scale(Reference Flint, Raben and Blundell17) at 09.00, 09.35, 10.05 and 10.35 to assess their perceptions of appetite (i.e. hunger, fullness and desire to eat sweet, sour, fatty and salty foods). Also, subjects completed another visual analogue scale at 09.00, 09.35, 10.05 and 10.35 to assess the perceptions of feelings of stomach condition (i.e., ‘Does your stomach feel uncomfortable?’, ‘Do you feel your stomach is expanding?’ and ‘Do you want to eat now?’). The verbal anchors ‘not at all’ and ‘extremely’ were placed at 0 and 100 mm on visual analogue scale, respectively.
Assessment of gastric motility and blood flow
Several previous studies suggest that the antrum is the most suitable area to evaluate the stomach capacity (for a review of this, see Van de Putte et al. (Reference Van De Putte and Perlas19)). Pyloric antral area measurements were performed using a 2D ultrasound machine (LOGIQ-e; GE Healthcare) and a 5·0 MHz sector transducer. All metals were removed from the surrounding area to avoid the possibility of interference during acquisition. To optimise precision, the transducer was positioned vertically to obtain a parasagittal image of the pyloric antrum, with the superior mesenteric vein and the abdominal aorta in a longitudinal section, as described previously(Reference Van De Putte and Perlas19). After obtaining these signals for measuring pyloric antral area for 1 min at 09.00, 09.35, 09.45, 09.55 and 10.05, the pyloric antral area (cm2) was determined using an image-editing software (ImageJ 1.47; National Institute of Mental Health). The gastric contractions of the pyloric antral area were defined as the frequency of contractions per 1 min.
The mean blood velocities (MBV) and vessel diameters of the coeliac artery (CA), which supplies blood flow to the stomach, liver and spleen, and superior mesenteric artery (SMA) that supplies to the entire small intestine, proximal portions of the colon and the pancreas were measured. Simultaneously pulsed and echo-Doppler ultrasound flowmetry (LOGIQ-e; GE Healthcare) was used to measure MBV in and vessel diameters of the CA and SMA, as described previously(Reference Hamada, Kashima and Hayashi20). A curved-array Doppler scan probe operated a pulse Doppler frequency of 3·3 MHz (LOGIQ-e; GE Healthcare). The Doppler beam insonation angle relative to the blood vessel was maintained at ≤60°. After obtaining these signals for the measurement of MBV for 1 min, a cross-sectional image of the vessel was recorded for 30 s. This process was performed at 09.00, 09.35, 09.45, 09.55 and 10.05. The HR and MBV were sampled at 20 kHz using an A/D converter (PowerLab 8/30; ADInstruments). The spectra of the MBV signals were analysed offline using our Doppler signal processing software, and beat-by-beat MBV values were calculated. MBV was determined by averaging the ten largest values per min to eliminate respiration-induced data variations. The images sent from the Doppler monitor were videotaped to enable later measurement of the pyloric antral area and vessel diameters using image-editing software (ImageJ 1.47; National Institute of Mental Health). The MBV of CA and SMA were calculated as π·r2·MBV × 60, where r is the radius of the artery. The same technician measured all gastric measurements (i.e. MBV of CA and SMA, pyloric antral area and gastric contractions) to minimise measurement errors. CV for gastric motility and blood flow were 0·5 % for pyloric antral area, 0·6 % for gastric contractions of the pyloric antral area, 0·6 % for MBV of CA and 0·6 % for MBV of SMA.
Biochemical analysis
For serum NEFA, venous blood samples were collected into tubes containing clotting activators for serum isolation. The collected samples were allowed to clot for 30 min at room temperature and then centrifuged at 1861 g for 10 min at 4°C. Serum was removed, divided into aliquots and stored at −80°C for later analysis. For plasma glucose, insulin and PYY measurements, venous blood samples were collected into sodium fluoride-EDTA tubes. The tubes were immediately centrifuged and treated as above. For plasma acylated ghrelin, venous blood samples were collected into EDTA tubes containing aprotinin to prevent degradation of ghrelin by protease. The tube was immediately centrifuged, and 100 μl of plasma was removed and was transferred into a tube containing 10 μl of hydrochloric acid. The sample was stored at −80°C for later analysis. Enzymatic, colorimetric assays were used to measure serum NEFA (NEFAHR; Wako Pure Chemical Industries, Ltd) and plasma glucose (GLU-HK(M); Shino-Test Corporation). ELISA was used to measure plasma acylated ghrelin (N750; SCETI K.K) and PYY (YK080; Yanaihara Institute Inc.).
Statistical analysis
The sample size was estimated with G*Power 3.1(Reference Faul, Erdfelder and Lang21), using the data from a previous cross-over study that investigated the effects of pre-meal fluid intake on energy intake(Reference Fujihira, Hamada and Yanaoka10). A sample size of ≥5 subjects was required to detect energy intake with a power of 80 % and an α level of 5 %. However, gastric motility varies among individuals(Reference Bateman22), and the magnitude changes in gastric motility influence subsequent energy intake(Reference Fujihira, Hamada and Yanaoka10). Therefore, we set our sample size to 12 based on our previous study that examined the changes in pre-meal water intake and gastric motility(Reference Fujihira, Hamada and Suzuki23). Data were analysed using the Predictive Analytics Software (PASW) version 23.0 for Windows (IBM SPSS Statistics 23.0; SPSS Japan Inc.). The Shapiro–Wilk test was used to check for the normality of distribution; all parameters were normally distributed. Repeated-measures one-factor ANOVA was used to assess differences among the three trials in energy intake and the length of meal. Repeated-measures, two-factor ANOVA was used to examine differences over time among the three trials in the cross-sectional pyloric antral area, frequency of gastric contractions, splanchnic blood flow, subjective appetite perceptions (i.e. hunger, fullness and desire to eat sweet, sour, fatty and salty foods) and subjective perception of the stomach. Where significant trial–time interactions and trial effects were found, the values were subsequently analysed with post hoc analysis for multiple comparisons using the Bonferroni method. The correlation coefficients were determined by using Pearson’s product-moment tests between the frequency of gastric contractions and energy intake. The 95 % CI for the mean absolute pairwise differences among the three trials were calculated using the t-distribution and degrees of freedom (n − 1). Effect sizes (ES) (Cohen’s d) were calculated to describe the magnitude of difference between trials. Effect sizes of 0·2 are considered the minimum important difference in all outcome measures, 0·5 moderate and 0·8 large(Reference Cohen24). Data were expressed as means and standard deviations. Statistical significance was set at P < 0·05.
Results
Dietary and physical activity data
The mean energy intake 15 h before each trial was 3·4 (sd 1·3) MJ. Subjects weighed and recorded all dietary intakes in the 15 h before the first trial, and these dietary intakes were subsequently replicated in the 15 h preceding the second and third trials. The food diary analyses of food diaries revealed that energy intake was the same in the 15 h preceding each trial. The energy intake equated to 36 (sd 24) % (36·7 (sd 30·7) g/d) from fat, 41 (sd 24) % (99·7 (sd 40·8) g/d) from carbohydrates and 14 (sd 6) % (30·8 (sd 15·0) g/d) from protein. The step counts, frequencies for light (levels 1–3) physical activity, moderate (levels 4–6) physical activity and vigorous (levels 7–9) physical activity recorded the day before each trial did not differ significantly among the trials (Table 2).
Table 2. Pre-trial physical activity, anthropometric, biochemical and perception of appetite and stomach values†
(Mean values and standard deviations, n = 12)
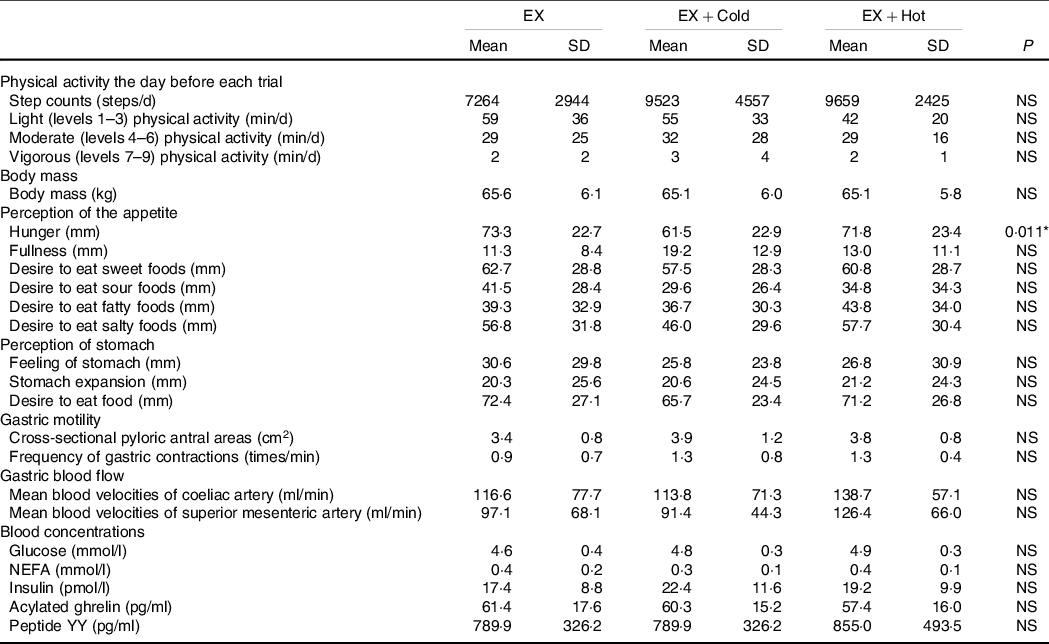
EX, running for 30 min at 80 % of maximum heart rate; Cold, 300 ml of protein-containing drink intake at 2°C; Hot, 300 ml of protein-containing drink intake at 60°C.
* No significant differences between trials (P > 0·05).
† Data were analysed using one-factor ANOVA.
Pre-trial
There were no significant differences in body mass among the exercise, exercise + cold drink and exercise + hot drink trials at 09.00 (i.e. pre-trial). At pre-trial, subjective appetite perceptions (i.e. fullness and desire to eat sweet, sour, fatty and salty foods) and perception of the stomach did not differ among the trials. Subjective appetite perception of hunger at the pre-trial differed among the trials. Post hoc analyses revealed no significant differences between trials (P > 0·05). Cross-sectional pyloric antral areas and frequency of gastric contractions were also not different at 09.00 (i.e. pre-trial) among the trials. MBV of CA and SMA were also not different at 09.00 (i.e. pre-trial) among the trials. There were no significant differences in glucose, NEFA, insulin, acylated ghrelin and PYY concentrations at the pre-trial among the trials. The body mass, fasting values for perception of the appetite and stomach, cross-sectional pyloric antral areas, frequency of gastric contractions, MBV of CA and SMA, glucose, NEFA, insulin, acylated ghrelin and PYY are shown in Table 2.
Exercise responses
There were no differences in mean HR (161 (sd 12) v. 161 (sd 15) v. 162 (sd 14) bpm for the exercise, exercise + cold drink and exercise + hot drink trials, respectively, ES = 0·007, P > 0·05) and ratings of perceived exertion (14 (sd 2) v. 14 (sd 2) v. 14 (sd 2) for the exercise, exercise + cold drink and exercise + hot drink trials, respectively, ES = 0·049, P > 0·05) during the treadmill running among the trials.
Energy intake
Energy intake differed among the trials (6·5 (sd 2·1) MJ v. 6·1 (sd 2·1) MJ v. 7·5 (sd 2·5) MJ for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; main effect of trial, ES = 0·501, P < 0·001). Post hoc tests revealed that energy intake in the exercise + hot drink trial was 14 % and 15 % higher than the exercise (P = 0·046, 95 % CI 4·010, 482·538) and exercise + cold drink (P = 0·001, 95 % CI 160·089, 517·111) trials, respectively (Fig. 1). The amount of cereal (282·2 (sd 87·8) g v. 269·3 (sd 95·7) g v. 338·2 (sd 133·4) g for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; main effect of trial, ES = 0·462, P = 0·001) consumed also differed among trials. The amount of milk consumed did not differ among trials (694·6 (sd 317·5) ml v. 627·1 (sd 256·2) ml v. 739·1 (sd 264·4) ml for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; main effect of trial, ES = 0·201, P > 0·05). There was no difference in the average time taken to feel comfortably full (25·5 (sd 6·5) min, 23·1 (sd 4·5) min and 23·9 (sd 6·1) min for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; ES = 0·162, P > 0·05) among the trials.
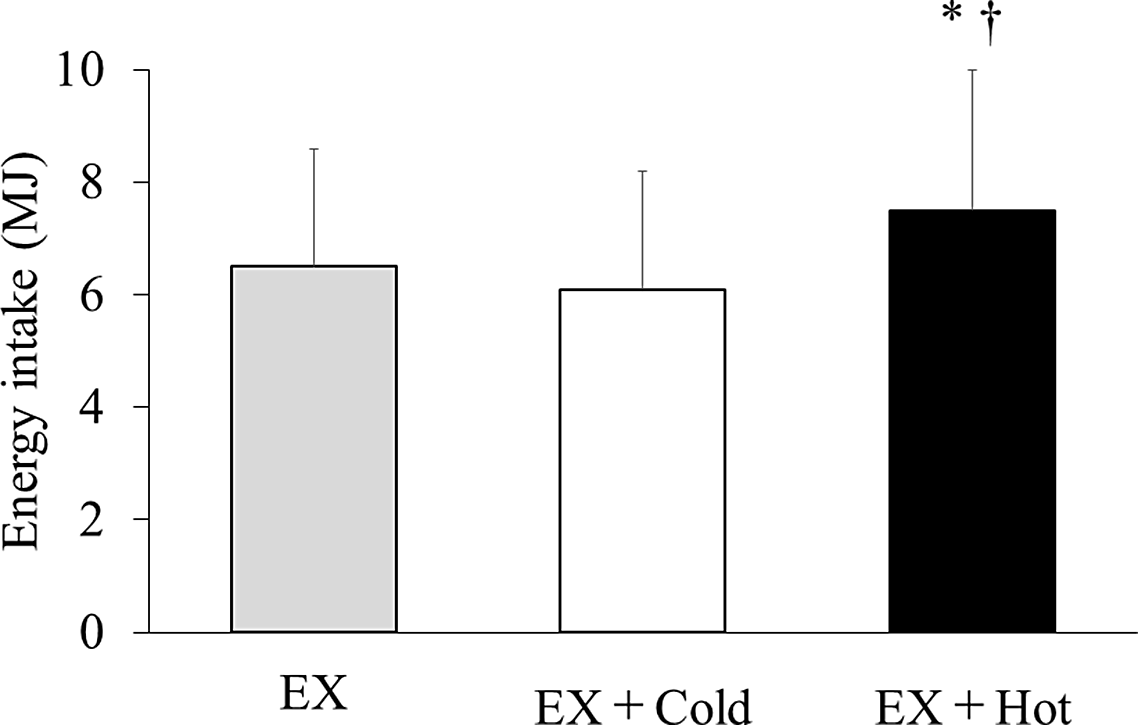
Fig. 1. Energy intake at test meal. Data are mean ± SD. Mean was compared using one-factor ANOVA for the main effect of trial followed by a multiple comparison test using the Bonferroni method. EX, running for 30 min at 80 % of maximum heart rate; Cold, 300 ml of protein-containing drink intake at 2°C; Hot, 300 ml of protein-containing drink intake at 60°C. *Significantly different between the EX and EX + Hot trials (p < 0·05). †Significantly different between the EX + Cold and EX + Hot trials (p < 0·05).
Subjective appetite perceptions
There were no significant differences in subjective appetite perceptions (i.e. hunger, fullness and desire to eat sweet, sour, fatty and salty foods) or perceptions of the stomach (i.e. ‘Dose your stomach feel uncomfortable?’, ‘Do you feel your stomach is expanding?’ and ‘Do you want to eat now?’) among the trials (P > 0·05).
Gastric antral area and gastric motility
For the cross-sectional pyloric antral areas, there were trial–time interactions (ES = 0·372, P = 0·011). Cross-sectional pyloric antral areas increased in the exercise + cold drink and exercise + hot drink trials compared with the exercise trial at 09.35 (i.e. after exercise) (4·3 (sd 2·1) cm2 v. 7·9 (sd 3·5) cm2 v. 9·8 (sd 3·7) cm2 for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; exercise v. exercise + cold drink: P = 0·024, 95 % CI 0·804, 8·431, exercise hot v. exercise + hot drink: P = 0·018, 95 % CI 1·148, 9·205), 09.45 (i.e. 15 min after exercise) (4·4 (sd 2·1) cm2 v. 9·0 (sd 2·3) cm2 v. 9·0 (sd 3·2) cm2 for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; exercise v. exercise + cold drink: P = 0·001, 95 % CI 3·350, 7·436, exercise v. exercise + hot drink: P = 0·004, 95 % CI 0·146, 8·361) and 09.55 (i.e. 25 min after exercise) (4·0 (sd 1·6) cm2 v. 8·0 (sd 1·4) cm2 v. 7·2 (sd 2·1) cm2 for the exercise, exercise + cold drink and exercise + hot drink trials, respectively; exercise v. exercise + cold drink: P = 0·003, 95 % CI 2·133, 6·447, exercise v. exercise + hot drink: P = 0·042, 95 % CI 0·136, 5·676) (Fig. 2). The frequency of gastric contractions differed significantly among the trials (main effect of trial, ES = 0·710, P = 0·001). Post hoc analyses indicated that the frequency of gastric contractions in the exercise + hot drink trial was higher than the exercise (P = 0·023, 95 % CI 0·176, 1·938) and exercise + cold drink (P = 0·007, 95 % CI 0·370, 1·801) trials (Fig. 3).
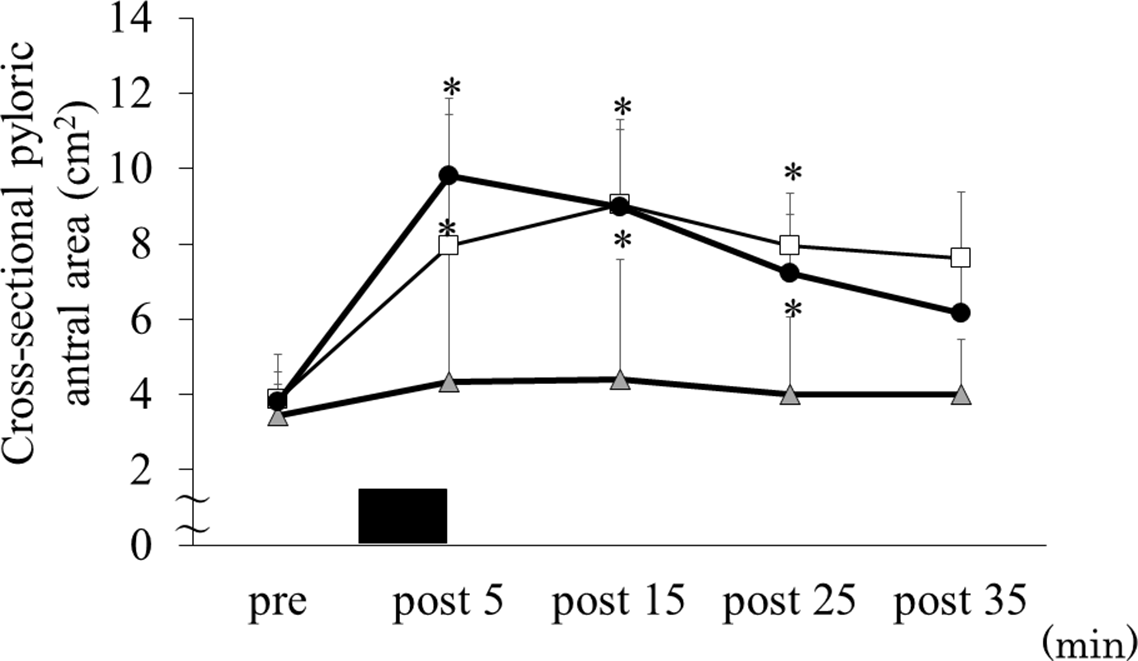
Fig. 2. Cross-sectional pyloric antral area before and after exercise. Data are mean ± SD. Black rectangle indicates consuming 300 ml of protein-containing drink in 5 min. Data were analysed using two-factor ANOVA followed by a multiple comparison test using the Bonferroni method. EX, running for 30 min at 80 % of maximum heart rate; Cold, 300 ml of protein-containing drink intake at 2°C; Hot, 300 ml of protein-containing drink intake at 60°C. There was a significant trial–time interaction (p = 0·011). *Significantly different from EX trial (p < 0·05). ![]() , EX;
, EX; ![]() , EX + Cold;
, EX + Cold; ![]() , EX + Hot.
, EX + Hot.
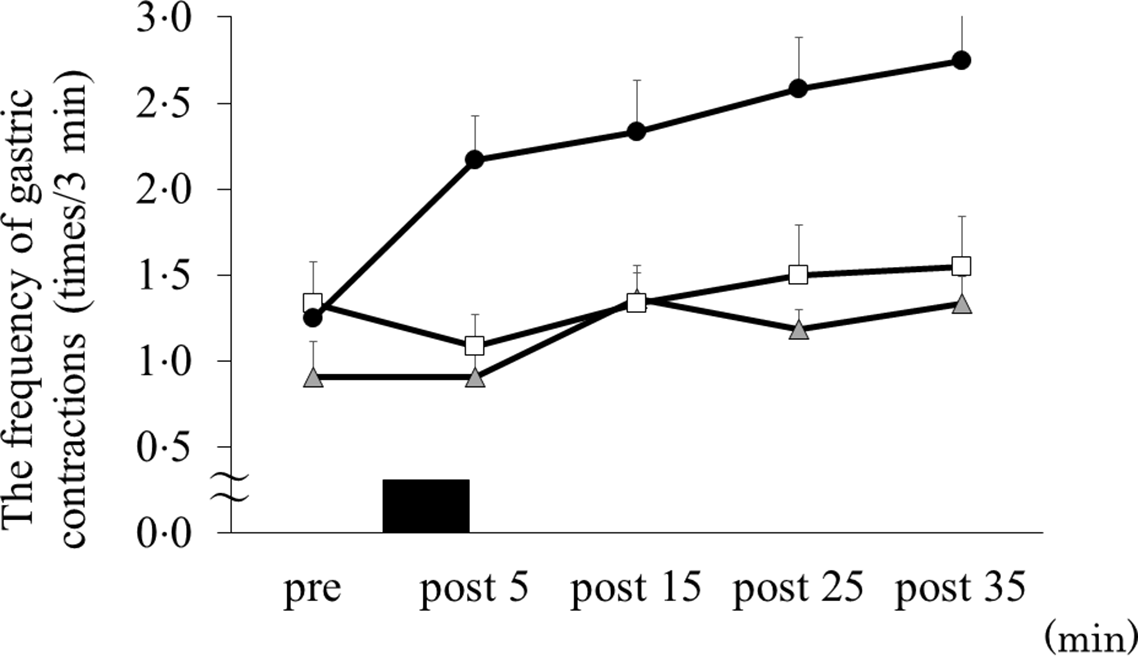
Fig. 3. The frequency of gastric contractions before and after exercise. Data are mean ± SD. Black rectangle indicates consuming 300 ml of protein-containing drink in 5 min. Data were analysed using two-factor ANOVA followed by a multiple comparison test using the Bonferroni method. EX, running for 30 min at 80 % of maximum heart rate; Cold, 300 ml of protein-containing drink intake at 2°C; Hot, 300 ml of protein-containing drink intake at 60°C. There was a significant main effect of trial (p = 0·001). ![]() , EX;
, EX; ![]() , EX + Cold;
, EX + Cold; ![]() , EX + Hot.
, EX + Hot.
Association between energy intake and frequency of the gastric contractions
There was a positive relationship between energy intake from the test meal and the total frequency of gastric contractions when the data from all trials were pooled (i.e. exercise, exercise + cold drink and exercise + hot drink trials) (r = 0·386, P = 0·022).
Splanchnic blood flow
There was no main effect of trial (ES < 0·001, P > 0·05), time (ES = 0·048, P > 0·05) and trial–time interaction (ES = 0·192, P > 0·05) for blood flow in the CA. There were no main effects of trial (ES = 0·315, P > 0·05), time (ES = 0·474, P > 0·05) and trial–time interaction (ES = 0·389, P > 0·05) for blood flow in the SMA.
Blood concentrations
The 2-h incremental AUC values for glucose, NEFA, insulin, acylated ghrelin and PYY are shown in Table 3. For the glucose, there was a main effect of time (ES = 0·345, P = 0·003). Post hoc analysis indicated that glucose increased after exercise (P < 0·05). For the NEFA, there was a main effect of time (ES = 0·885, P < 0·001) and trial–time interactions (ES = 0·338, P < 0·001). Post hoc analysis revealed that NEFA did not differ between trials at all time points (P > 0·05). For the insulin, there was a main effect of trial (ES = 0·338, P = 0·016). Post hoc analysis indicated that insulin tended to be higher in the exercise + cold drink (P = 0·062, 95 % CI –0·257, 11·590) and exercise + hot drink (P = 0·079, 95 % CI –0·867, 17·822) trials than the exercise trial. There were no between-trials differences for the acylated ghrelin. For the PYY, there was a main effect of time (ES = 0·865, P < 0·001). Post hoc analysis revealed that PYY increased after exercise and the test meal.
Table 3 Incremental AUC values of blood measurements over 2 h†
(Mean values and standard deviations, n = 12)

EX, running for 30 min at 80 % of maximum heart rate; Cold, 300 ml of protein-containing drink intake at 2°C; Hot, 300 ml of protein-containing drink intake at 60°C.
* Different from the EX trial (P < 0·05).
† Data were analysed using one-factor ANOVA followed by a multiple comparison test using the Bonferroni method.
Discussion
To our knowledge, the present study is the first to investigate how different temperatures of protein-containing drink after exercise influence gastric motility and subsequent energy intake in healthy men. The present study demonstrated that consuming 300 ml of protein-containing drink at 60°C after 80 % of HRmax treadmill running increased subsequent ad libitum energy intake by 14 % and 19 % compared with no preload and consuming 300 ml of protein-containing drink at 2°C, respectively. The present study also showed that increased energy intake after consuming protein-containing drink at 60°C was accompanied by changes in gastric contractions. These findings add new knowledge to the literature, demonstrating that drink temperature may play an important role in modulating post-exercise gastric motility and subsequent energy intake. The inability to replenish the energy consumed by exercise from the diet after exercise can cause negative physical consequences, including delayed physical recovery. Our study provides useful information for improving appetite and can aid in proper energy replenishment after exercise.
Three laboratory-based studies have examined the effects of protein-containing drink ingestion on subsequent energy intake(Reference Monteyne, Martin and Jackson3–Reference Clayton, Stensel and Watson5), with discrepancies in the results. Although three available studies examined the effect of post-exercise protein-containing drink ingestion on energy intake in humans(Reference Monteyne, Martin and Jackson3–Reference Clayton, Stensel and Watson5), only one study has specified the temperature of water used in the study (i.e. about 5°C)(Reference Rumbold, Shaw and James4). Alternatively, given the well-documented slower rate of gastric emptying and lower energy intake at cold water (i.e. at 2°C) compared with warm water (i.e. at 37°C and 60°C)(Reference Fujihira, Hamada and Yanaoka10), the temperature of ingested protein-containing drink may be the proposed reason for the discrepant findings among the studies. In addition, oral sensations such as taste related to appetite(Reference Yin, Hewson and Linforth25) are known to be affected by meal temperature, especially sweetness which increases in intensity with increasing temperature(Reference Green and Marriott26). Although the present study did not measure the effects of different temperatures of protein-containing drinks on oral sensation, it may explain the differences in energy intake caused by drink temperatures.
Changes in gastric motility might be the most plausible reason why the different temperatures of protein-containing drink affected ad libitum energy intake. Gastric motility is known as a key mediator of appetite control(Reference Janssen, Vanden Berghe and Verschueren27). Several previous studies have shown that gastric emptying and gastric motility can influence exercise-induced energy intake changes before and during exercise(Reference Horner, Schubert and Desbrow7). The present study demonstrated that the gastric contraction increased after consuming a 300 ml of protein-containing drink at 60°C compared with other trials. There was a positive relationship between energy intake from the test meal and the frequency of the total gastric contractions. Moreover, our previous study demonstrated that consuming 500 ml of water at 37°C and 60°C increased subsequent energy intake compared with 500 ml of water at 2°C. Increased energy intake after consuming water at 37°C and 60°C was accompanied by a change in gastric contractions(Reference Fujihira, Hamada and Yanaoka10). The temperature of a drink is known to be one of the major factors affecting gastric motility(Reference Verhagen, Luijk and Samsom8,Reference Sun, Penagini and Hebbard16) . Two laboratory-based studies that investigated the effects of hot drink intake on gastric motility measured by electrogastrogram have demonstrated increased gastric contraction after consuming a hot drink (50–55°C) compared with consuming cold drink or warm drink (4–37°C)(Reference Verhagen, Luijk and Samsom8,Reference Sun, Penagini and Hebbard16) . Although we measured gastric motility via an ultrasound imaging system instead of an electrogastrogram in the present study, our findings were similar to these previous studies(Reference Verhagen, Luijk and Samsom8,Reference Sun, Penagini and Hebbard16) . Peristaltic motions of the stomach propagate due to induction by slow waves from the cardia to pyloric antral area and controlled gastric motility. Nakamura et al. collected the smooth muscle from guinea pigs and examined peristalsis changes with elevated temperature(Reference Nakamura, Kito and Hashitani28). This study indicated that elevated the temperature from 24°C to 42°C increased the frequency and maximum rate of rising of the upstroke phase of slow waves(Reference Nakamura, Kito and Hashitani28). Sun et al. reported that the temperature in the stomach reached 43°C after consuming 400 ml of orange juice at 50°C(Reference Sun, Houghton and Read29). Based on these findings, a similar gastric temperature can be expected after consuming 300 ml of protein-containing drink at 60°C in the present study. These results suggest that increased gastric motility might be caused by elevated gastric temperatures. Although the rate of gastric emptying is influenced by the fluid temperature(Reference Mishima, Amano and Takahashi9,Reference Bateman22,Reference Sun, Houghton and Read29) and affects the subjective feeling of hunger(Reference Bergmann, Chassany and Petit30), the cross-sectional pyloric antral area, an indicator of gastric emptying, did not change between the protein-containing drink at 60°C and 2°C in the present study. These discrepancies observed between the present study and previous studies(Reference Mishima, Amano and Takahashi9,Reference Bateman22,Reference Sun, Houghton and Read29) may be explained by the differences in the measurement condition (i.e. exercise or rest). Exercise is one of the factors that leads to delayed gastric emptying(Reference Horner, Schubert and Desbrow7), and immediate post-exercise carbohydrate-protein drink ingestion delays gastric emptying compared with ingestion 30 min after exercise(Reference Kashima, Harada and Miyamoto15). Therefore, gastric emptying is temporarily delayed after exercise(Reference Kashima, Harada and Miyamoto15), and gastric emptying may be less affected by the temperature of drink after exercise. Another reason for the discrepant findings may be explained by the effects of sensory perceptions of the drinks. Although the macronutrients of drink (i.e. protein, fat and carbohydrate) are less effective for gastric emptying(Reference Goetze, Steingoetter and Menne31), the differences in oral sensation, such as thickness and creaminess, due to differences in nutrients, affect gastric emptying rate(Reference Monteyne, Martin and Jackson3). In the present study, the thickness and creaminess of protein-containing drinks may have contributed to gastric emptying differences due to temperature. Future studies should examine the effect of the differences in oral sensations due to drink temperature on gastric emptying.
Several possible mechanisms explain how gastric contraction affects energy intake. First, appetite-regulating hormones secreted from the gastrointestinal tract might affect gastric contraction and/or energy intake(Reference Guyton and Hall32). However, in the present study, the trials did not differ in the concentrations of acylated ghrelin, a hormone that increases gastric contraction and enhances energy intake, and PYY which reduces gastric contraction and suppresses energy intake. Second, gastrointestinal blood flow, which is delivering appetite-regulating hormones, has been reported to decrease after high-intensity exercise and has been implicated as a contributing factor to anorexia after exercise(Reference Hazell, Islam and Townsend33). However, after 30 min of running at 80 % of HRmax conducted in the present study, the blood flow in the SMA and CA, which are represented by gastrointestinal blood flow, did not change. Also, the gastrointestinal blood flow was not affected by the different temperatures of the consumed drink after exercise. These results suggest that gastrointestinal blood flow has little effect on the increase in energy intake from hot protein-containing drinks ingested after exercise. Collectively, our findings suggest that increased mechanical gastric contractions other than appetite-regulating hormones and gastrointestinal blood flow may play an important role in increased energy intake.
The present study has several strengths. We examined the effects of different temperatures of protein-containing drinks on both gastric motility and energy intake. The macronutrient content of drinks, ambient temperature and the amount of water taken during exercise are often considered factors for the influence of exercise on energy intake(Reference Clayton, Stensel and Watson5,Reference Horner, Schubert and Desbrow7,Reference Halse, Wallman and Guelfi34,Reference Kojima, Kasai and Kondo35) . To our knowledge, the present study is the first to examine the effects of different temperatures of protein-containing drinks after exercise on subsequent energy intake. Moreover, we have tried to address the role of gastric motility, a potential mechanism underpinning the modulation of energy intake, in energy intake after exercise. Therefore, the present findings may provide important insight into the role that the drink temperature plays in modulating energy intake after exercise. Although future research is needed to examine the effects of palatability, easiness and taste which are associated with drink temperature on feelings of appetite and energy intake in order to evaluate the practical relevance of warm drink in daily exercise, the results of the present study indicate that hot meals such as hot drinks and hot soups may be useful as one of the methods of post-exercise nutrient ingestion to enhance appetite. However, the present study has several limitations. First, energy intake, appetite-regulating hormones, gastric motility and gastrointestinal blood flow were assessed for lasting approximately 2 h. These variables may need to be assessed over a more extended period to detect the effects of different temperatures of protein-containing drink after exercise, if any, on appetite. Second, we did not provide water to subjects during the meal to avoid stomach distention caused by water intake, and this lack of water might affect energy intake after exercise(Reference Pérez-Luco, Díaz-Castro and Jorquera36). Another limitation is the generalisability of our study since this study examined the acute effect of the pre-meal drink volume on gastric motility and energy intake in healthy individuals. Further studies should examine the chronic effects in various populations, including different age groups and health conditions.
In conclusion, consuming 300 ml of protein-containing drink at 60°C after 80 % of HRmax treadmill running increased gastric contractions and ad libitum energy intake compared with no preload and an identical drink at 2°C after 80 % of HRmax treadmill running in healthy young men. Therefore, the post-exercise consumption of hot drinks is useful for post-exercise appetite enhancement.
Acknowledgements
This study was supported by the Japan Society of Promotion for Science (grant number 18J12735) and the Human Performance Laboratory, Organization for University Research Initiatives, Waseda University (grant number 2019-8). We wish to express our gratitude to all individuals who participated in our study.
K. F. supervised the data collection, performed the data analysis and interpretation and wrote the manuscript. Y. H. and M. H. assisted with all aspects of the data collection. K. S. advised the data analysis and interpretation to K. F. and revised the manuscript. M. M. conceived the study, advised the data analysis and interpretation to K. F. and revised the manuscript. All authors approved the final version of the manuscript.
All authors declare that there is no conflict of interest.









