Introduction
Eating disorders are psychiatric illnesses marked by dysregulated appetite, eating behaviours, and shape and weight concerns. Anorexia nervosa is characterized by significantly low body weight and intense fear of weight gain (Dalle Grave et al., Reference Dalle Grave, Calugi, Conti, Doll and Fairburn2013) and affects approximately 1% females and 0.3% males (Hudson et al., Reference Hudson, Hiripi, Pope and Kessler2007; Preti et al., Reference Preti, Girolamo, Vilagut, Alonso, Graaf, Bruffaerts, Demyttenaere, Pinto-Meza, Haro, Morosini and Investigators2009; Thomas et al., Reference Thomas, Lee and Becker2016) worldwide. Bulimia nervosa, affecting approximately 1.5% females and 0.5% males (Hudson et al., Reference Hudson, Hiripi, Pope and Kessler2007; Preti et al., Reference Preti, Girolamo, Vilagut, Alonso, Graaf, Bruffaerts, Demyttenaere, Pinto-Meza, Haro, Morosini and Investigators2009; Thomas et al., Reference Thomas, Lee and Becker2016), is characterized by recurrent episodes of uncontrollable binge eating coupled with compensatory behaviours (e.g. purging, fasting and excessive exercise) to prevent consequent weight gain (Dalle Grave et al., Reference Dalle Grave, Calugi, Conti, Doll and Fairburn2013). Both eating disorders have been associated with increased premature mortality and suicidal risk (Arcelus et al., Reference Arcelus, Mitchell, Wales and Nielsen2011; Whiteford et al., Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari, Erskine, Charlson, Norman, Flaxman, Johns, Burstein, Murray and Vos2013; Keshaviah et al., Reference Keshaviah, Edkins, Hastings, Krishna, Franko, Herzog, Thomas, Murray and Eddy2014; Yao et al., Reference Yao, Kuja-Halkola, Thornton, Runfola, D'Onofrio, Almqvist, Lichtenstein, Sjolander, Larsson and Bulik2016) and medical (Kessler et al., Reference Kessler, Berglund, Chiu, Deitz, Hudson, Shahly, Aguilar-Gaxiola, Alonso, Angermeyer, Benjet, Bruffaerts, de Girolamo, de Graaf, Maria Haro, Kovess-Masfety, O'Neill, Posada-Villa, Sasu, Scott, Viana and Xavier2013; Sheehan and Herman, Reference Sheehan and Herman2015; Westmoreland et al., Reference Westmoreland, Krantz and Mehler2016) and psychiatric comorbidities (Herzog et al., Reference Herzog, Keller, Sacks, Yeh and Lavori1992; Striegel-Moore et al., Reference Striegel-Moore, Garvin, Dohm and Rosenheck1999; Kaye et al., Reference Kaye, Bulik, Thornton, Barbarich and Masters2004; Keel et al., Reference Keel, Klump, Miller, McGue and Iacono2005; Hudson et al., Reference Hudson, Hiripi, Pope and Kessler2007; Bulik-Sullivan et al., Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day, Loh, Duncan, Perry, Patterson, Robinson, Daly, Price and Neale2015; Cederlof et al., Reference Cederlof, Thornton, Baker, Lichtenstein, Larsson, Ruck, Bulik and Mataix-Cols2015).
Although anorexia nervosa and bulimia nervosa are defined as two distinct eating disorders, they share common symptoms. For instance, both disorders include over-evaluation of body weight and shape and behaviours to control weight; anorexia nervosa can include binge eating and purging behaviours, and bulimia nervosa can also involve restrictive eating behaviours. Diagnostic crossover has also been observed across the course of illness. Although pure forms of the illnesses exist, 10–54% of individuals with anorexia nervosa develop bulimia nervosa and 2–27% of individuals with bulimia nervosa develop anorexia nervosa during the course of illness (Tozzi et al., Reference Tozzi, Thornton, Klump, Fichter, Halmi, Kaplan, Strober, Woodside, Crow, Mitchell, Rotondo, Mauri, Cassano, Keel, Plotnicov, Pollice, Lilenfeld, Berrettini, Bulik and Kaye2005; Eddy et al., Reference Eddy, Dorer, Franko, Tahilani, Thompson-Brenner and Herzog2008; Peat et al., Reference Peat, Mitchell, Hoek and Wonderlich2009; Fichter et al., Reference Fichter, Quadflieg, Crosby and Koch2017). The two eating disorders also share common comorbid conditions such as mood disorders, anxiety disorder, substance use disorders and attention-deficit hyperactivity disorder (Hudson et al., Reference Hudson, Hiripi, Pope and Kessler2007; Yao et al., Reference Yao, Kuja-Halkola, Martin, Lu, Lichtenstein, Norring, Birgegard, Yilmaz, Huebel, Watson, Baker, Almqvist, Thornton, Magnusson, Bulik and Larsson2019) In terms of treatment, transdiagnostic approaches such as enhanced cognitive behavioural therapy are associated with favourable outcomes in both anorexia nervosa and bulimia nervosa (Dalle Grave et al., Reference Dalle Grave, Calugi, Conti, Doll and Fairburn2013; Fairburn et al., Reference Fairburn, Cooper, Doll, O'Connor, Palmer and Dalle Grave2013; Fairburn et al., Reference Fairburn, Bailey-Straebler, Basden, Doll, Jones, Murphy, O'Connor and Cooper2015). Together, these observations suggest considerable, but not complete, overlap between anorexia nervosa and bulimia nervosa.
Observed familial co-aggregation of the two eating disorders further implies their aetiological overlap; relatives of individuals with anorexia nervosa have increased risk (over four times) of bulimia nervosa compared to the same types of relatives of individuals without anorexia nervosa, and vice versa (Stein et al., Reference Stein, Lilenfeld, Plotnicov, Pollice, Rao, Strober and Kaye1999; Strober et al., Reference Strober, Freeman, Lampert, Diamond and Kaye2000). To our knowledge, only one study has quantified the genetic and environmental effects on the overlap between anorexia nervosa and bulimia nervosa (Bulik et al., Reference Bulik, Thornton, Root, Pisetsky, Lichtenstein and Pedersen2010). Using self-ratings of eating behaviours in a Swedish twin sample, the study reported high genetic [0.78, 95% confidence interval (CI) (0.51–1.00)] and moderate unique environmental [0.44 (0.20–0.65)] correlations between anorexia nervosa and bulimia nervosa. However, due to the relatively low prevalence of both disorders and of twinning, clinical diagnoses have rarely been used in quantitative genetic studies of eating disorders.
In the current work, we used clinical diagnoses of anorexia nervosa and bulimia nervosa in a large Swedish female cohort containing full-sisters (sisters born to the same biological parents) and maternal half-sisters (sisters born to the same biological mother but different biological fathers). The application of diagnostic information considerably expands the clinical significance of the results. Using non-twin siblings significantly increased sample size and improved precision in estimating the underlying morbidity liabilities. We quantified the contribution of genetic and environmental effects on anorexia nervosa and bulimia nervosa and their overlap and estimated the genetic and environmental correlations between the two eating disorders.
Methods and materials
The current study was approved by the Regional Ethics Review Board in Stockholm, Sweden.
Register data
We acquired information from several Swedish national registers linked by the unique individual identification number. The Swedish Population Register provided birth year and month, death date and migration information (Ludvigsson et al., Reference Ludvigsson, Almqvist, Bonamy, Ljung, Michaelsson, Neovius, Stephansson and Ye2016). The Multi-Generation Register contained information on biological parents for each individual who was born after 1932 and lived in Sweden since 1961. The National Patient Register (NPR) included inpatient psychiatric diagnoses since 1973 and outpatient psychiatric diagnoses since 2001; diagnoses of eating disorders in the NPR were based on the Swedish versions of the International Classification of Diseases, Ninth Revision (ICD-9 1987–1996) and Tenth Revision (ICD-10, since 1997) (Ludvigsson et al., Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim, Reuterwall, Heurgren and Olausson2011). The Swedish National Quality Register for Eating Disorder Treatment (Riksät, since 1999) and quality assurance system for eating disorders (Stepwise, since 2005) were two treatment quality registers for eating disorder treatments from specialized centres across Sweden (Birgegard et al., Reference Birgegard, Bjorck and Clinton2010; Emilsson et al., Reference Emilsson, Lindahl, Koster, Lambe and Ludvigsson2015; Javaras et al., Reference Javaras, Runfola, Thornton, Agerbo, Birgegard, Norring, Yao, Rastam, Larsson, Lichtenstein and Bulik2015); diagnoses in the treatment quality registers were based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Data from the registers were updated until 2013–12–31.
Anorexia nervosa diagnosis was identified with ICD-9 code 307B or ICD-10 codes F50.0 or F50.1 from the NPR, or meeting DSM-IV-TR criteria for anorexia nervosa or sub-threshold anorexia nervosa in Eating Disorder Not Otherwise Specified (EDNOS) from the treatment quality registers. We were unable to distinguish between restricting and binge-eating and purging anorexia nervosa from the ICD diagnosis in the NPR. Bulimia nervosa diagnosis was identified with ICD-10 codes F50.2 or F50.3 from the NPR, or meeting DSM-IV-TR criteria for bulimia nervosa or sub-threshold bulimia nervosa in EDNOS from the treatment quality registers. Bulimia nervosa was not an independent eating disorder category in the Swedish version of ICD-9 (1987). Therefore, its diagnostic period was shorter than that of anorexia nervosa, which could lead to under-detection of bulimia nervosa cases relative to anorexia nervosa cases.
Study population
Through the Swedish Population Register and the Multi-Generation Register, we identified all full-sisters (sisters born to the same parents) and maternal half-sisters (sisters born to the same mother but different fathers) who were born in Sweden between 1970 and 2005. We excluded individuals who emigrated or died before age 6, adopted individuals and individuals whose biological parents were not identifiable from the registers. We did not have access to information on race and ethnicity; however, being born in Sweden might suggest that our study population was comprised primarily of individuals of Scandinavian/Nordic ancestry. We then randomly selected one pair of maternal half-sisters per mother [57 036 pairs, mean age 25.2 years by the end of follow-up in year 2013, standard deviation (s.d.) = 9.3]. We then randomly selected one pair of full-sisters per mother in the remaining mothers after excluding mothers of the selected maternal half-sisters. We excluded twin pairs from full-sister pairs, resulting in 334 433 pairs of full-sisters for analysis (mean age 25.5 years by year 2013, s.d. = 9.6). No individual was included in more than one pair. The selected full-sisters and maternal half-sisters comprised the study population, where 6104 (0.91%) individuals among the full-sisters and 938 (0.82%) among the maternal half-sisters had been diagnosed with anorexia nervosa; 3142 (0.47%) among the full-sisters and 579 (0.51%) among the maternal half-sisters had been diagnosed with bulimia nervosa; 679 (0.10%) among the full-sisters and 122 (0.11%) among the half-sisters had been diagnosed with both disorders (Table 1).
Table 1. Descriptive statistics and correlations

The presented correlations are tetrachoric correlations (with standard error). Phenotypic correlation was the correlation of anorexia nervosa and bulimia nervosa within an individual; cross-sister-within-trait correlation was the correlation of one disorder between the two sisters in a pair; cross-sister-cross-trait correlation was the correlation between anorexia nervosa in one sister and bulimia nervosa in the other sister in a pair. We presented both the crude correlations and the correlations adjusted for birth year.
a A concordant pair was a pair where both sisters had the disorder; a discordant pair was a pair where only one of the sisters had the disorder.
b The number (and prevalence) of individuals with both anorexia nervosa and bulimia nervosa.
c Here a concordant pair was a pair where one sister had anorexia nervosa and the other had bulimia nervosa, and a discordant pair was a pair where one sister had anorexia or bulimia nervosa, and the other sister did not have the other disorder.
Statistical analysis
We first examined the distribution of anorexia nervosa and bulimia nervosa and their correlations in full-sisters and maternal half-sisters. We estimated phenotypic correlations (i.e. correlation between anorexia nervosa and bulimia nervosa within an individual), cross-sister-within-trait correlations (i.e. correlation of one disorder between the two individuals in a pair) and cross-sister-cross-trait correlations (i.e. correlation between anorexia nervosa in one individual and bulimia nervosa in the other individual in the pair). The correlations were tetrachoric correlations in this study as the diagnoses were binary variables (whether the individual received the diagnosis or not). The estimates were obtained with a liability-threshold model where normally distributed underlying liabilities to the binary phenotypes were assumed (Neale and Cardon, Reference Neale and Cardon1992; Bulik et al., Reference Bulik, Sullivan, Wade and Kendler2000). We adjusted for birth year of the individuals.
We then applied structural equation modelling (Neale and Cardon, Reference Neale and Cardon1992) to decompose the aforementioned correlations and estimate the genetic and environmental effects on anorexia nervosa and bulimia nervosa and their overlap. Specifically, four effects were estimated in the study. Additive genetic effects (A) represented the cumulative influence of multiple genes, each of which was presumed to have a small effect on the phenotype. Dominance genetic effects (D) were modelled to measure the genetic effects deviated from the additive effects. Shared environmental effects (C) were defined as environmental impact on both individuals in a pair (i.e. which made the two sisters similar to each other), whereas unique environmental effects (E, including measurement error) influence one individual but not the other in a pair (Plomin et al., Reference Plomin, DeFries, Knopik and Neiderhiser2013). Full-sisters share 50% of their segregating alleles on average and therefore 50% additive genetic effects and 25% dominance genetic effects; whereas maternal half-sisters share 25% of additive genetic effects on average and no dominance genetic effects. As most maternal half-siblings live in the same family just as full-siblings in our study population (1994), the two types of sisters were assumed to have equal shared environmental effects. Structural equation models quantified the proportions of phenotypic variation attributable to all or some of the A, D, C and E components.
Given the data of two types of siblings, at most three of the four components could be estimated at a time. We fitted three bivariate structural equation models, namely models including components A, C and E (an ACE model), A, D and E (an ADE model), and A and E (an AE model), using the OpenMx package (version 2.8.3) (Neale et al., Reference Neale, Hunter, Pritikin, Zahery, Brick, Kirkpatrick, Estabrook, Bates, Maes and Boker2016) in R (version 3.3.3). Weighted least squares method was used for model fitting and estimating the standard errors. Scripts are available from the corresponding author upon reasonable request. Figure 1 illustrates the path diagram of the A component in the models; other components included in the models follow the same logic but are not shown in the figure for simplicity.
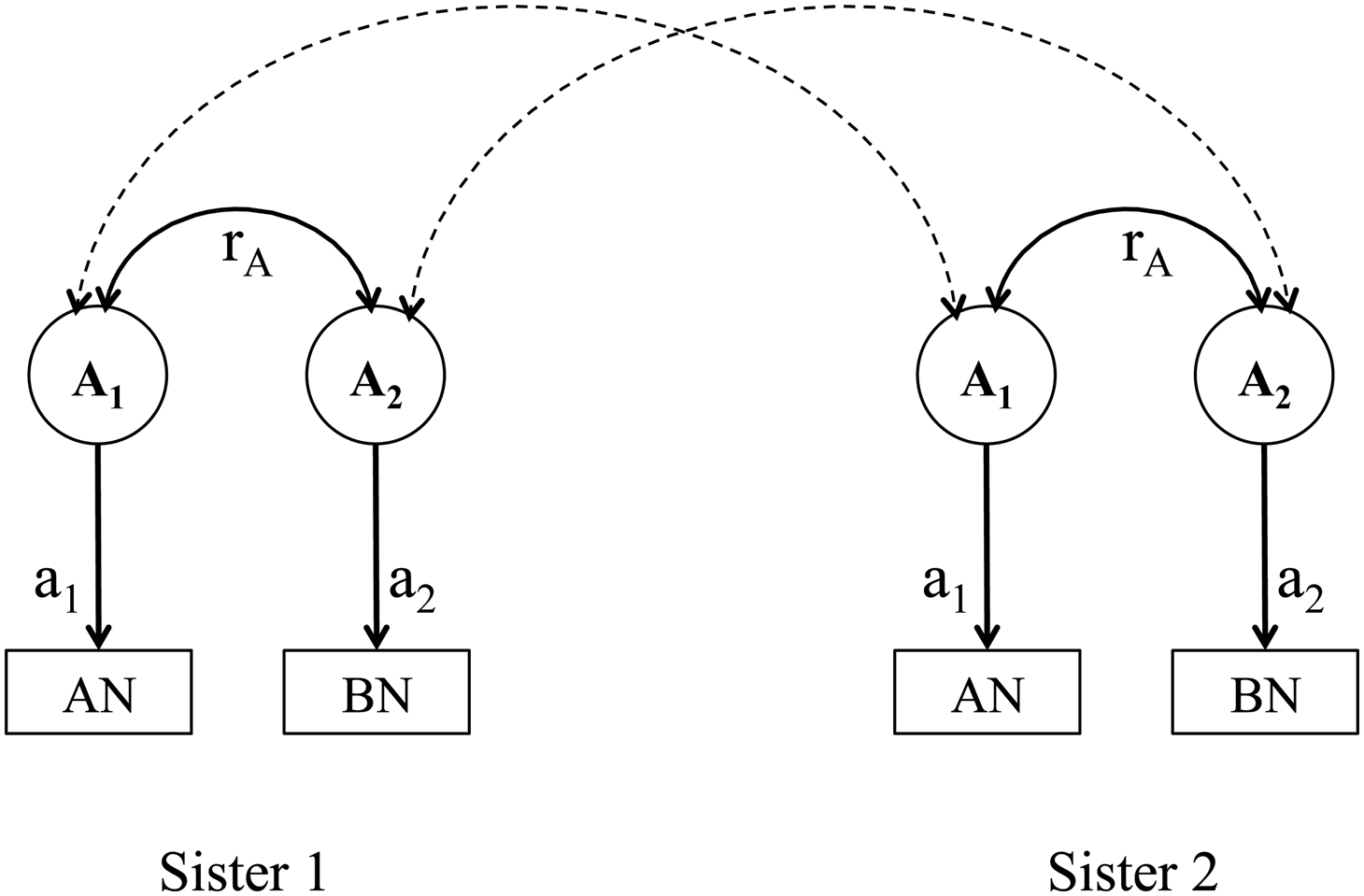
Fig. 1. Path diagram of the additive genetic effects and genetic correlations in a bivariate model of anorexia nervosa and bulimia nervosa. AN, anorexia nervosa; BN, bulimia nervosa. A 1 represents the latent additive genetic effect that contributes a1 to anorexia nervosa; A 2 represents the latent additive genetic effect that contributes a2 to bulimia nervosa. The additive genetic correlation between anorexia nervosa and bulimia nervosa is represented by r A. Parameters a1, a2 and r A are the unknown parameters. The dashed double arrows indicate the genetic sharing between two sisters in a pair; they are 0.5 for full-sisters and 0.25 for maternal half-sisters.
We detected that the prevalence of the disorders differed slightly between full-sisters and maternal half-sisters (χ2 test, p = 0.003 for anorexia nervosa, and p = 0.090 for bulimia nervosa), and therefore allowed the thresholds to be freely estimated across relative types in all models. Likelihood ratio test was performed for each bivariate model against the saturated model to evaluate whether the bivariate model fitted the data similarly well compared to the saturated model (p > 0.05 indicates that the fittings were similarly well). We interpreted the results of the model with the lowest Akaike Information Criterion (AIC), which comprehensively reflects the goodness of fit (also reflected by likelihood ratio test) and model parsimony.
Results
Genetic and environmental effects on clinically diagnosed anorexia nervosa and bulimia nervosa
In the current study, we included clinical diagnostic information from 334 433 pairs of full-sisters and 57 036 pairs of maternal half-sisters. The cross-sister-within-trait correlation for anorexia nervosa was estimated as 0.22 [standard error (s.e.) = 0.02] in full-sisters and 0.03 (s.e. = 0.06) in maternal half-sisters; the cross-sister-within-trait correlation for bulimia nervosa was estimated as 0.20 (s.e. = 0.03) in full-sisters and 0.13 (s.e. = 0.07) in maternal half-sisters (Table 1). The higher resemblance in full-sisters than in maternal half-sisters indicates that genetic factors contribute to the liability to the eating disorders, because full-sisters on average share more segregating genes than maternal half-sisters, whereas the environmental contribution within a pair was assumed to be equal between the two types of sisters.
The models fitted the data similarly well compared to the saturated model (p values >0.05 in likelihood ratio tests against the saturated model). The AE model was selected for interpretation because it had the lowest AIC (Table 2). The model revealed moderate genetic and unique environmental effects on both anorexia nervosa and bulimia nervosa. The heritability of anorexia nervosa was estimated as 43% [95% CI (36–50%)], suggesting that 43% of the variance of anorexia nervosa in the population was attributable to the genetic variance in the population and the remaining variance [57% (50–64%)] was attributable to unique environmental variance. Approximately 41% (31–52%) of the observed variance of bulimia nervosa was explained by genetic variance and the remaining variance [59% (48–70%)] was attributable to unique environmental variance (AE model in Table 3).
Table 2. Model fitting statistics

ACE model included components of additive genetic effects (A), shared environmental effects (C) and unique environmental effects (E). ADE model included components of A, dominant genetic effects (D) and E. AE model included components of A and E.
a The goodness of fit of ACE, ADE and AE models were similar to that of the saturated model (p values >0.05 in likelihood ratio tests against the saturated model). AE model had the lowest AIC, suggesting it was more parsimonious than ACE and ADE models. We therefore selected the estimates from AE model as the main results for interpretation.
Table 3. Genetic and environmental contributions to anorexia nervosa, bulimia nervosa, and their overlap, and the genetic and environmental correlations between the two eating disorders estimated from bivariate ACE, ADE and AE models (with 95% CI)
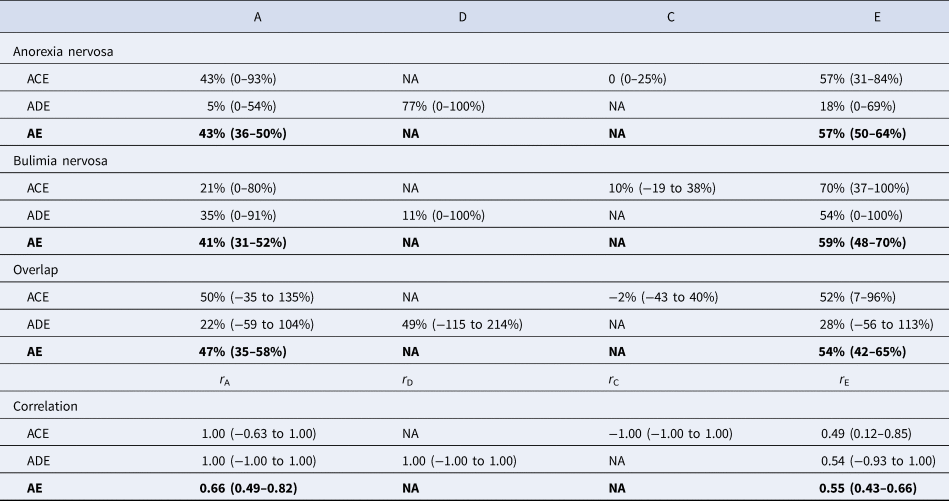
A stands for additive genetic effects; C stands for shared environmental effects; D stands for dominant genetic effects; E stands for unique environmental effects. The effect of each on anorexia nervosa, bulimia nervosa and their overlap was estimated, adjusted for birth year. We primarily interpret results from AE model (with the lowest AIC).
Genetic and environmental effects on the overlap between anorexia nervosa and bulimia nervosa
We observed statistically significant phenotypic correlations between clinically diagnosed anorexia nervosa and bulimia nervosa for both types of sisters (0.59, s.e. = 0.01 for full-sisters and 0.60, s.e. = 0.02 for maternal half-sisters), illustrating the diagnostic transition between the two eating disorders within individuals regardless of the type of siblings. The cross-sister-cross-trait correlation further characterizes the nature of the association between the disorders across the individuals in a pair. A higher cross-sister-cross-trait correlation was observed in full-sister pairs (0.14, s.e. = 0.02) than in maternal half-sisters (0.03, s.e. = 0.06), suggesting genetic effects on the overlap between the two eating disorders (Table 1).
The contribution of genetic effect to the overlap between anorexia nervosa and bulimia nervosa was measured by the proportion of the correlation of the two disorders explained by the covariance of the genetic effect on anorexia nervosa and the genetic effect on bulimia nervosa. The contribution of the unique environmental effect on the overlap was defined analogously. The overlap between anorexia nervosa and bulimia nervosa was about equally explained by genetic and unique environmental factors: approximately 46% [95% CI (35–58%)] of the phenotypic correlation of the two disorders was explained by their genetic covariance and the remaining 54% [95% CI (42–65%)] was explained by their environmental covariance (Table 3). We found significant genetic correlation [0.66, 95% CI (0.49–0.82)] and environmental correlation [0.55, 95% CI (0.43–0.66); Table 3] between anorexia nervosa and bulimia nervosa.
Discussion
In this study, we quantified the genetic and environmental effects on clinically diagnosed anorexia nervosa and bulimia nervosa and their overlap. The findings of significant genetic [0.66, 95% CI (0.49–0.82)] and environmental correlations [0.55 (0.43–0.66)] suggest an aetiological overlap between anorexia nervosa and bulimia nervosa and underscore the importance of vigilance for transitions between the two eating disorders during treatment. Using large population data, we significantly improved the precision of the estimates. We reported heritability for clinically diagnosed anorexia nervosa as 43% (36–50%) and bulimia nervosa as 41% (31–52%), which was slightly lower than, but within the confidence interval of, the heritability found in the twin study based on self-reported questionnaire data [57% (0–81%) for anorexia nervosa and 62% (8–70%) for bulimia nervosa] (Bulik et al., Reference Bulik, Thornton, Root, Pisetsky, Lichtenstein and Pedersen2010). The single-nucleotide polymorphism (SNP)-based heritability for anorexia nervosa has been estimated between 11% and 17% (s.e. 1%) in the most updated genome-wide association study (GWAS) (Watson and Yilmaz, Reference Watson, Yilmaz, Thornton, Hubel, Coleman, Gaspar, Bryois, Hinney, Leppa, Mattheisen, Medland, Ripke, Yao, Giusti-Rodriguez, Anorexia Nervosa Genetics, Hanscombe, Purves, Adan, Alfredsson, Ando, Andreassen, Baker, Berrettini, Boehm, Boni, Perica, Buehren, Burghardt, Cassina, Cichon, Clementi, Cone, Courtet, Crow, Crowley, Danner, Davis, de Zwaan, Dedoussis, Degortes, DeSocio, Dick, Dikeos, Dina, Dmitrzak-Weglarz, Docampo, Duncan, Egberts, Ehrlich, Escaramis, Esko, Estivill, Farmer, Favaro, Fernandez-Aranda, Fichter, Fischer, Focker, Foretova, Forstner, Forzan, Franklin, Gallinger, Giegling, Giuranna, Gonidakis, Gorwood, Mayora, Guillaume, Guo, Hakonarson, Hatzikotoulas, Hauser, Hebebrand, Helder, Herms, Herpertz-Dahlmann, Herzog, Huckins, Hudson, Imgart, Inoko, Janout, Jimenez-Murcia, Julia, Kalsi, Kaminska, Kaprio, Karhunen, Karwautz, Kas, Kennedy, Keski-Rahkonen, Kiezebrink, Kim, Klareskog, Klump, Knudsen, La Via, Le Hellard, Levitan, Li, Lilenfeld, Lin, Lissowska, Luykx, Magistretti, Maj, Mannik, Marsal, Marshall, Mattingsdal, McDevitt, McGuffin, Metspalu, Meulenbelt, Micali, Mitchell, Monteleone, Monteleone, Munn-Chernoff, Nacmias, Navratilova, Ntalla, O'Toole, Ophoff, Padyukov, Palotie, Pantel, Papezova, Pinto, Rabionet, Raevuori, Ramoz, Reichborn-Kjennerud, Ricca, Ripatti, Ritschel, Roberts, Rotondo, Rujescu, Rybakowski, Santonastaso, Scherag, Scherer, Schmidt, Schork, Schosser, Seitz, Slachtova, Slagboom, Slof-Op't Landt, Slopien, Sorbi, Swiatkowska, Szatkiewicz, Tachmazidou, Tenconi, Tortorella, Tozzi, Treasure, Tsitsika, Tyszkiewicz-Nwafor, Tziouvas, van Elburg, van Furth, Wagner, Walton, Widen, Zeggini, Zerwas, Zipfel, Bergen, Boden, Brandt, Crawford, Halmi, Horwood, Johnson, Kaplan, Kaye, Mitchell, Olsen, Pearson, Pedersen, Strober, Werge, Whiteman, Woodside, Stuber, Gordon, Grove, Henders, Jureus, Kirk, Larsen, Parker, Petersen, Jordan, Kennedy, Montgomery, Wade, Birgegard, Lichtenstein, Norring, Landen, Martin, Mortensen, Sullivan, Breen and Bulik2019), which appeared lower than the heritability estimated in quantitative genetic studies. SNP-based heritability is not yet available for bulimia nervosa, as currently there is no GWAS on bulimia nervosa. The discrepancy between SNP-based heritability and heritability estimated from twin and sibling models has been observed for various phenotypes (Manolio et al., Reference Manolio, Collins, Cox, Goldstein, Hindorff, Hunter, McCarthy, Ramos, Cardon, Chakravarti, Cho, Guttmacher, Kong, Kruglyak, Mardis, Rotimi, Slatkin, Valle, Whittemore, Boehnke, Clark, Eichler, Gibson, Haines, Mackay, McCarroll and Visscher2009). One possible explanation is that SNP-based heritability did not capture the heritability from alleles whose effects were too small to pass stringent significant tests (Yang et al., Reference Yang, Benyamin, McEvoy, Gordon, Henders, Nyholt, Madden, Heath, Martin, Montgomery, Goddard and Visscher2010).
Our finding of significant genetic correlation between clinically diagnosed anorexia nervosa and bulimia nervosa is in line with the previous twin study based on self-reported disordered eating conditions (Bulik et al., Reference Bulik, Thornton, Root, Pisetsky, Lichtenstein and Pedersen2010). The significant genetic and environmental correlations found in both studies may reflect nosological overlap, but may also be explained by biological pleiotropic effects on both disorders and even other explanations, such as a causal influence of one disorder on the other (Martin et al., Reference Martin, Taylor and Lichtenstein2018). Nevertheless, there is no clear hypothesis on a directed causal effect between the two disorders, as transmission between anorexia nervosa and bulimia nervosa has been observed in both directions in clinical settings; approximately 10–54% patients with anorexia nervosa were later diagnosed with bulimia nervosa and approximately 2–27% with bulimia nervosa were later diagnosed with anorexia nervosa (Peat et al., Reference Peat, Mitchell, Hoek and Wonderlich2009). It was also difficult to determine the temporal order of the onset of the two disorders based on registered diagnosis in the treatment-seeking sample, as the date of diagnosis was not necessarily the date of onset. Therefore, we did not attempt to discern the directionality of phenotypic association. Studies with accurate measures of the temporal order of the disorders (e.g. age at onset of the eating disorders) and pathological questions may be able to elucidate the developmental process underlying a directed association between anorexia nervosa and bulimia nervosa.
The observed genetic correlation between the two eating disorders was higher than that found between eating disorders and other psychiatric disorders. The highest genetic correlations were found between anorexia nervosa and obsessive-compulsive disorder [0.52 (0.26–0.81)] and between anorexia nervosa and major depression (around 0.5 in multiple studies) (Wade et al., Reference Wade, Bulik, Neale and Kendler2000; Cederlof et al., Reference Cederlof, Thornton, Baker, Lichtenstein, Larsson, Ruck, Bulik and Mataix-Cols2015; Thornton et al., Reference Thornton, Welch, Munn-Chernoff, Lichtenstein and Bulik2016), which were comparable but lower than the genetic correlation we estimated between the two eating disorders [0.66, 95% CI (0.49–0.82)]. SNP-based genetic correlation between anorexia nervosa and schizophrenia was lower [0.19 (0.11–0.27)] (Bulik-Sullivan et al., Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day, Loh, Duncan, Perry, Patterson, Robinson, Daly, Price and Neale2015), as well as the genetic correlations with attention-deficit hyperactivity disorder in the same study population [0.14 (0.05–0.22) for anorexia nervosa and 0.37 (0.31–0.42) for bulimia nervosa] (Yao et al., Reference Yao, Kuja-Halkola, Martin, Lu, Lichtenstein, Norring, Birgegard, Yilmaz, Huebel, Watson, Baker, Almqvist, Thornton, Magnusson, Bulik and Larsson2019). Such differences might reflect greater degrees of pleiotropy between the two eating disorders than between eating disorders and other psychiatric disorders.
In addition, we reported significant unique environmental effects, both disorder-specific and common to the two eating disorders. Previous research suggested various potential environmental risk factors for eating disorders, such as dieting and events that trigger mood change and thin-ideal internalization (Chua et al., Reference Chua, Touyz and Hill2004; Striegel-Moore and Bulik, Reference Striegel-Moore and Bulik2007; Haedt-Matt et al., Reference Haedt-Matt, Zalta, Forbush and Keel2012). Notably, environmental events shared between siblings might produce unique effects in each individual (Klump et al., Reference Klump, Wonderlich, Lehoux, Lilenfeld and Bulik2002). For instance, people might have different degrees of thin-ideal internalization under the same social or media exposure. Such differences might be influenced by the age, sex, personality traits and other factors of the individuals, which may also interact with genetic risks (Culbert et al., Reference Culbert, Racine and Klump2015) and need to be considered in risk factor research.
Using extended familial relatedness drastically increased the available sample size of the models in our study, and the use of clinical diagnostic information increased the clinical significance of the results. Our results are consistent with the prior twin study on the overlap between anorexia nervosa and bulimia nervosa based on self-reported measures of eating behaviours (Bulik et al., Reference Bulik, Thornton, Root, Pisetsky, Lichtenstein and Pedersen2010). The consistency confirms the comparability between self-reported eating behavioural measures and clinical measures. Our definition of anorexia nervosa mirrors the definition used in the anorexia nervosa GWAS (Boraska et al., Reference Boraska, Franklin, Floyd, Thornton, Huckins, Southam, Rayner, Tachmazidou, Klump, Treasure, Lewis, Schmidt, Tozzi, Kiezebrink, Hebebrand, Gorwood, Adan, Kas, Favaro, Santonastaso, Fernandez-Aranda, Gratacos, Rybakowski, Dmitrzak-Weglarz, Kaprio, Keski-Rahkonen, Raevuori, Van Furth, Slof-Op't Landt, Hudson, Reichborn-Kjennerud, Knudsen, Monteleone, Kaplan, Karwautz, Hakonarson, Berrettini, Guo, Li, Schork, Komaki, Ando, Inoko, Esko, Fischer, Mannik, Metspalu, Baker, Cone, Dackor, DeSocio, Hilliard, O'Toole, Pantel, Szatkiewicz, Taico, Zerwas, Trace, Davis, Helder, Buhren, Burghardt, de Zwaan, Egberts, Ehrlich, Herpertz-Dahlmann, Herzog, Imgart, Scherag, Scherag, Zipfel, Boni, Ramoz, Versini, Brandys, Danner, de Kovel, Hendriks, Koeleman, Ophoff, Strengman, van Elburg, Bruson, Clementi, Degortes, Forzan, Tenconi, Docampo, Escaramis, Jimenez-Murcia, Lissowska, Rajewski, Szeszenia-Dabrowska, Slopien, Hauser, Karhunen, Meulenbelt, Slagboom, Tortorella, Maj, Dedoussis, Dikeos, Gonidakis, Tziouvas, Tsitsika, Papezova, Slachtova, Martaskova, Kennedy, Levitan, Yilmaz, Huemer, Koubek, Merl, Wagner, Lichtenstein, Breen, Cohen-Woods, Farmer, McGuffin, Cichon, Giegling, Herms, Rujescu, Schreiber, Wichmann, Dina, Sladek, Gambaro, Soranzo, Julia, Marsal, Rabionet, Gaborieau, Dick, Palotie, Ripatti, Widen, Andreassen, Espeseth, Lundervold, Reinvang, Steen, Le Hellard, Mattingsdal, Ntalla, Bencko, Foretova, Janout, Navratilova, Gallinger, Pinto, Scherer, Aschauer, Carlberg, Schosser, Alfredsson, Ding, Klareskog, Padyukov, Courtet, Guillaume, Jaussent, Finan, Kalsi, Roberts, Logan, Peltonen, Ritchie, Barrett, Estivill, Hinney, Sullivan, Collier, Zeggini and Bulik2014; Duncan et al., Reference Duncan, Yilmaz, Gaspar, Walters, Goldstein, Anttila, Bulik-Sullivan, Ripke, Thornton, Hinney, Daly, Sullivan, Zeggini, Breen and Bulik2017; Watson and Yilmaz, Reference Watson, Yilmaz, Thornton, Hubel, Coleman, Gaspar, Bryois, Hinney, Leppa, Mattheisen, Medland, Ripke, Yao, Giusti-Rodriguez, Anorexia Nervosa Genetics, Hanscombe, Purves, Adan, Alfredsson, Ando, Andreassen, Baker, Berrettini, Boehm, Boni, Perica, Buehren, Burghardt, Cassina, Cichon, Clementi, Cone, Courtet, Crow, Crowley, Danner, Davis, de Zwaan, Dedoussis, Degortes, DeSocio, Dick, Dikeos, Dina, Dmitrzak-Weglarz, Docampo, Duncan, Egberts, Ehrlich, Escaramis, Esko, Estivill, Farmer, Favaro, Fernandez-Aranda, Fichter, Fischer, Focker, Foretova, Forstner, Forzan, Franklin, Gallinger, Giegling, Giuranna, Gonidakis, Gorwood, Mayora, Guillaume, Guo, Hakonarson, Hatzikotoulas, Hauser, Hebebrand, Helder, Herms, Herpertz-Dahlmann, Herzog, Huckins, Hudson, Imgart, Inoko, Janout, Jimenez-Murcia, Julia, Kalsi, Kaminska, Kaprio, Karhunen, Karwautz, Kas, Kennedy, Keski-Rahkonen, Kiezebrink, Kim, Klareskog, Klump, Knudsen, La Via, Le Hellard, Levitan, Li, Lilenfeld, Lin, Lissowska, Luykx, Magistretti, Maj, Mannik, Marsal, Marshall, Mattingsdal, McDevitt, McGuffin, Metspalu, Meulenbelt, Micali, Mitchell, Monteleone, Monteleone, Munn-Chernoff, Nacmias, Navratilova, Ntalla, O'Toole, Ophoff, Padyukov, Palotie, Pantel, Papezova, Pinto, Rabionet, Raevuori, Ramoz, Reichborn-Kjennerud, Ricca, Ripatti, Ritschel, Roberts, Rotondo, Rujescu, Rybakowski, Santonastaso, Scherag, Scherer, Schmidt, Schork, Schosser, Seitz, Slachtova, Slagboom, Slof-Op't Landt, Slopien, Sorbi, Swiatkowska, Szatkiewicz, Tachmazidou, Tenconi, Tortorella, Tozzi, Treasure, Tsitsika, Tyszkiewicz-Nwafor, Tziouvas, van Elburg, van Furth, Wagner, Walton, Widen, Zeggini, Zerwas, Zipfel, Bergen, Boden, Brandt, Crawford, Halmi, Horwood, Johnson, Kaplan, Kaye, Mitchell, Olsen, Pearson, Pedersen, Strober, Werge, Whiteman, Woodside, Stuber, Gordon, Grove, Henders, Jureus, Kirk, Larsen, Parker, Petersen, Jordan, Kennedy, Montgomery, Wade, Birgegard, Lichtenstein, Norring, Landen, Martin, Mortensen, Sullivan, Breen and Bulik2019), which also allowed for diagnostic crossover with bulimia nervosa. The largest and most recent GWAS (Watson and Yilmaz, Reference Watson, Yilmaz, Thornton, Hubel, Coleman, Gaspar, Bryois, Hinney, Leppa, Mattheisen, Medland, Ripke, Yao, Giusti-Rodriguez, Anorexia Nervosa Genetics, Hanscombe, Purves, Adan, Alfredsson, Ando, Andreassen, Baker, Berrettini, Boehm, Boni, Perica, Buehren, Burghardt, Cassina, Cichon, Clementi, Cone, Courtet, Crow, Crowley, Danner, Davis, de Zwaan, Dedoussis, Degortes, DeSocio, Dick, Dikeos, Dina, Dmitrzak-Weglarz, Docampo, Duncan, Egberts, Ehrlich, Escaramis, Esko, Estivill, Farmer, Favaro, Fernandez-Aranda, Fichter, Fischer, Focker, Foretova, Forstner, Forzan, Franklin, Gallinger, Giegling, Giuranna, Gonidakis, Gorwood, Mayora, Guillaume, Guo, Hakonarson, Hatzikotoulas, Hauser, Hebebrand, Helder, Herms, Herpertz-Dahlmann, Herzog, Huckins, Hudson, Imgart, Inoko, Janout, Jimenez-Murcia, Julia, Kalsi, Kaminska, Kaprio, Karhunen, Karwautz, Kas, Kennedy, Keski-Rahkonen, Kiezebrink, Kim, Klareskog, Klump, Knudsen, La Via, Le Hellard, Levitan, Li, Lilenfeld, Lin, Lissowska, Luykx, Magistretti, Maj, Mannik, Marsal, Marshall, Mattingsdal, McDevitt, McGuffin, Metspalu, Meulenbelt, Micali, Mitchell, Monteleone, Monteleone, Munn-Chernoff, Nacmias, Navratilova, Ntalla, O'Toole, Ophoff, Padyukov, Palotie, Pantel, Papezova, Pinto, Rabionet, Raevuori, Ramoz, Reichborn-Kjennerud, Ricca, Ripatti, Ritschel, Roberts, Rotondo, Rujescu, Rybakowski, Santonastaso, Scherag, Scherer, Schmidt, Schork, Schosser, Seitz, Slachtova, Slagboom, Slof-Op't Landt, Slopien, Sorbi, Swiatkowska, Szatkiewicz, Tachmazidou, Tenconi, Tortorella, Tozzi, Treasure, Tsitsika, Tyszkiewicz-Nwafor, Tziouvas, van Elburg, van Furth, Wagner, Walton, Widen, Zeggini, Zerwas, Zipfel, Bergen, Boden, Brandt, Crawford, Halmi, Horwood, Johnson, Kaplan, Kaye, Mitchell, Olsen, Pearson, Pedersen, Strober, Werge, Whiteman, Woodside, Stuber, Gordon, Grove, Henders, Jureus, Kirk, Larsen, Parker, Petersen, Jordan, Kennedy, Montgomery, Wade, Birgegard, Lichtenstein, Norring, Landen, Martin, Mortensen, Sullivan, Breen and Bulik2019) has reported eight genome-wide-significant genetic polymorphism loci for anorexia nervosa and supported a polygenic aetiology. The genetic correlation found in this study and previous twin research should be revisited using molecular genetic approaches once the GWAS of bulimia nervosa is available.
Limitations of the current study would arise if there are violations of basic assumptions of the models, such as the assumption of equal shared environmental factors across different types of siblings [equal environment assumption, EEA (Plomin et al., Reference Plomin, DeFries, Knopik and Neiderhiser2013)] and the assumption of random mating in the population (Nordsletten et al., Reference Nordsletten, Larsson, Crowley, Almqvist, Lichtenstein and Mataix-Cols2016). Previous research has validated EEA in twin studies for multiple psychiatric outcomes (Kendler et al., Reference Kendler, Neale, Kessler, Heath and Eaves1993; Conley et al., Reference Conley, Rauscher, Dawes, Magnusson and Siegal2013). Although we were unable to validate EEA in full- and maternal half-siblings in our study population, we argue that EEA is as likely to be valid in these two types of sisters as most (91%) children lived with mothers after parental separation (1994). Violation of the random mating assumption threatens the validity of heritability estimation (Plomin et al., Reference Plomin, DeFries, Knopik and Neiderhiser2013). Non-random mating has been observed in several psychiatric traits (Frisell et al., Reference Frisell, Pawitan, Langstrom and Lichtenstein2012; Nordsletten et al., Reference Nordsletten, Larsson, Crowley, Almqvist, Lichtenstein and Mataix-Cols2016) and could lead to underestimated heritability in twin studies, as it makes dizygotic twins more genetically similar but does not influence the genetic similarity between monozygotic twins (Plomin et al., Reference Plomin, DeFries, Knopik and Neiderhiser2013). However, its influence on heritability estimated from full- and half-sibling data is less predictable. The genetic correlation between two full-sisters might be higher than 0.5 due to non-random mating. However, whether the genetic correlation between two maternal half-sisters in a pair is higher or lower than 0.25 depends on whether their fathers are more or less genetically similar than two random individuals in the population. In addition, the direction and magnitude of bias in heritability estimation due to non-random mating in sibling designs are influenced by how much the genetic correlation between half-sisters deviated from 0.25 in relation to how much the genetic correlation between full-sisters deviated from 0.5. We did not examine the effect of non-random mating on the results, but previous research has suggested that its impact on heritability estimation was mostly mild in twin and sibling studies (Frisell et al., Reference Frisell, Pawitan, Langstrom and Lichtenstein2012; Peyrot et al., Reference Peyrot, Robinson, Penninx and Wray2016). Given that the binge-eating and purging type of anorexia nervosa share some core features with bulimia nervosa, distinguishing between different subtypes of anorexia nervosa could potentially provide more insight in understanding the aetiological overlap. However, limited by the available ICD-based diagnostic information in the NPR, we were unable to distinguish the anorexia nervosa subtypes. Nevertheless, the largest GWAS of anorexia nervosa yet conducted (Watson and Yilmaz, Reference Watson, Yilmaz, Thornton, Hubel, Coleman, Gaspar, Bryois, Hinney, Leppa, Mattheisen, Medland, Ripke, Yao, Giusti-Rodriguez, Anorexia Nervosa Genetics, Hanscombe, Purves, Adan, Alfredsson, Ando, Andreassen, Baker, Berrettini, Boehm, Boni, Perica, Buehren, Burghardt, Cassina, Cichon, Clementi, Cone, Courtet, Crow, Crowley, Danner, Davis, de Zwaan, Dedoussis, Degortes, DeSocio, Dick, Dikeos, Dina, Dmitrzak-Weglarz, Docampo, Duncan, Egberts, Ehrlich, Escaramis, Esko, Estivill, Farmer, Favaro, Fernandez-Aranda, Fichter, Fischer, Focker, Foretova, Forstner, Forzan, Franklin, Gallinger, Giegling, Giuranna, Gonidakis, Gorwood, Mayora, Guillaume, Guo, Hakonarson, Hatzikotoulas, Hauser, Hebebrand, Helder, Herms, Herpertz-Dahlmann, Herzog, Huckins, Hudson, Imgart, Inoko, Janout, Jimenez-Murcia, Julia, Kalsi, Kaminska, Kaprio, Karhunen, Karwautz, Kas, Kennedy, Keski-Rahkonen, Kiezebrink, Kim, Klareskog, Klump, Knudsen, La Via, Le Hellard, Levitan, Li, Lilenfeld, Lin, Lissowska, Luykx, Magistretti, Maj, Mannik, Marsal, Marshall, Mattingsdal, McDevitt, McGuffin, Metspalu, Meulenbelt, Micali, Mitchell, Monteleone, Monteleone, Munn-Chernoff, Nacmias, Navratilova, Ntalla, O'Toole, Ophoff, Padyukov, Palotie, Pantel, Papezova, Pinto, Rabionet, Raevuori, Ramoz, Reichborn-Kjennerud, Ricca, Ripatti, Ritschel, Roberts, Rotondo, Rujescu, Rybakowski, Santonastaso, Scherag, Scherer, Schmidt, Schork, Schosser, Seitz, Slachtova, Slagboom, Slof-Op't Landt, Slopien, Sorbi, Swiatkowska, Szatkiewicz, Tachmazidou, Tenconi, Tortorella, Tozzi, Treasure, Tsitsika, Tyszkiewicz-Nwafor, Tziouvas, van Elburg, van Furth, Wagner, Walton, Widen, Zeggini, Zerwas, Zipfel, Bergen, Boden, Brandt, Crawford, Halmi, Horwood, Johnson, Kaplan, Kaye, Mitchell, Olsen, Pearson, Pedersen, Strober, Werge, Whiteman, Woodside, Stuber, Gordon, Grove, Henders, Jureus, Kirk, Larsen, Parker, Petersen, Jordan, Kennedy, Montgomery, Wade, Birgegard, Lichtenstein, Norring, Landen, Martin, Mortensen, Sullivan, Breen and Bulik2019) did not find evidence for different polygenic architecture between the restricting and the binge-eating and purging subtypes of anorexia nervosa, although the result should be interpreted carefully in light of low statistical power. Another limitation was that we were only able to detect individuals who were listed in the medical registers. Individuals who had the disorders but were not listed in the registers were therefore misclassified as not having the disorder. Such misclassification might be more common for bulimia nervosa than anorexia nervosa, as we observe a lower than expected prevalence (Smink et al., Reference Smink, van Hoeken and Hoek2012) and bulimia nervosa was not an independent diagnosis in the Swedish version of ICD-9. We were unable to test how such misclassification influenced the results. The issue of misclassification is related to how well a clinical diagnosis reflects the symptom spectrum. A Norwegian twin study showed high genetic correlations between clinical diagnosis and interview-based diagnosis (around 0.8) for major depression and autism spectrum disorder, even though the two methods identified different individuals; however, the genetic correlation between the two measures for alcohol use disorder was lower with wide confidence interval (Torvik et al., Reference Torvik, Ystrom, Gustavson, Rosenstrom, Bramness, Gillespie, Aggen, Kendler and Reichborn-Kjennerud2018). Nevertheless, we argue that clinical diagnoses might reflect the more severe end of the spectrum of disordered eating behaviours, and the estimated heritability and genetic correlation might be restricted to the more severe cases.
To our knowledge, this is the first quantitative genetic study on clinically diagnosed anorexia nervosa and bulimia nervosa and their overlap. The moderate and statistically significant genetic and environmental correlations between the two eating disorders might suggest aetiological overlaps and emphasize clinical vigilance of the transition between the two disorders.
Acknowledgements
We acknowledge the financial support and access to data: S.Y. acknowledges financial support from the China Scholarship Council. C.M.B. acknowledges funding from the Swedish Research Council (VR Dnr: 538-2013-8864). H.L. acknowledges financial support from the Swedish Research Council (2014-3831). The project has also received funding from the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework Grant no. 340-2013-5867.







