Introduction
In 2020, 4,006 ha of strawberry [Fragaria × ananassa (Weston) Duchesne ex Rozier ssp. ananassa] were grown in Florida, with 88% of the crop grown in Hillsborough County (USDA-NASS 2021). Florida ranks as the second-largest strawberry producer in the United States, with an estimated economic value of $240 million (USDA-NASS 2021). In Florida, strawberries are grown through the winter months, with the season lasting from late September to April. Beds are formed, fumigated, and covered with polyethylene mulch in August or September, with most of the crop transplanted in September. Berries are typically harvested from December through late March.
Weed management can be a serious issue for strawberry growers throughout the state. Growers utilize a variety of management tools such as cover crops, fumigants, mulches, and herbicides. The use of polyethylene mulches enhances fumigant efficacy but also provides excellent control of grass and broadleaf weeds on the bed, except where the plastic is punctured for transplant (Schonbeck Reference Schonbeck1999). Weeds that emerge in the planting holes can be managed with a variety of preemergence herbicides, such as flumioxazin, oxyfluorfen, or napropamide, that are applied to the bed top immediately before the beds are covered with polyethylene mulch (Boyd et al. Reference Boyd, Sharpe and Kanissery2021; Boyd and Reed Reference Boyd and Reed2016; Daugovish et al. Reference Daugovish, Fennimore and Mochizuki2008; Stall et al. Reference Stall, Hochmuth, Gilreath and Crocker1995). Purple (Cyperus rotundus L.) and yellow (Cyperus esculentus L.) nutsedge are the only species that can not only emerge in planting holes but also pierce the polyethylene mulch. Preemergence herbicides can suppress Cyperus spp., but fumigants are generally the most effective management option.
Management of Cyperus spp., grass, and broadleaf weeds with preemergence herbicides tends to be inconsistent (Boyd and Reed Reference Boyd and Reed2016; Khatri et al. Reference Khatri, Peres, Noling and Boyd2020), and if adequate control is not achieved with preemergence herbicides, there are very few postemergence herbicides available (Manning and Fennimore Reference Manning and Fennimore2001). Clopyralid, clethodim, and sethoxydim are the only postemergence herbicides registered for banded applications over the bed in Florida. Clopyralid has activity on select species such as black medic (Medicago lupulina L.) and Carolina geranium (Geranium carolinianum L.) (Sharpe et al. Reference Sharpe, Boyd and Dittmar2016) but does not control Cyperus spp., grass, or many other broadleaf species. A combination of preemergence and postemergence herbicides are typically recommended for season-long Cyperus spp. control in Florida crops (Yu et al. Reference Yu, Sharpe and Boyd2020), but this is not an option in strawberry due to a lack of registered postemergence herbicides with activity on Cyperus spp.
Weeds also emerge between the raised, plastic-covered beds (row middles), where they are typically controlled with a combination of preemergence and postemergence herbicides. Weeds in row middles can reduce crop yields, interfere with harvest operations, hinder plastic removal, and host crop pests. Row-middle herbicide trials have been conducted in vegetable crops in Florida (Boyd Reference Boyd2016; Sharpe and Boyd Reference Sharpe and Boyd2019b), but to the authors’ knowledge there are no published reports on row-middle weed management in strawberry, and limited information exists on current practices.
Weed surveys have been used in a variety of crops and environments to identify the weed flora present (McCully et al. Reference McCully, Samson and Watson1991; Thomas Reference Thomas1985; Webster Reference Webster2010), enhance our understanding of critical issues such as herbicide resistance (Boutsalis et al. Reference Boutsalis, Gill and Preston2012), and facilitate improved management practices (Osten et al. Reference Osten, Walker, Storrie, Widderick, Moylan, Robinson and Galea2007). A weed survey can provide a snapshot of the flora present within a defined region or crop and highlight areas where growers should focus management efforts. To the authors’ knowledge, no weed survey has ever been conducted in Florida strawberry fields. Surveys conducted by the Weed Science Society of America (WSSA) have reported that M. lupulina, G. carolinianum, dogfennel [Eupatorium capillifolium (Lam.) Small], goosegrass [Eleusine indica (L) Gaertn.], and common purslane (Portulaca oleracea L.) were the most common weeds in strawberry, whereas M. lupulina, G. carolinianum, E. indica, Cyperus spp., and wild radish (Raphanus raphanistrum L.) were the most troublesome (Van Wychen Reference Van Wychen2019). This information is useful but is based upon the experience of weed scientists rather than actual field data.
The objective of this survey was to estimate the frequency, density, field uniformity, and relative abundance of weeds in commercial strawberry fields in Florida and to determine whether the abovementioned variables vary with weed management practice.
Materials and Methods
Sampling Procedure
A weed survey was conducted in commercial strawberry fields located in central Florida, in February and March 2020 (Figure 1). The survey was conducted near the end of the strawberry growing season and represents weed species that were not controlled and persisted throughout the season or emerged late in the season. This time period was selected because weed density tends to be highest late in the growing season, and this population cohort is most likely to survive long enough to flower and produce seeds. Weed emergence periods for many weed species that occur in Florida strawberry are unknown (Sharpe et al. Reference Sharpe and Boyd2019a), but weeds that emerge and grow rapidly during the first few months of crop growth are likely to be hand pulled during runner-cutting operations, and as a result, this portion of the weed population would not be accounted for in this survey. For the row middles, this weed survey represents weeds that survived initial management efforts or emerged after crop flowering, as most preemergence and postemergence row middle herbicides are applied before this time period.

Figure 1. Sites surveyed at the end of the 2019–2020 season.
Fourteen farms and a total of 41 fields, 36 conventional and 5 certified organic, were surveyed. Farms were selected to include small (<20.2 ha), medium (>20.2 ha, <162 ha), and large (>162 ha) farms in the region. Participating growers provided information on weed management, including fumigant, herbicide and polyethylene mulch usage, and field age and hectarage. Fields were surveyed following the methodology of McCully et al. (Reference McCully, Samson and Watson1991) and Thomas (Reference Thomas1985) with modifications. Twenty, 1 by 0.82 m quadrats were randomly placed along an “inverted W” pattern in each field. The first quadrat was placed after walking 20 paces from one corner of the field, along the first leg of the “W.” Five quadrats were placed along each of the four legs. The distance between quadrats varied with the size and shape of each field to accommodate any obstacles, gaps, or structures. Therefore, larger fields had greater distances between quadrats. Each quadrat was placed to cover one-half of the bed top and the entire row middle on one side of the raised bed. The raised, plastic-covered beds were spaced 1.22 m apart, with each bed 0.81-m wide at the base. All weeds within each quadrat were counted and identified. Weed species outside the quadrats or located along the perimeter of the field that did not occur within the quadrats were also identified but not counted. When a weed species could not be identified in the field, photographs and samples were taken, and the species were identified by weed scientists at the University of Florida.
Categorization Criteria
Mean weed density was grouped based on management practices or field characteristics. Fields were grouped by: (1) production type (organic vs. conventional), (2) fumigant applied the year of the survey (96% isothiocyanate, 31% chloropicrin [Pic] + 64% isothiocyanate, 35% Pic + 63% 1,3-dichloropropene [1,3-D], 60% Pic + 39% 1,3-D, 80% Pic + 20% 1,3-D, 21% Pic + 78% dimethyl disulfide, 3.5% thyme oil, and 54% metam potassium or 42% metam sodium), and (3) number of consecutive years strawberries were grown on the field (<10 yr, 10 to 25 yr, 26 to 50 yr, >50 yr). Categories for field age were selected based on: (1) the ability to trace strawberry production for a maximum of 50 to 60 consecutive years on a limited number of farms and (2) grower perception that farms <10 yr of age were generally considered younger fields.
Data Analysis
The data were analyzed using multiple quantitative measures, including frequency, density, field uniformity, and relative abundance. Frequency (F) is the number of fields (f) in which the species (s) occurs expressed as a percentage of the total number of fields surveyed (T). It was calculated as:
Frequency does not consider the density of a given species within a field or the size of the weeds that occur. Field uniformity (U) is the number of sample locations within a field in which species s occurs (q) expressed as a percentage of the total number of samples taken in the given field (t). Occurrence within any given field where species s occurred was calculated as:
Average uniformity for all fields where species s occurred was calculated. Field uniformity was also calculated as a percentage of all fields present in the study (T = 41). Throughout the paper, these two calculations are referred to as U(occurrence fields) and U(all fields), respectively. U(occurrence fields) provides an estimate of the uniformity of the species within fields where it occurs, whereas U(all fields) provides an estimate of the uniformity of the species across the entire production region.
Density within any given field was calculated as:
Where k is the total number plants species s within a given field. Mean field density (MFD) for occurrence fields was calculated as:
where the sum of the densities for species s is divided by the number of fields where species s occurs (T). MFD for all fields was calculated using the sum of the densities for species s divided by the total number of fields present in the study (T = 41). These two variables are referred to as MFD(occurrence fields) and MFD(all fields), respectively.
The relative abundance (RA) was also calculated to provide a unitless ranking of all species included in the survey (Thomas Reference Thomas1985). It was calculated as follows:
Relative frequency for species s (RF s ):
Relative uniformity for species s (RU s ):
Relative mean density for species s (RMD s ):
And RA for species s was calculated as follows:
This formula facilitates the comparison of individual weed species relative to each other and assumes that relative frequency, uniformity, and density are of equal importance.
Mean weed density for the various farm categories was compared using 95% confidence intervals. The confidence intervals were calculated using the Proc Means CLM statement in SAS (v. 9.4, SAS Institute, Cary, NC).
Results and Discussion
General Production
The total area surveyed represented 805 and 102 ha of conventional and organic strawberry production, respectively. This was approximately 23% of all planted hectarage in Florida. Soil fumigants were used in all conventional fields, and 72% of the hectarage was fumigated with chloropicrin and 1,3-dichloropropene combinations (Table 1). The 60% Pic + 39% 1,3-D combination was the most common, with 31% of the hectarage fumigated with this product. Sixteen percent of the hectarage was fumigated with 21% Pic + 78% dimethyl disulfide, but dimethyl disulfide is no longer available for use as a soil fumigant in Florida. Our survey verified the widespread adoption of fumigants as a critical component of integrated weed management (IWM) programs for intensive strawberry production systems in Florida. However, fumigant efficacy on weeds is often variable (Khatri et al. Reference Khatri, Vallad, Noling and Boyd2021a; Wu et al. Reference Wu, Qushim, Guan, Boyd, Vallad, MacRae and Jacoby2020), though there is strong evidence that fumigants kill weed seeds (Khatri et al. Reference Khatri, Vallad, Peres, Desaegaer, Regmi and Boyd2021b; Klose et al. Reference Klose, Ajwa, Browne, Subbarao, Martin, Fennimore and Westerdahl2008). Field trials are needed to evaluate the long-term impacts of fumigants on weed population dynamics.
Table 1. Fumigants used on surveyed conventional strawberry fields in central Florida during the 2019–2020 season.

A range of polyethylene films were used, with totally impermeable films (TIF), very impermeable films, and low-density polyethylene films used on 549, 253, and 105 ha, respectively. This represented a wide-scale transition to TIF films, which is a beneficial development, as TIF films reduce emissions, retain fumigants more effectively, and facilitate reduced fumigant rates (Qian et al. Reference Qian, Kamel, Stafford, Nguyen, Chism, Dawson and Smith2011; Stevens et al. Reference Stevens, Freeman and Dittmar2016).
Several preemergence herbicides are registered for use under plastic mulch (Dittmar et al. Reference Dittmar, Freeman, Paret and Smith2020), and research conducted in Florida has proven that strawberry exhibits good tolerance to many preemergence herbicides such as flumioxazin and oxyfluorfen (Boyd and Reed Reference Boyd and Reed2016; Boyd et al. Reference Boyd, Sharpe and Kanissery2021). However, the only preemergence herbicide applied under the plastic film was sulfentrazone, and it was only used on 12% of the surveyed area. Growers throughout the region rely on the use of plastic mulches with narrow slits for crop transplant rather than holes, fumigation, and hand weeding, typically combined with runner cutting early in the season, for weed control. Although this approach is effective, weeds remain a serious issue in some fields, and hand pulling weeds, even if combined with runner-cutting operations, adds significantly to labor costs. Increased use of preemergence herbicides is likely to reduce labor inputs early in the season.
Conversely, herbicides were applied in the row middles on 100% of the conventional acreage, with glyphosate applied on 99% of conventional fields (Table 2). Preemergence flumioxazin applications were used on 38% of the acreage. Carfentrazone and clethodim were applied on 8% and 2% of conventional fields, respectively. Herbicide application timing varied across sites, but the majority of preemergence and postemergence herbicides were applied before transplanting or shortly after overhead irrigation, which is used for crop transplant establishment in Florida, was turned off for the season. Additional postemergence herbicides were applied as needed early in the season, with very few herbicide applications occurring after the start of the berry harvest. An acetic acid formulation was the only organic herbicide applied, and it was only used in row middles on one certified organic farm on 9 ha (9% of organic fields), whereas all other organic farms controlled weeds in row middles with repeated cultivation. Cultivators were all designed on farm and consisted of an arrangement of three to four sweeps per row middle with various devices used to smooth and pack the soil behind the cultivator.
Table 2. Herbicide active ingredients used on surveyed conventional strawberry fields in central Florida during the 2019–2020 season.

Frequency, Field Uniformity, and Density
A total of 47 weed species were identified in Florida strawberry fields, with 16 of the 47 only occurring on field boundaries (Tables 3 and 4). Of these 16 species, common groundsel (Senecio vulgaris L.), which can be a serious issue in northern states, was observed but was uncommon. Purple cudweed [Gamochaeta purpurea (L.) Cabrera] was also only noted on field borders in this survey but has been observed emerging in planting holes at low densities in commercial fields. Ragweed parthenium (Parthenium hysterophorus L.) has only recently been identified in strawberry fields in Florida and has formed dense populations in row middles in a limited number of commercial fields, but in this survey was only observed on field borders. This weed is a concern because it exhibits tolerance to glyphosate or paraquat (Fernandez et al. Reference Fernandez, Odero, MacDonald, Ferrell and Gettys2015; Palma-Bautista et al. Reference Palma-Bautista, Hoyos, Plaza, Vázquez-García, Rosario, Rojano-Delgado and De Prado2020), both commonly used for postemergence weed control in row middles, and it appears to be spreading to more fields each year.
Table 3. Weed species observed in Florida strawberry fields or on field borders that did not occur in the quadrats during a weed survey conducted in the 2019 and 2020 season.

Table 4. Frequency, field uniformity, density, and relative abundance of weeds in commercial strawberry fields in Florida during the 2019 and 2020 season.
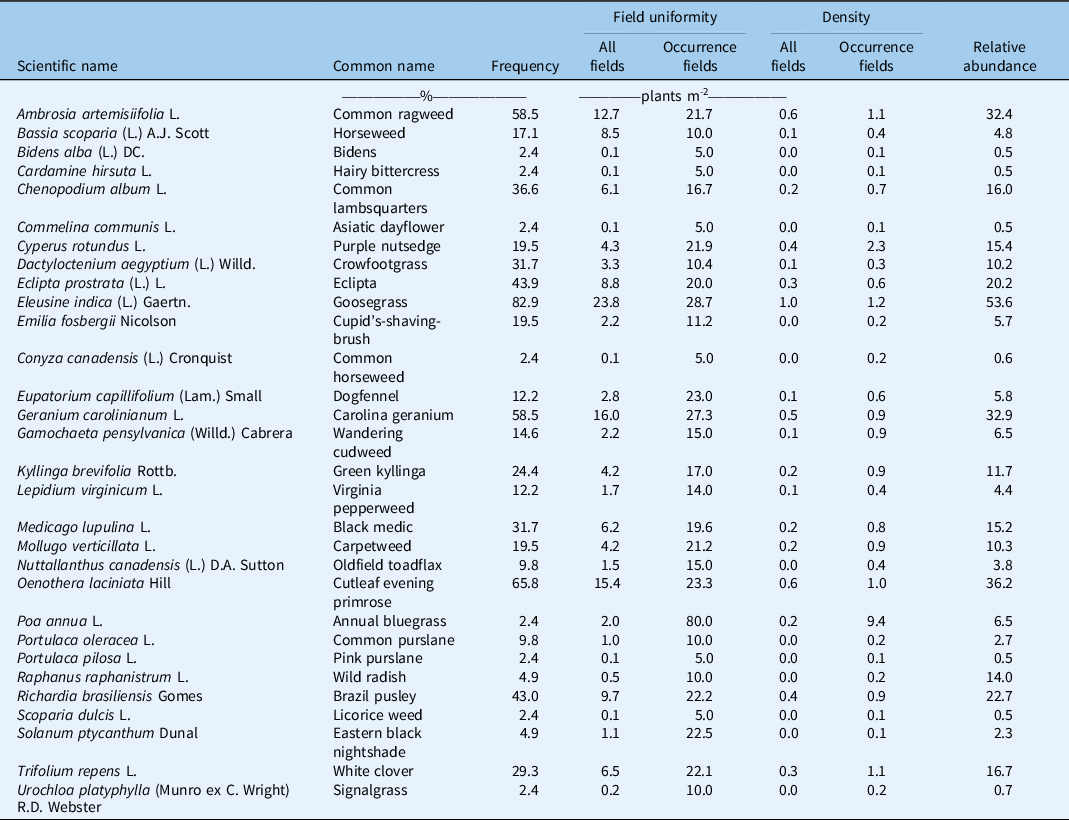
The five weed species with the highest frequency observed in the quadrats were E. indica, cutleaf evening primrose (Oenothera laciniata Hill), G. carolinianum, common ragweed (Ambrosia artemisiifolia L.), and eclipta (Eclipta prostrata L.), which occurred in 83%, 63%, 58%, 58%, and 46% of all fields surveyed (Table 4). The top five species with the highest overall relative abundance were similar and included E. indica, O. lanciniata, G. carolinium, A. artemisiifolia, and R. brasiliensis. Only E. indica and G. carolinium were included in the top five most common weed species in a survey conducted by the WSSA that relied on the professional opinion of weed scientists (Van Wychen Reference Van Wychen2019). Eupatorium capillifolium, P. oleracea, and Cyperus spp. were listed as the remaining most common species in the WSSA survey but were found to be far less common than previously expected. The divergence between the two surveys highlights the importance of collecting field data.
Eleusine indica had the highest frequency and relative abundance and tended to have high uniformity across all fields and within occurrence fields, suggesting it is widely distributed across the industry. This is a significant concern, because E. indica is a prolific seed producer (Chin Reference Chin and Kwee1979) and difficult to remove by hand, and dense populations occurred on farms where preemergence herbicides were applied, which suggests preemergence applications early in the season do not provide season-long control. In addition, populations with resistance to paraquat have been reported in Florida (Buker et al. Reference Buker, Steed and Stall2002), and this herbicide is widely used for crop termination. It is likely that resistant plants would survive crop termination and set seed, which may provide a potential explanation for the widespread occurrence of this species. Given the prevalence of this species, research efforts should be undertaken to determine whether herbicide-resistant populations occur within strawberry fields and identify effective management options in the presence or absence of herbicide resistance.
Oenothera laciniata had the second highest frequency and relative abundance, though uniformity was not as high as for some other species. This species tends to be difficult to control in many vegetable crops, which may explain the relative abundance. Geranium carolinianum and A. artemisiifolia were similar in frequency, uniformity, and relative abundance. Both species are susceptible to postemergence applications of clopyralid, but strawberry growers tend not to use this product due to crop damage concerns. Eclipta prostrata had the fifth highest frequency. Uniformity tended to be high in occurrence fields but much lower when averaged across all fields, which means E. prostrata is not as widespread as some of the other species but is potentially a serious problem where it occurs. Cyperus rotundus only occurred in 20% of the fields, but uniformity tended to be high in occurrence fields, which suggests it can be a serious issue in problem fields. Eupatorium capillifolium, annual bluegrass (Poa annua L.), eastern black nightshade (Solanum ptycanthum Dunal), and white clover (Trifolium repens L.) occurred in fewer fields but tended to have relatively high field uniformity in occurrence fields, which suggests growers have difficulty controlling these species when present.
Overall, mean field densities tended to be relatively low across all fields, with densities within occurrence fields ranging from 0 to 9.4 plants m−2 per species, with most species below 1 plant m−2. The methodology utilized for this weed survey does not allow us to distinguish between weeds occurring in the row middle and in the transplant holes, but weed emergence in all fields was more common in the row middles. The low overall densities observed suggest that the IWM approach adopted by most growers—fallow period cover crops or herbicides, fumigation, polyethylene mulches with narrow slits, hand weeding, and multiple preemergence + postemergence herbicides in the row middle—is effective.
Conventional versus Organic Fields
We hypothesized that weed density would be higher in organic fields, but this was not the case, with similar densities observed within both production systems (Figure 2). The lack of difference between the two systems could possibly be attributed to increased weed management effort in the organic fields. We did not collect data on time or labor associated with weed control, costs associated with herbicide use, or hand-weeding frequency in the two production systems and subsequently have no way to determine whether labor or weed management input costs differed between the systems. There were very few associations between production system and weed species. One exception was the high density of P. annua, which only occurred on one organic farm where they were unable to control it. Cyperus rotundus was another exception, and the plant density was much higher in organic (3.4 plants m−2) than conventional fields (0.5 plants m−2). This can be partially attributed to the lack of Cyperus management options for organic growers that rely predominately on cultivation in row middles and cover crops during the fallow period for Cyperus control. Cover crops hinder Cyperus spp. growth but are not an effective management option, as Cyperus spp. grow beneath cover crop canopies, and cover crops do not reduce tuber densities (Collins et al. Reference Collins, Chase, Stall and Hutchinson2007; Yu et al. Reference Yu, Sharpe and Boyd2021). Conversely, conventional growers have the option to utilize fumigants, in-crop herbicides, and fallow period glyphosate applications to reduce Cyperus shoot and tuber densities. Research is needed to develop Cyperus spp. management programs for organic growers.

Figure 2. Mean weed density in organic and conventional strawberry fields during the 2019–2020 season. Diamonds are the mean weed density across all sites, hollow circles are the mean weed density within each site, and the error bars are the 95% confidence intervals.
Farm Size and Age
A range of field ages were included in the study, with some fields in continuous strawberry production for more than 50 yr (Figure 3). However, there were no clear differences in weed density associated with field age. There were also very few clear associations of field age with weed species, although C. rotundus densities tended to be higher in fields that had been in production less than 10 yr. It is important to note that organic fields also tended to have higher C. rotundus densities and tended to be in production less than 10 yr, so it is difficult to determine whether C. rotundus density was associated with field age or management practice.

Figure 3. Mean weed density in fields with strawberries grown for different consecutive periods of time during the 2019–2020 season. Diamonds are the mean weed density across all sites, hollow circles are the mean weed density within each site, and the error bars are the 95% confidence intervals.
Fumigant Type
It is important to note that fumigant selection can have cumulative effects over time. The current survey only evaluated the effects of the fumigant used in the season the survey was conducted (Figure 4). This may explain the lack of difference noted between the different fumigants and the lack of association of any weed species with a given fumigant. In general, the confidence intervals for 21% Pic + 78% dimethyl disulfide (DMDS), metam potassium or metam sodium (MITC products), and 60% Pic + 39% 1,3-D were narrower, which indicates that these products may provide more consistent overall weed control than the other fumigants included in the survey.

Figure 4. Mean weed density in fields treated with different fumigants during the 2019–2020 season. Diamonds are the mean weed density across all sites, hollow circles are the mean weed density within each site, and the error bars are the 95% confidence intervals. 1,3-D, 1,3-dichloropropene; dimethyl disulfide (DMDS); metam sodium or metam potassium (MITC); Pic, chloropicrin.
This weed survey provides insight into the most common and problematic weeds on commercial strawberry farms in Florida. The results differ substantially from previous weed surveys that rely on the opinions of weed scientists rather than actual data. This information can be used to focus future weed research efforts for this crop.
Acknowledgments
The authors would like to thank Alicia Whidden for assistance with contacting growers and the Florida Strawberry Growers Association for their support with the project. The authors would also like to thank all of the strawberry growers who allowed us to conduct the survey on their land. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. No conflicts of interest have been declared.











