Patients with medically unexplained symptoms of 3 months' duration or more are common in primary care (Reference Peveler, Kilkenny and KinmonthPeveler et al, 1997) and expensive to health services (Reference Barsky, Orav and BatesBarsky et al, 2005). Patients are too numerous for specialist care and prefer to see their general practitioner (GP) rather than any other health professional (Reference Kirmayer and RobbinsKirmayer & Robbins, 1996; Reference Arnold, Speckens and van HemertArnold et al, 2004). Reattribution is a structured consultation delivered by GPs which aims to provide a psychological explanation to patients with somatised mental disorder (Reference Goldberg, Gask and O'DowdGoldberg et al, 1989). Preliminary evidence suggested that it may be effective in reducing mental disorder and health costs associated with medically unexplained symptoms. It may also increase function, and both GP and patient satisfaction (Morriss et al, Reference Morriss, Gask and Ronalds1998, Reference Morriss, Gask and Ronalds1999; Reference BlankensteinBlankenstein, 2001; Reference Morriss and GaskMorriss & Gask, 2002; Reference Larisch, Schweickhardt and WirschingLarisch et al, 2004; Reference Rosendal, Bro and SokolowskiRosendal et al, 2005). However, these studies have methodological limitations such as lack of randomisation, contamination and failure to demonstrate that communication in the consultation changed after training. Reattribution was taught by experts who would not train GPs in routine practice and the training covered more than reattribution.
METHOD
Aims
The current study aimed to determine the effects of reattribution training on doctor–patient communication as the primary outcome, and clinical outcomes and service use as secondary outcomes in patients with medically unexplained symptoms of 3 months' duration or longer, using non-expert trainers to train all GPs in a practice compared with treatment as usual. We hypothesised that reattribution would improve doctor–patient communication in a consultation by providing an explanation that would change the beliefs of patients about their bodily symptoms, and that in turn patient satisfaction with GP care would improve, emotional distress would reduce and use of healthcare resources would diminish (Reference Morriss and GaskMorriss & Gask, 2002; Reference Morriss, Dowrick and SalmonMorriss et al, 2006). The changes in symptom beliefs held by the patient were in relation to the nature of the problem (from physical to emotional and ‘don't know’ to emotional), timeline (to a shorter duration), consequences (less severe impact on their life) and controllability (more under the patient's control) (Reference Morriss, Dowrick and SalmonMorriss et al, 2006).
Study design
The study is a cluster randomised controlled trial (MUST; ISRCTN44384258) with the practice as the unit of randomisation. Practice and patient recruitment, method and rationale for the study design, details of outcome measures, method, uptake and acceptability of the training intervention are described elsewhere (Reference Morriss, Dowrick and SalmonMorriss et al, 2006). In summary, 16 practices were recruited in the north-west of England from four areas with similar socio-demographic characteristics: East Lancashire, Greater Manchester, Liverpool and Wirral. Eight practices were randomised to reattribution training (by G.D.) using a computer-generated sequence and eight practices were controls. Two practices from each of the four areas were randomised to reattribution training and two practices to the control group. The randomisation sequence was communicated to the trial coordinator and trainers by telephone but to no other member of the research team until all patients completed follow-up. Once reattribution training was completed, patients were recruited by a researcher by screening consecutive patients attending a surgery in the waiting room. They were interviewed again at 1 month and completed a postal questionnaire at 3 months. Health records for each patient were examined at the end of the study. In addition, qualitative interviews were performed with participating and non-participating GPs and participating patients to explore barriers and drivers to the delivery and effectiveness of reattribution training (Reference Morriss, Dowrick and SalmonMorriss et al, 2006). The methods and results of the qualitative studies will be reported separately. The study received ethical approval from the North-West Multi-centre Research Ethics Committee.
Inclusion/exclusion criteria
Practices were included if all GP principals were willing to attend reattribution training and be randomised to either arm of the study. Practices were excluded if one or more GP had received the training previously. Patients were included if:
-
(a) the primary reason for consultation was a physical symptom(s) of 3 months' duration or longer;
-
(b) they were 18 years of age or older;
-
(c) an independent research GP (H.C.-J.), on the basis of the history obtained 1 month after the baseline consultation and all information in the practice notes, decided that the physical symptom and/or the impairment associated with the physical symptom were not explained by physical pathology.
Patients were excluded if:
-
(a) they refused to give written informed consent for data collection;
-
(b) they were already receiving psychological treatment or had been prescribed a new psychotropic drug in the preceding 3 months;
-
(c) their GP or the research GP stated that they had definite physical pathology that explained the presence of the symptom and the associated impairment.
Patients were recruited from January 2004 to July 2005. Follow-up data were collected by May 2006.
Outcome measures
The primary outcome data were the audiotaped and transcribed index consultations between GP and patient. All names and places were removed from the transcript so that both raters (L.G. and R.C.) were masked to the intervention group. The raters then assessed the transcribed consultation according to terms defined in a manual (Reference Morriss, Dowrick and SalmonMorriss et al, 2006). For the training to be regarded as successful, we required a difference between training and control groups on the primary outcome variable, the overall proportion of the consultation that was consistent with the reattribution model on a five-point scale (none, isolated, some, most, all) and a difference in the total score for each communication behaviour at three stages of the consultation (feeling understood, broadening the agenda, making the link) according to the reattribution model. We also examined the following individual items of communication that were specific to reattribution in previous studies (Reference Kaaya, Goldberg and GaskKaaya et al, 1992; Reference Morriss, Gask and RonaldsMorriss et al, 1999):
-
(a) exploring health beliefs (yes/no);
-
(b) summarising family and social factors (yes/no);
-
(c) quality of the ‘making the link’ explanation (0, = no attempt or incomplete attempt; 1, = at least one complete explanation given).
Secondary outcome measures were: (a) satisfaction of the patient with seven aspects of GP communication, including whether overall the patient received the help they wanted (Patient Satisfaction Questionnaire; Reference Morriss and GaskMorriss & Gask, 2002); (b) patients' symptom beliefs (Reference Morriss and GaskMorriss & Gask, 2002), notably the proportion of patients endorsing a physical, emotional or ‘don't know’ cause for their symptoms, and beliefs about timeline, consequences and ability to control symptoms (Reference Moss-Morris, Weinman and PetrieMoss-Morris et al, 2002); (c) caseness for anxiety or depression, measured as a score of 8 or more on the Hospital Anxiety Scale or Hospital Depression Scale (Reference Zigmond and SnaithZigmond & Snaith, 1983); (d) health anxiety measured by the 14-item Whitely Index (Reference PilowskyPilowsky, 1967); (e) quality of life on the EQ–5D (EuroQol Group, 1990), which yields an index score and a visual analogue scale score of overall health (health perception); (f) records of prescriptions, investigations and health contacts obtained from patient interview and primary care records (Reference Morriss, Gask and RonaldsMorriss et al, 1998).
Training intervention
Three nurses and a psychologist (health facilitators) with professional experience in primary care or liaison psychiatry but no reattribution training were trained by an expert (L.G.) in 5 days over a 2-month period immediately prior to the training of practices. The training covered the reattribution training package (Reference Morriss, Dowrick and SalmonMorriss et al, 2006), including the specifically prepared videotaped training materials, the reattribution model (Table 1), opportunities to role-play in order to learn specific communication skills and opportunities for videotaped feedback of actual performance with role-played or real-life patients. The aim of reattribution training is to generate the information to provide a simple three-stage psychological explanation (symptom, psychosocial problem, physiological or temporal mechanism linking symptom to psychosocial problem) for the patient's medically unexplained symptoms through negotiation between the GP and patient (Reference Goldberg, Gask and O'DowdGoldberg et al, 1989). The health facilitators were also taught methods in adult education to change skills, attitudes, knowledge, and to facilitate groups of adult learners, the principles of academic detailing and skills-based training, and practical issues in relation to interacting with primary care.
Table 1 Content of the reattribution intervention

| Stage | Content |
|---|---|
| Feeling understood | Elicit physical symptoms, psychosocial problems, mood state, beliefs held by patient about their problem, relevant physical examination and investigations |
| Broadening the agenda | Summarise physical and psychosocial findings. Negotiate these findings with patient |
| Making the link | Give explanation relating physical symptom to psychosocial problems of lifestyle because of link in time or physiology |
| Negotiating further treatment | Arrange follow-up or treatment of symptoms, psychosocial problems or mental disorder |
Each health facilitator trained two practices separately in one of four geographical areas in north-west England (Liverpool, Wirral, Greater Manchester and East Lancashire). They delivered three 2-hour training sessions at the practice work base to groups of GPs from the same practice at a time when all GPs in the practice were released from routine work. If a GP missed a training session, the health facilitator and GP would arrange a similar training session on a one-to-one basis, ideally before the next practice training session.
All eligible GPs (n=34) and one nurse practitioner in the eight allocated practices completed the training; 32 (91%) attended all three training sessions and 3 received individual training for one session and practice training for the other two sessions. Immediate postal feedback on the training was independently completed by 27 (77%) of the practitioners and revealed that after training 22 practitioners felt confident or very confident in managing patients with medically unexplained symptoms, although 5 (18%) were uncertain or unchanged in confidence.
Statistical analysis
The study was powered to examine communication outcomes. Assuming communication behaviour was consistent with reattribution in 70% of consultations after training (Reference BlankensteinBlankenstein, 2001) and 30% in the control group (Reference Kaaya, Goldberg and GaskKaaya et al, 1992; Reference Morriss, Gask and RonaldsMorriss et al, 1999), 65 consultations were required (90% power, 5% significance level, two-tailed chi-squared test). A correction factor for clustering of two (Reference Morriss, Dowrick and SalmonMorriss et al, 2006) doubled the sample size to 130 consultations; 140 consultations were required to allow for technical failures in audiotaping and transcribing in 5–10% of consultations.
All statistical analyses were carried out on an intention-to-treat basis using Stata Version 8. Treatment effects (either group differences for quantitative outcomes or odds ratios for binary outcomes) were estimated using Stata's gllamm (generalised linear latent and mixed models) command (Reference Rabe-Hesketh, Skrondal and PicklesRabe-Hesketh et al, 2002) by fitting three-level random effects models (with appropriate specification of distribution and link function depending on whether outcomes were binary or quantitative) allowing for clustering (random effects) at the level of both practice and individual GP. All models included age and gender as covariates and assumed any missing data were ‘missing at random’, i.e. the probability of a missing value is independent of actual outcome given fixed and random effects specified by the model. All data on use of healthcare resources other than consultation time were highly skewed so bootstrapping sampling using 1000 replications was used to estimate the effect size and 95% CI.
RESULTS
Recruitment, flow and follow-up
Practice recruitment has been described previously (Reference Morriss, Dowrick and SalmonMorriss et al, 2006). Sixteen practices, 74 GPs and 1 nurse practitioner were recruited. Patient recruitment, flow into the study and follow-up are shown in Fig. 1. We recruited 141 patients with medically unexplained symptoms. The main presenting symptoms were pain (n=80, 57%), bowel problems (n=13, 9%) and fatigue (n=10, 7%) with a wide range of other symptoms. Table 2 shows baseline characteristics of patients. Multiple presenting symptoms were offered by 32 (23%) patients. Patients who entered the trial did not differ from those who attended the surgeries run by the GPs in terms of age, but there were 10% more females in the study (data not shown).

Fig. 1 Trial CONSORT diagram. GP, general practitioner.
Table 2 Baseline characteristics of patients with medically unexplained symptoms
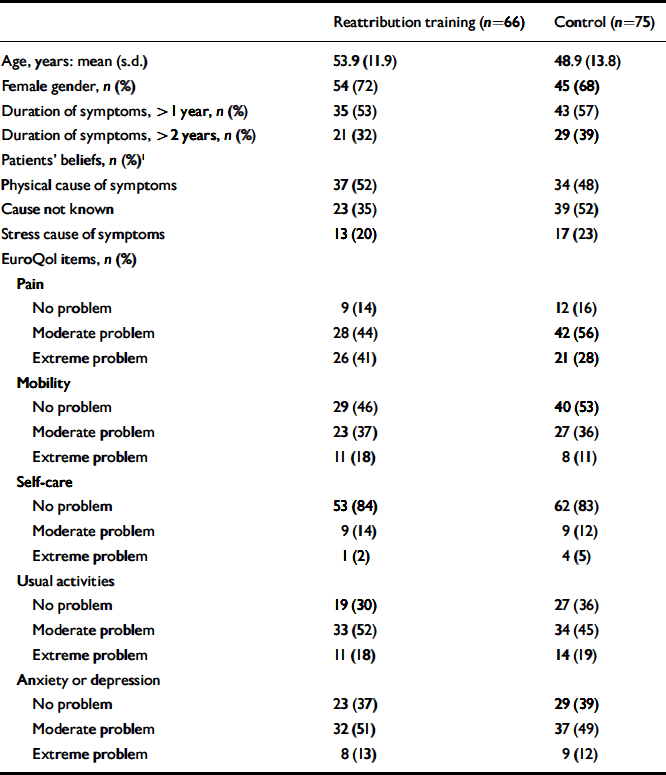
| Reattribution training (n=66) | Control (n=75) | |
|---|---|---|
| Age, years: mean (s.d.) | 53.9 (11.9) | 48.9 (13.8) |
| Female gender, n (%) | 54 (72) | 45 (68) |
| Duration of symptoms, > 1 year, n (%) | 35 (53) | 43 (57) |
| Duration of symptoms, > 2 years, n (%) | 21 (32) | 29 (39) |
| Patients' beliefs, n (%) 1 | ||
| Physical cause of symptoms | 37 (52) | 34 (48) |
| Cause not known | 23 (35) | 39 (52) |
| Stress cause of symptoms | 13 (20) | 17 (23) |
| EuroQol items, n (%) | ||
| Pain | ||
| No problem | 9 (14) | 12 (16) |
| Moderate problem | 28 (44) | 42 (56) |
| Extreme problem | 26 (41) | 21 (28) |
| Mobility | ||
| No problem | 29 (46) | 40 (53) |
| Moderate problem | 23 (37) | 27 (36) |
| Extreme problem | 11 (18) | 8 (11) |
| Self-care | ||
| No problem | 53 (84) | 62 (83) |
| Moderate problem | 9 (14) | 9 (12) |
| Extreme problem | 1 (2) | 4 (5) |
| Usual activities | ||
| No problem | 19 (30) | 27 (36) |
| Moderate problem | 33 (52) | 34 (45) |
| Extreme problem | 11 (18) | 14 (19) |
| Anxiety or depression | ||
| No problem | 23 (37) | 29 (39) |
| Moderate problem | 32 (51) | 37 (49) |
| Extreme problem | 8 (13) | 9 (12) |
1. Patients' beliefs about the causes of their medically unexplained symptoms were not mutually exclusive
Doctor–patient communication
Interrater agreement on ten audiotapes for the proportion of the consultation that was consistent with reattribution was 100% to one point on the five-point scale at the beginning of recruitment and 90% at the end of recruitment. Table 3 shows that there were substantial improvements with training in the overall proportion of the doctor–patient consultation mostly consistent with the reattribution model, the quality of the first three stages of reattribution and two (exploring health beliefs, quality of making the link explanation itself) of the three characteristic features of reattribution consultation behaviour. In the group with the reattribution training the feeling understood stage of consultation was completed in 46 (71%) consultations compared with only 21 (32%) in the control group. The proportion of the consultation that was consistent with reattribution did not change with the length of time since training was delivered (up to 18 months later).
Table 3 Effects of reattribution training on doctor–patient communication at index consultation
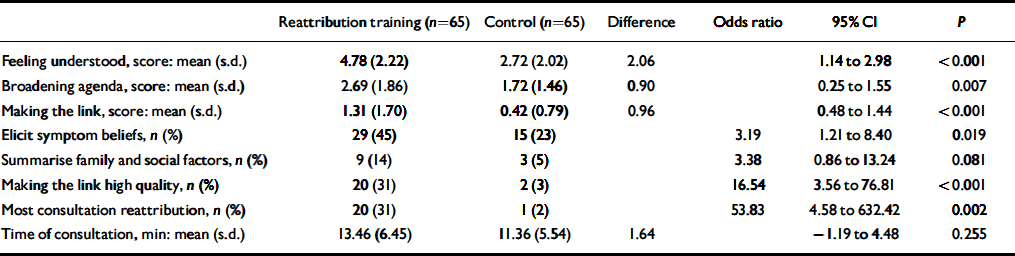
| Reattribution training (n=65) | Control (n=65) | Difference | Odds ratio | 95% CI | P | |
|---|---|---|---|---|---|---|
| Feeling understood, score: mean (s.d.) | 4.78 (2.22) | 2.72 (2.02) | 2.06 | 1.14 to 2.98 | <0.001 | |
| Broadening agenda, score: mean (s.d.) | 2.69 (1.86) | 1.72 (1.46) | 0.90 | 0.25 to 1.55 | 0.007 | |
| Making the link, score: mean (s.d.) | 1.31 (1.70) | 0.42 (0.79) | 0.96 | 0.48 to 1.44 | <0.001 | |
| Elicit symptom beliefs, n (%) | 29 (45) | 15 (23) | 3.19 | 1.21 to 8.40 | 0.019 | |
| Summarise family and social factors, n (%) | 9 (14) | 3 (5) | 3.38 | 0.86 to 13.24 | 0.081 | |
| Making the link high quality, n (%) | 20 (31) | 2 (3) | 16.54 | 3.56 to 76.81 | <0.001 | |
| Most consultation reattribution, n (%) | 20 (31) | 1 (2) | 53.83 | 4.58 to 632.42 | 0.002 | |
| Time of consultation, min: mean (s.d.) | 13.46 (6.45) | 11.36 (5.54) | 1.64 | –1.19 to 4.48 | 0.255 |
Secondary outcome measures
Table 4 shows that the expected pattern of improvement in secondary clinical outcomes with reattribution was not seen by 3 months. Reattribution training was associated non-significantly with improved patient satisfaction with the help they received from their GP (and on each of the other six items of the satisfaction scale), and a greater proportion of patients knew the cause of their symptoms and endorsed an emotional cause. However, reattribution training was associated with worse self-rating of overall health and, non-significantly, with more possible cases of anxiety and beliefs that problems might last longer, have more serious consequences or be less under their control. Training had no effects on caseness for depression, health anxiety (Table 4) or on use of healthcare resources (Table 5).
Table 4 Intention to treat analysis of patient outcomes following reattribution training of general practitioners
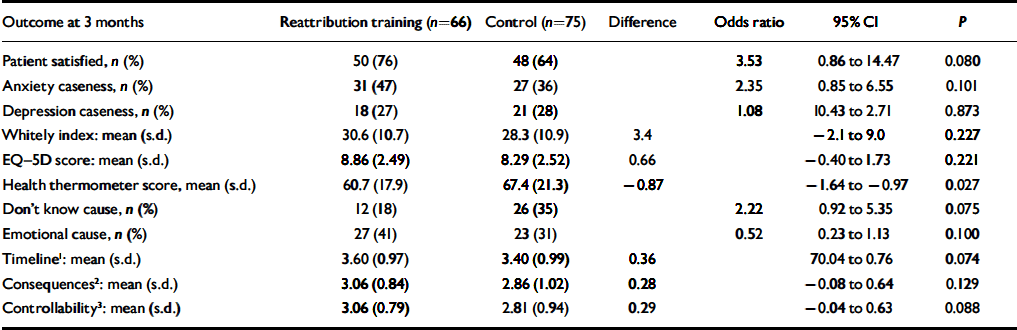
| Outcome at 3 months | Reattribution training (n=66) | Control (n=75) | Difference | Odds ratio | 95% CI | P |
|---|---|---|---|---|---|---|
| Patient satisfied, n (%) | 50 (76) | 48 (64) | 3.53 | 0.86 to 14.47 | 0.080 | |
| Anxiety caseness, n (%) | 31 (47) | 27 (36) | 2.35 | 0.85 to 6.55 | 0.101 | |
| Depression caseness, n (%) | 18 (27) | 21 (28) | 1.08 | 10.43 to 2.71 | 0.873 | |
| Whitely index: mean (s.d.) | 30.6 (10.7) | 28.3 (10.9) | 3.4 | –2.1 to 9.0 | 0.227 | |
| EQ–5D score: mean (s.d.) | 8.86 (2.49) | 8.29 (2.52) | 0.66 | –0.40 to 1.73 | 0.221 | |
| Health thermometer score, mean (s.d.) | 60.7 (17.9) | 67.4 (21.3) | –0.87 | –1.64 to −0.97 | 0.027 | |
| Don't know cause, n (%) | 12 (18) | 26 (35) | 2.22 | 0.92 to 5.35 | 0.075 | |
| Emotional cause, n (%) | 27 (41) | 23 (31) | 0.52 | 0.23 to 1.13 | 0.100 | |
| Timeline 1 : mean (s.d.) | 3.60 (0.97) | 3.40 (0.99) | 0.36 | 70.04 to 0.76 | 0.074 | |
| Consequences 2 : mean (s.d.) | 3.06 (0.84) | 2.86 (1.02) | 0.28 | –0.08 to 0.64 | 0.129 | |
| Controllability 3 : mean (s.d.) | 3.06 (0.79) | 2.81 (0.94) | 0.29 | –0.04 to 0.63 | 0.088 |
1. Shorter duration of symptoms
Table 5 Intention to treat analysis of the use of health services by patients following reattribution training of general practitioners
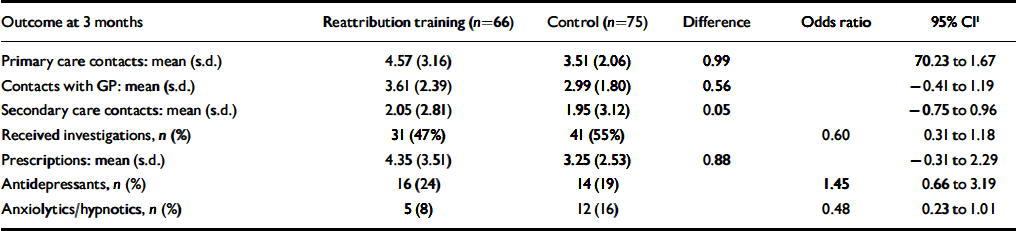
| Outcome at 3 months | Reattribution training (n=66) | Control (n=75) | Difference | Odds ratio | 95% CI 1 |
|---|---|---|---|---|---|
| Primary care contacts: mean (s.d.) | 4.57 (3.16) | 3.51 (2.06) | 0.99 | 70.23 to 1.67 | |
| Contacts with GP: mean (s.d.) | 3.61 (2.39) | 2.99 (1.80) | 0.56 | –0.41 to 1.19 | |
| Secondary care contacts: mean (s.d.) | 2.05 (2.81) | 1.95 (3.12) | 0.05 | –0.75 to 0.96 | |
| Received investigations, n (%) | 31 (47%) | 41 (55%) | 0.60 | 0.31 to 1.18 | |
| Prescriptions: mean (s.d.) | 4.35 (3.51) | 3.25 (2.53) | 0.88 | –0.31 to 2.29 | |
| Antidepressants, n (%) | 16 (24) | 14 (19) | 1.45 | 0.66 to 3.19 | |
| Anxiolytics/hypnotics, n (%) | 5 (8) | 12 (16) | 0.48 | 0.23 to 1.01 |
1. Confidence intervals obtained using a non-parametric bootstrap. The 95% CI indicate that the effect of the training is non-significant
2. Less severe impact on life
3. More under the patient's control
DISCUSSION
Reattribution training was effective in changing clinical communication so that in a greater proportion of consultations doctor–patient communication was mostly consistent with reattribution. In itself this was an achievement given the relative ineffectiveness of brief training in changing doctor's communication skills in relation to mental health problems (Reference Hodges, Inch and SilverHodges et al, 2001). However, there was no evidence of improvement in patient outcome or service use after reattribution training, which was disappointing given that reattribution is used internationally for the management of medically unexplained symptoms in primary care (Reference BlankensteinBlankenstein, 2001; Reference Larisch, Schweickhardt and WirschingLarisch et al, 2004; Reference Rosendal, Bro and SokolowskiRosendal et al, 2005; Reference Aiarzaguena, Grandes and GamindeAiarzaguena et al, 2007). The possible explanations for the negative result are discussed.
Effectiveness of training
Reattribution training for non-expert health professionals over 6 hours has been shown to be feasible and successful (Reference Morriss, Dowrick and SalmonMorriss et al, 2006). Reattribution training was completed for all GPs in all practices with positive feedback from all but a few. The first stage of reattribution was completed by GPs in over 70% consultations as predicted and as found previously when training was delivered to GPs by experts in the Netherlands (Reference BlankensteinBlankenstein, 2001). With reattribution training there was increased delivery of the first three stages of reattribution and two of the three characteristic features. The third feature, summarising family and social factors, is used more rarely than the other two so the failure to demonstrate its increased use might be due to lack of statistical power rather than a failure of training. The delivery of retribution training by non-experts in practices seems as effective as training delivered to individual GPs by experts outside the practice. Nevertheless, the full reattribution model was employed in only 31% of the trained group and 2% of the control group, indicating some problems in implementing reattribution in a single consultation. Some patients needed further investigation or were not ready for all stages of the reattribution model in one consultation, but in other instances GPs reported that reattribution did not address the needs of the patient.
Possible methodological limitations
Compared with previous studies, the current randomised controlled trial (MUST) has many methodological strengths (Reference Morriss, Dowrick and SalmonMorriss et al, 2006). In previous studies, a volunteer GP in a practice would receive reattribution training but patients with medically unexplained symptoms would also consult GPs who had not received the training. Thus contamination between reattribution training and treatment as usual might have obscured a treatment effect. In this study, all GPs in the practice were trained so contamination did not occur. In some previous studies, randomisation was not used (Reference Morriss, Gask and RonaldsMorriss et al, 1999) or was compromised (Reference BlankensteinBlankenstein, 2001). The effects of the intervention might have been overestimated by not allowing for clustering (Reference TorgersonTorgerson, 2001) but clustering was accounted for in this study (MUST). There was an imbalance in age of patients between the intervention groups but this was controlled for in the analysis. Some randomised controlled trials investigating interventions by GPs for patients with medically unexplained symptoms have demonstrated selection and ascertainment bias because the GPs delivering the intervention also selected the patients for the study (Reference Smith, Monson and RaySmith et al, 1986) and because GPs use different criteria to diagnose medically unexplained symptoms. In our study, consecutive attenders were screened in the waiting room before the index consultation and were only recruited after a final decision by an independent research GP. Therefore selection and ascertainment bias were avoided. High rates of follow-up mean that the study (MUST) did not suffer from attrition bias.
The study might have been underpowered to examine some clinical outcomes. The odds ratios of 2 or more suggest that reattribution training might have had benefits for knowledge about the nature of the bodily symptoms and improved patient satisfaction but detrimental effects on other symptom beliefs and anxiety, as well as perception of health. However, even if the study was underpowered, the results leave no doubt that reattribution training did not produce the benefits in clinical outcome and service use that have previously been reported.
Patient recruitment
Around 20% of consecutive attenders in primary care have medically unexplained symptoms (Reference Peveler, Kilkenny and KinmonthPeveler et al, 1997) although only 2.6% are frequent consulters (four or more occasions per year) with such symptoms (Reference Verhaak, Meijer and VisserVerhaak et al, 2006). Only 2.6% of consecutive attenders were recruited in our study. Of these, 83% had consulted their GP at least twice in the previous 3 months and half had consulted their GP three or more times, with no difference between the intervention groups. Therefore, the majority of our sample belongs to a group of patients who frequently consult primary care practitioners and have medically unexplained symptoms. It is notable that we screened 4483 patients to obtain 141 with medically unexplained symptoms. Although such symptoms may be the subject of many consultations, it is the more conspicuous frequently attending group that we were able to engage, raising questions concerning the recognition of less severe medically unexplained symptoms. It is possibile that reattribution might be effective in patients who have not previously, or have rarely, consulted with medically unexplained symptoms, but does not improve outcomes in patients who frequently consult their GP. The group we recruited did not differ in age but included more females compared with other primary care attenders as would be expected among frequent attenders with medically unexplained symptoms (Reference Verhaak, Meijer and VisserVerhaak et al, 2006).
Reattribution was originally designed for delivery to patients with somatised depressive and anxiety disorder rather than all patients with medically unexplained symptoms. However, in the MUST trial the training had no effects on possible depressive disorder and there was a trend for an increase in anxiety disorder. Therefore, it is not plausible that reattribution has beneficial effects on clinical outcome in patients with somatised mental disorder.
Package of care
The low rate of overall completion of reattribution in a single consultation indicates that the training might not address the complexity of some patients' presentations. Treatment as usual improved health perception over time, unlike reattribution training where health perception remained at the same poor level, particularly in patients who identified problems with anxiety or depression at baseline. In a separate study, our group has shown that patients with medically unexplained symptoms had a greater need for emotional support than patients with medically explained symptoms (Reference Salmon, Ring and DowrickSalmon et al, 2005). In the study reported here, we found that the main aims of GPs delivering treatment as usual were to eliminate physical illness and to use a variety of listening and other communication skills to convey empathy (Reference Salmon, Peters and CliffordSalmon et al, 2007). The ruling out of physical illness by the GP and demonstration of empathy may legitimise the patient's complaints and convey emotional support. Although reattribution training would also have the aim of carrying out these tasks, didactic and somatic-focused communication rather than negotiated and emotion-focused communication might be more effective in delivering emotional support to people with somatic complaints and high baseline anxiety (Reference Graugaard, Eide and FinsetGraugaard et al, 2003). There are trends in the data to suggest that reattribution might make some patients more worried about their health and more pessimistic about their outcome. Reattribution is ineffective as an intervention when it is given alone and the patient's other problems and agendas are not addressed.
Another important difference between this trial and previous studies of reattribution which have shown more positive results is the extensive previous experience of GPs in mental health (e.g. Reference Morriss, Gask and RonaldsMorriss et al, 1999; Reference Larisch, Schweickhardt and WirschingLarisch et al, 2004). Reattribution may be a useful technique when it complements a range of other approaches to medically unexplained symptoms, such as problem-solving (Reference Wilkinson and Mynors-WallisWilkinson & Mynors-Wallis, 1994) or cognitive–behavioural therapy to manage health anxiety (Reference BlankensteinBlankenstein, 2001), but may be ineffective on its own. When experienced health professionals learn reattribution, they may be able to use it effectively with other mental health interventions to improve patient outcome. There is also evidence that improved patient outcome for conditions such as depressive disorder require organisational change in primary care practice as well as the delivery of evidence-based interventions at the individual patient level (Reference Lin, Katon and SimonLin et al, 1997). It is likely that the same would also apply to the management of medically unexplained symptoms in primary care (Reference Smith, Lyles and GardinerSmith et al, 2006).
Implications
Reattribution alone is ineffective in patients who frequently attend their GP and have medically unexplained symptoms. Effective approaches for managing medically unexplained symptoms in primary care are likely to require a broad range of interventions and involve the whole primary care team, including the GP and nurses with specialist training (Reference Smith, Lyles and GardinerSmith et al, 2006). However, GPs in many healthcare systems in the world are only likely to attend relatively brief training concerning the assessment and management of medically unexplained symptoms. Qualitative data from participating patients and GPs in this trial will provide further information on the barriers to reattribution and indicate ways in which such brief training could be improved. The practice-based training methods developed may be an efficient method for implementing the training of practice staff in brief interventions. More comprehensive training would then be reserved for health professionals giving more specialist interventions in primary care, which is a possibility given the huge financial cost of somatisation for healthcare symptoms (Reference Barsky, Orav and BatesBarsky et al, 2005).
Acknowledgements
The study was funded by the Medical Research Council (Grant Reference Number G0100809, ISRCTN44384258), Mersey Care NHS Trust and Mersey Primary Care Research Organisation, and the Department of Health. We thank the following practices and their patients that took part in the study: Bousfield Health Centre; Brownhill Surgery; Brownlow Group Practice; Ellesmere Medical Centre; Heaton Medical Centre; Manor Health Centre; Moore Street Surgery; Pendleside Medical Practice; Primrose Bank Medical Centre; Roe Lee Surgery; Somerville Group Practice; Stonehill Medical Centre; Unsworth Medical Centre; Victoria Park Health Centre; Villa Medical Centre; Westmoreland General Practice.









eLetters
No eLetters have been published for this article.