Conventional antipsychotic drugs remain one of the mainstays of treatment of schizophrenia and related psychotic disorders. The therapeutic efficacy of these drugs is well established, both for treatment of acute symptoms and in relapse prevention. Unfortunately, they are associated with a broad range of side-effects, the most prominent of which is the development of a variety of movement disorders (see Box 1). Compared with the conventional antipsychotic agents, the newer, atypical antipsychotics have a lower liability for the acute extrapyramidal side-effects (EPS) and, for a few of the new drugs, there is some evidence of a lower risk of tardive dyskinesia (Reference Barnes and McPhillipsBarnes & McPhillips, 1999). Nevertheless, even with these newer agents, movement disorders are seen in a significant proportion of patients.
The clinical management of patients receiving these drugs thus involves careful assessment to optimise the balance of potential benefits and risks. The motor disturbances are relatively common, can be disabling and distressing and may be a disincentive to compliance. Some of the motor phenomena can be misinterpreted as signs and symptoms of psychotic illness, thus confounding clinical assessment and decisions regarding adjustment of medication. The assessment of drug-related movement disorders in psychosis is further complicated by the occurrence of a range of motor disorders that are inherent in the psychotic illness. These range from a lack of coordination, simple tic-like movements and grimacing to chorea (Reference Casey, Hansen, Jeste and WyattCasey & Hansen, 1984).
A medication-related aetiology is assumed in the acute syndrome, which are temporally more closely related to drug administration and some of which respond quickly to anticholinergic medication.
Greater difficulty arises in attributing a medication-related aetiology to the chronic motor disorders, especially tardive dyskinesia. Aetiological explanations for this disorder refer to the consequences of chronic medication, the disease process of the psychotic illness and the effects of advancing age, or an interaction between these variables. Clinical investigation of these issues and of the relative contribution of each to the totality of tardive dyskinesia has been hampered by the lack of availability of control groups of non-medicated patients with psychoses. The debate regarding aetiology and also the constraint placed on long-term treatment, which is partially medico-legal, has yielded a number of studies of selected groups of neuroleptic-näve patients. These include retrospective case record reviews (Reference Fenton, Wyatt and McGlashanFenton et al, 1994) and studies of long-term institutionalised patients (Reference Owens, Johnstone and FrithOwens et al, 1982) and samples in community settings (Reference McCreadie, Thara and KamathMcCreadie et al, 1996). While there are conflicting results, the rate of non-medication-related, or ‘spontaneous’, dyskinesia seems to be higher than previously thought. Attempting to attribute aetiological causes in an individual patient is difficult and may bias ratings of observed movements. Fortunately, most currently available rating scales for abnormal involuntary movements tend to avoid discrimination of aetiology.
Box 1. Extrapyramidal side-effects of antipsychotic drugs
-
• Parkinsonism
-
• Acute akathisia
-
• Acute dystonia
Acute movement disorders
-
• Tardive dystonia
-
• Chronic akathisia
-
• Tardive dyskinesia
Chronic movement disorders
The observable features of acute parkinsonism, such as limb stiffness, slowness of movement and mask-like faces, are a social and functional handicap. The same is true of the restless movements and agitation associated with acute akathisia. However, acute EPS can have less evident mental aspects: Parkinsonian patients report slowness of thinking or ‘feeling like a zombie’, while patients with akathisia describe inner restlessness and unease. These subjective phenomena are difficult to quantify, but some attempts have been made to study their relationship to the accompanying motor phenomena and their negative effects on quality of life, compliance and outcome (Reference Hogan and AwadHogan & Awad, 1992). While patients are often unaware of the movements in tardive dyskinesia and are usually not distressed by them, relatives often find them distressing. These movements can be very obvious to onlookers and can set patients apart socially, possibly adding to the stigma of severe psychiatric illness. An association between tardive dyskinesia and poorer quality of life has been reported (Reference Browne, Roe and LaneBrowne et al, 1996).
Despite the difficulties in attribution of aetiology outlined above, the systematic and reliable assessment of drug-induced movement disorders is essential in both clinical and research settings. In treatment studies, it is necessary for the investigation of the side-effect profiles of antipsychotic drugs and of the relationships between motor disorders and other clinical variables. Clinically, rating scales may act as a diagnostic aid and allow more systematic monitoring of movement disorders during individual therapeutic trials.
This review describes the characteristic features of the drug-induced movement disorders, some of the problems in their assessment and the clinical utility of a few selected rating scales. Particular emphasis is placed on three of the most widely used and easily applicable scales and a combined examination procedure is described.
Assessment
Drug-induced parkinsonism
The signs of drug-induced parkinsonism may develop within days of starting antipsychotic treatment; the literature records widely varying incidence figures of up to 40% in clinical practice. The condition resembles idiopathic Parkinson's disease in its main symptoms, although some of the characteristic features may not be as common in the drug-induced condition (see Box 2).
Box 2. Symptoms of idiopathic parkinsonism observed in the drug-induced condition
-
• Muscle rigidity
-
• Tremor
-
• Postural abnormalities
-
• Bradykinesia
Symptoms commonly observed
-
• Festinant, hurrying gait
-
• 3-5 Hz resting tremor
-
• Reduction in the size of handwriting
-
• Rhythmic disturbance of handwriting, related to tremor
Symptoms less frequently observed
On physical examination, rigidity of the limbs, which are resistant to passive movement, is perhaps the most obvious feature of drug-induced parkinsonism. It can occur as sustained resistance, described as ‘lead pipe’ rigidity, or as a succession of resistances that are rapidly overcome by passive movement, known as ‘cog-wheel’ rigidity. Milder forms of rigidity may be best detected on activation. The rigidity tends to become more obvious when the subject is engaged in moving the opposite limb, for example, tapping the knee with the opposite hand. The examination may be hampered by the subject's inability to relax, or a tendency to move voluntarily with the examiner or resist passive movement. For these reasons it is better to carry out passive movements in an unpredictable way by varying the speed and order of movements around each joint.
Rating scales for drug-induced parkinsonism
A number of general scales have been developed for extrapyramidal symptoms which incorporate items for rating drug-induced parkinsonism. These include Chouinard et al's Extrapyramidal Rating Scale (Reference Chouinard, Ross-Chouinard and AnnableChouinard et al, 1980), the Targeting Abnormal Kinetic Effects (TAKE) scale (Reference Wojcik, Gelenberg and La BrieWojcik et al, 1980), the Extrapyramidal Symptoms Scale (Reference Adler, Angrist and ReiterAdler et al, 1989) and the Neurological Rating Scale for Extrapyramidal Effects (Simpson-Angus scale; Simpson & Angus, 1970). The scales vary in the emphasis they place on the different features of drug-induced parkinsonism. The TAKE scale, for example, places more emphasis on akinesia and bradykinesia, while the Simpson-Angus scale places the majority of emphasis on rigidity. The choice of scale may vary according to the purposes of the clinician or investigator, but the Simpson-Angus scale, which was the first to be developed, remains the most widely used.
The Simpson-Angus scale was devised to measure drug-induced parkinsonism, providing standardised ratings for rigidity, tremor and salivation. The scale is entirely sign led. It contains 10 items, each rated on a 5-point scale (0-4), with descriptive anchors for each point and a clearly described examination procedure for each item. Six of the 10 items rate rigidity: arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness and neck rigidity. There is a single item for gait, which is the only measure for bradykinesia, and is in fact a compound item incorporating gait, posture and loss of arm swing. The other three items measure tremor, glabellar tap and salivation.
Simpson and Angus (1970) validated the their scale by demonstrating that it separated three groups of patients on different doses of antipsychotic medication. Interrater reliability was established by two examiners rating 14 patients on two occasions. Almost all items achieved acceptable levels of agreement, and the ratings of rigidity tended to show the highest correlation coefficients. Perhaps the main criticism of the scale is that it is not particularly comprehensive in assessing the full range of symptoms, overemphasising rigidity. Some authors view bradykinesia as the main feature of the condition and criticise the scale for only indirectly measuring this through the item on gait (Reference OwensOwens, 1999).
The TAKE scale rates many manifestations of bradykinesia, but few manifestations of rigidity. Bradykinesia is characterised by features such as paucity of gesture, a mask-like facies and slow monotonous speech. These features may be mistaken for retarded, or ‘akinetic’, depression. There is also a potential overlap between these symptoms and negative features of schizophrenia such as affective flattening, poverty of speech and lack of drive (Reference Barnes and McPhillipsBarnes & McPhillips, 1995). Whether current assessment scales for negative features allow differentiation of affective flattening from these more subjective features of drug-induced parkinsonism is in some doubt. It may be that the concentration on more objective features, such as rigidity in the Simpson-Angus scale, confers an advantage of lack of measurement redundancy.
The examination method for assessing neck rigidity recommended in the Simpson-Angus scale is flawed both in terms of its utility in rating this item and of disrupting professional rapport with the patient. The procedure is that the patient Òlies on a well padded table (if such a thing is to hand) and his head is raised by the examiner's hand and then allowed to drop”. In normal subjects the head will fall onto the table, whereas in mild parkinsonism the movement is delayed and in severe cases it is absent. Apart from the fact that the procedure is only likely to work once in the same patient, the differential diagnosis might also include the ‘psychological pillow’ sign described in catatonic schizophrenia. Neck rigidity can be assessed just as accurately by holding the patient's head and passively moving it around the neck joint, as outlined in the assessment method described in the Appendix.
The Simpson-Angus scale has only one item for tremor, relating to tremor of the fingers and arm or whole body tremor, but no items relating to titubation or tongue tremor. There is no mechanism for differentiating between mild Parkinsonian tremor and tremor with other causes (such as anxiety, lithium or benign essential tremor). While this may be seen as a disadvantage, it again can add to objectivity, as the examiner rates what is seen rather than changing the rating according to a presumed cause. The Simpson-Angus scale also lays emphasis on unobtrusive observation while the patient is walking into the room: this may exaggerate the score for tremor due to postural tremor, which is relatively common in psychiatric patients on medication.
Salivation is rated in a single item according to observation of pooling of saliva in the mouth, difficulty with speaking because of excess salivation or actual drooling. The mechanism of excess salivation in drug-induced parkinsonism is unknown, but one explanation is that saliva accumulates because the patient is swallowing less frequently. Excess salivation may occur for reasons other than parkinsonism. For example, it occurs in about a third of patients taking clozapine, which may lead to difficulties if this item artificially inflates the total score for parkinsonism in the Simpson-Angus scale. Analysis of results in studies involving clozapine may need to take this into account.
The conventional scoring system for the Simpson-Angus scale is to calculate a global score by summing the individual item scores and then dividing by the total number of items. Simpson and Angus considered that a final score of up to 0.3 was Òwithin the normal range”. This method of scoring is quite arbitrary and does not allow for separate monitoring of the features of rigidity, tremor and salivation.
Acute and chronic akathisia
The diagnosis and assessment of akathisia should take account of both its subjective (Box 3) and objective components.
Box 3. Subjective components of akathisia
-
• Sense of inner restlessness
-
• Mental unease
-
• Unrest or dysphoria
-
• Feeling unable to keep still
-
• An irresistible urge to move the legs
-
• Mounting inner tension when required to stand still
Commonly experienced (Reference Halstead, Barnes and SpellerHalstead et al, 1994)
-
• Tension and discomfort in the limbs
-
• Parasthesiae and unpleasant pulling or drawing sensations in the muscles of the legs
Less commonly experienced
The restless movements most commonly associated with the subjective experience of akathisia are not dyskinetic or stereotypical, but resemble normal patterns of restless movement. Most typical are lower-limb movements, such as rocking from foot to foot and walking on the spot when standing, and shuffling and tramping of the legs or swinging one leg on the other when sitting. Pacing rapidly up and down is a characteristic of severe akathisia, and in the worst cases, patients are unable to feel comfortable in any position, such as sitting, lying or standing, for more than a few minutes. In addition, trunk rocking and fidgety movements of the upper limbs may be seen. The severity of the movements in akathisia can vary according to the situation and the patient's degree of arousal. For example, the movements may be less obvious during an interview or while concentrating on some mental task, and more evident when standing engaged in conversation on neutral topics (Reference BarnesBarnes, 1992). Thus, limiting the assessment of akathisia to a brief, formal examination runs the risk of underestimating the presence and severity of the condition. For this reason, a period standing with the patient and engaging in undemanding conversation on neutral topics has been included in the examination instructions of some akathisia rating scales.
Akathisia has most frequently been considered as a relatively common acute extrapyramidal problem, but it can also be a persistent problem in those receiving maintenance antipsychotic treatment (Reference Barnes and BraudeBarnes & Braude, 1985). There are no marked differences in the motor phenomena of acute and chronic akathisia, although it has been suggested that the accompanying subjective sense of restlessness may be less intense in the latter. Thus, as with acute akathisia, the motor features most commonly involve the lower limbs, and included marching in place and crossing and uncrossing the legs when sitting. Other movements include trunk rocking, respiratory grunting and complex hand movements, such as face rubbing and scratching and rubbing the thighs. In a relatively small number of individuals, repetitive, restless movements are observed that are characteristic of akathisia, but are not accompanied by any sense of inner restlessness or a compulsion to move. This presentation is referred to as pseudoakathisia. The condition seems to be more common in males and older patients with higher scores on negative symptoms and is likely to coexist with tardive dyskinesia (Reference Barnes and BraudeBarnes & Braude, 1985; Reference Brown and WhiteBrown & White, 1991; Reference Halstead, Barnes and SpellerHalstead et al, 1994).
Rating scales for antipsychotic-induced akathisia
For many years, akathisia was assessed by a single item within a more general scale for extrapyramidal symptoms. This tradition has been continued with the TAKE scale, which conceptualises akathisia as part of the Parkinsonian syndrome and rates it on the basis of subjective symptoms (Reference OwensOwens, 1999). More recently, scales specifically dedicated to the condition have been developed: the Rating Scale for Drug-Induced Akathisia (the Barnes scale; Barnes, 1989), the Hillside Akathisia Scale (Reference Fleischhacker, Bergmann and PerovichFleischhacker et al, 1989) and the Prince Henry Hospital Akathisia scale (Reference SachdevSachdev, 1994). All three scales include both subjective and objective items and a global item. The psychometric properties and clinical utility of these scales have been compared and contrasted in the literature (Reference Barnes, Kane, Barnes and NelsonBarnes & Kane, 1994; Reference SachdevSachdev, 1995; Reference OwensOwens, 1999).
The Barnes scale is the most widely used, and both Sachdev (1995) and Owens (1999) consider that it has strong face validity, is simple and easy to use, with clear instructions for examination, and has well-defined, relevant anchor points. Unlike the other scales, the Barnes scale differentiates between the experience of restlessness and any associated distress. The global item score may be used as an overall severity measure and has a diagnostic threshold, with a score of 2 or more indicating the presence of akathisia.
Acute and tardive dystonia
Acute dystonic reactions are involuntary movements characterised by sustained muscle action (to the point of maximal contraction)(Box 4). Repetitive contorting, twisting movements are seen, which vary from fleeting disturbance to maintained abnormal postures. The incidence in acute psychiatric patients receiving conventional antipsychotics may be between 25 and 40% (Reference Addonzio and AlexopoulosAddonzio & Alexopoulos, 1988); the problem is more common in young adults and children. Owens (1999) delineates the subjective aspects of the condition, including prodromal symptoms of anxiety and a sense of something non-specific, but imminent. Awareness of motor symptoms, such as uncomfortable muscle stiffness and postural distortion, which can be painful, may make patients agitated and frightened. In clinical practice, the condition may well be missed in patients not progressing beyond the prodromal stage, or with only mild signs. Furthermore, the condition may be misdiagnosed as dissociative phenomena, malingering, seizures, tetany, posturing associated with psychosis, or an attempt to persuade doctors to prescribe anticholinergic medication.
Box 4. Common motor presentations of dystonia
-
• Torticollis (dystonic distortion of the neck)
-
• Trismus (contraction of the masticatory muscles)
-
• Blepharospasm (forceful eye closure)
-
• Dystonia of the trunk and limbs (less common)
Acute dystonia
-
• Blepharospasm
-
• Torticollis
-
• Retrocollis
-
• Oculogyric spasm
Chronic (tardive) dystonia
The muscles of the head and neck are most commonly affected, and involvement of the laryngeal and pharyngeal muscles may lead to serious problems, such as respiratory distress and asphyxia, and dysphagia and choking.
Like akathisia, dystonia is generally viewed as an acute, relatively transient extrapyramidal side-effect. The occurrence of persistent, or tardive, dystonia as a distinct phenomenon in patients receiving long-term antipsychotic treatment has been accepted only in the past 20 years and its reported prevalence is about 1.5-4% (Reference RajaRaja, 1995). The motor presentations are similar to those seen in acute dystonia, and are clearly distinguishable from them only by their duration. The condition is apparently identical to idiopathic torsion dystonia or secondary dystonia associated with conditions such as Huntington's disease or Wilson's disease, and there is some overlap with the features of tardive dyskinesia, with which it may coexist (Reference BarnesBarnes, 1990).
Rating scales for antipsychotic-induced dystonia
No rating scale has been devised specifically to assess antipsychotic-induced dystonia. This may be partly because acute dystonias are often transitory phenomena, of relatively rapid onset and rapidly responding to anticholinergic medication, and therefore not particularly suited to formal cross-sectional assessment. Also, until recently, such dystonias were perceived as relatively uncommon problems.
Burke et al (1985) have tested the reliability and validity of a scale for primary torsion dystonia called the Dystonia Movement Scale (or Fahn-Marsden Scale), which has been applied to drug-related disorder (Reference van Harten, Matroos and Hoekvan Harten et al, 1996). However, it is arguably appropriate only for cases of tardive dystonia that are severe and persistent.
Tardive dyskinesia
Tardive dyskinesia is the main late-onset condition among the EPS. The diagnosis has been applied loosely to a range of involuntary movements (Box 5) occurring more commonly in older patients on antipsychotic medication (Reference BarnesBarnes, 1990). While patients tend to be unaware of these movements, they can be quite distressing to relatives and may contribute to stigma and social handicap. The cause of these movements has been a subject of debate in the literature and a stimulus for continuing research. Explanations of the aetiopathogenesis of these movements include the effects of chronic medication, the disease process of the illness and effects of advancing age, or an interaction of these factors.
Box 5. Common motor presentations of tardive dyskinesia
-
• Protrusion or twisting of the tongue
-
• Smacking, pursing and sucking movements of the lips
-
• Puffing of the cheeks
-
• Lateral, chewing movements of the jaw
-
• Grimacing movements of the face
Orofacial dyskinesia (bucco-linguo-masticatory syndrome)
-
• Purposeless, jerky, stereotypical choreiform (choreo-athetoid) movement
-
• Athetosis of the extremities
-
• Limb and axial dystonias
-
• Abnormalities of gait
-
• Lordosis
-
• Rocking
-
• Shoulder shrugging
-
• Rotatory movements of the pelvis
Limb and trunk movements
Perhaps the main difficulty in the assessment of tardive dyskinesia, especially orofacial, is that indistinguishable movements, termed spontaneous dyskinesia, are seen in 5-15% of elderly individuals who have never been medicated with antipsychotics (Reference Kane, Weinhold and KinonKane et al, 1982). There is also recent evidence that these spontaneous movements occur in about 7% of antipsychotic-näve individuals with schizophrenia at onset of their illness (Reference Gervin, Browne and LaneGervin et al, 1998; Reference Puri, Barnes and ChapmanPuri et al, 1999). Attempts to attribute a cause, such as spontaneous or tardive, while rating involuntary movements are problematic and may bias raters. In research studies it is better to rate what is seen and be blind to treatment status.
Rating scales for tardive dyskinesia
Of the instruments used to assess dyskinesia, the 12-item Abnormal Involuntary Movements Scale (AIMS; Guy, 1976) is the most popular. Several other scales are commonly used, such as the Tardive Dyskinesia Rating Scale (TDRS; Simpson et al, 1979) and Chouinard et al's (1980) Extrapyramidal Rating Scale. While generally referred to as a multi-item scale, the AIMS might best be characterised as a global impression scale, albeit with its items ordered regionally. The main advantage of these scales is that they provide a comprehensive rating of abnormal involuntary movements in various body sites. Many of them also provide a recommended examination procedure, standardising the clinical assessment and enabling comparison between different studies.
The AIMS assesses abnormal involuntary movements in three body regions: orofacial movements, rated on four separate items; extremity movements, on two separate items; and trunk movements, on one item. Each item is rated on a 5-point scale (0-4), with instructions to rate the highest severity observed and to score movements that occur upon activation one less than those observed spontaneously. Three separate items score global severity, the subject's awareness, and incapacitation due to involuntary movements (each on a 5-point scale). Two additional items cover the subject's dental status, as movements in the orofacial area are more obvious in edentulous patients.
There are several versions of guidelines for conducting the AIMS examination (Reference Lane, Glazer and HansenLane et al, 1985; Reference Munetz and BenjaminMunetz & Benjamin, 1988). Gardos and Cole (1980) summarised the reliability of these multi-item scales as Òvariable”, but satisfactory levels of interrater reliability have been demonstrated for the more popular instruments, such as the AIMS (Reference Smith, Kucharski and OswoldSmith et al, 1979; Reference Lane, Glazer and HansenLane et al, 1985) and the TDRS (Reference Simpson, Lee and ZoubokSimpson et al, 1979). These scales take little time to complete and are applicable to most patients, making them particularly useful as screening instruments for tardive dyskinesia. Some of the problems with assessing tardive dyskinesia lie more in the variability of the disorder itself than in the scales. Individual patients may show a wide variability in site and severity of involuntary movements, related to adjustment of medication, anxiety, posture and mobility. There may also be spontaneous fluctuations from day to day or even at different times within one day. It has been suggested that repeated ratings should be carried out over time and, as far as possible, carried out at the same time of day by the same rater. Bergen et al (1984) used the AIMS to quantify spontaneous fluctuations in tardive dyskinesia and found that intrarater variability dominated intrapatient variability.
The AIMS, as with many of the other multi-item scales, is not a diagnostic instrument, but usefully quantifies involuntary movements, giving ratings that can then be used with diagnostic criteria. The use of threshold scores or diagnostic cut-off points can result in heterogeneous groups in terms of regional distribution of dyskinesia. The topographical distribution of involuntary movements may be a critical issue in the light of evidence suggesting that orofacial and limb-truncal dyskinesia are sub-syndromes of tardive dyskinesia that are pathophysiologically distinct, with different clinical correlates and differential response to drug treatments (Reference BarnesBarnes, 1990).
Examination procedure for extrapyramidal side-effects
The process of examining for dyskinetic movements requires methodical and close observation of specific body areas, while simultaneously scanning the body as a whole for movements occurring in other regions. It is important to differentiate the irregular, relatively stereotyped movements of dyskinesia from the regular rhythmic movements seen in tremor and the more purposeful, restless movements of akathisia. Training in the examination of dyskinetic movements can be greatly aided by the use of videotaped assessments of patients, which allow repeated observation and discussion. A comprehensive examination procedure to facilitate the assessment of involuntary movements and other EPS has been described in detail by Owens (1999). Over a number of years of use, we have developed a similar, relatively brief, standardised style of examination (see Appendix). Adopting a systematic head-to-toe approach makes the routine easy to remember. Our procedure is largely based on the AIMS examination to rate tardive dyskinesia, but incorporates procedures that help identify other extrapyramidal symptoms.
Appendix
Examination procedure for assessing extrapyramidal syndromes in people with schizophrenia receiving antipsychotic medication
Either before or after completing the Examination Procedure unobtrusively observe the patient at rest (e.g. in the waiting room or while taking history) and observe the gait on entering or leaving the examination room.
The chair used in the examination should be firm, relatively high and without arms. It should be placed at a slight angle to the rater, rather than face on, to allow for more discreet observation.
Throughout the examination look out for the characteristic restless movements of akathisia.
-
A Examine the patient while you are both seated on chairs a sufficient distance apart to allow you to view all body areas. When examining specific body sites, continue to scan all areas.
-
1 Ask the patient if there is anything in his/her mouth (such as gum or a sweet) and if there is ask him/her to remove it.
-
2 Ask the patient about the current condition of his/her teeth. Ask if he/she wears dentures. Ask if teeth or dentures are causing discomfort.
-
3 Have the patient sit on the chair with hands on knees, legs slightly apart and feet flat on the floor. Look at entire body for movements in this position.
-
4 Ask the patient to sit with hands unsupported, hanging down by the side (opening and closing the fist once may help to relax the hands); then ask him/her to place the hands hanging over the knees. Observe hands and other body areas.
-
5 Asking the patient to remove shoes and socks can reveal subtle bradykinesia and allows clear observation of any subtle peripheral movements. However, such a request may be bothersome for some patients and jeopardise further cooperation; also, the procedure can be time consuming.
-
6 Ask the patient to open his/her mouth. Observe tongue at rest within the mouth. Do this twice.
-
7 Ask the patient to protrude the tongue. Observe abnormalities of tongue movement. Do this twice.
-
8 Ask the patient to tap his/her thumb with each finger, as rapidly as possible for 10-15 seconds; repeat for right and left hands. Tapping the fingers engages the patient in an activity that helps release involuntary movements elsewhere. Observe facial and leg movements as this is done.
For AIMS ratings, the convention is to rate movements that occur upon activation one less than those observed spontaneously.
-
-
B Approach the patient while he/she is still seated.
-
1 Perform the glabellar tap examination by standing behind the patient out of the line of vision and tapping gently but rapidly on his/her forehead (in the midline, between the eyebrows), asking the patient to try not to blink.
-
2 Examine the patient's neck for rigidity by gently holding the head and flexing and extending the neck in coronal and sagittal planes and then gently rotating the neck.
-
3 Examine under the patient's tongue for pooling of saliva. If involuntary tongue movements are observed during this, rate them on the AIMS one less than those observed spontaneously.
-
-
C Ask the patient to stand up while you are still standing beside him/her.
-
1 Examine the patient's arms for rigidity by flexing and extending the elbow and the hand and rotating the shoulder.
-
-
D Ask the patient to sit on an examination couch or a desk so that his/her feet are not touching the floor.
-
1 Examine the patient's legs for rigidity by first passively flexing and extending the knee while palpating the quadriceps for stiffness, then swinging the leg in a pendular motion and observing for freeness of swing.
-
-
E With both you and the patient standing, move to a distance sufficient to allow you to view all body areas.
-
1 Ask the patient to turn around through 380¼ in four stages. View from the front, the side, the back, the other side and then face on again. Observe truncal involuntary movements while continuing to scan all areas.
-
2 Ask the patient to stretch both arms out in front of him/her and to spread the fingers. Observe tremor.
-
3 Ask the patient to extend both both arms to the side, raising them to shoulder level and then allowing them to fall freely down to the sides again. Observe freeness of fall, audible contact and rebound. Do this twice.
-
4 To examine a patient's natural gait it may be best unobtrusively to observe them walking to and from the examination room. Otherwise, ask the patient to walk casually along a nearby corridor and then back as if in a hurry. Observe for swinging of arms and Parkinsonian gait. Do this twice.
-
-
F While standing with the patient engage in conversation on neutral topics.
-
1 Observe for characteristic restless movements of akathisia.
-
2 Ask the patient if he/she notices any movements in the mouth, face, hands or feet. If yes, ask him/her to describe them and to what extent they usually bother the patient or interfere with activities.
-
3 Enquire about the patient's subjective sense of restlessness:
-
(a) non-specific inner restlessness;
-
(b) awareness of a particular inability to keep the legs still, or a desire to move the legs, and/or inner restlessness aggravated specifically by being required to stand still (for example, when in a queue);
-
(c) awareness of an intense compulsion to move most of the time and/or a strong desire to walk or pace most of the time.
-
-
If the patient reports any of these, determine to what extent this distresses him/her.
After the examination, rate the movements observed according to the anchors on each item of the AIMS. Other extrapyramidal side-effects such as parkinsonism and akathisia can be rated on appropriate scales.
Multiple choice questions
-
1. The assessment of extrapyramidal side-effects may be enhanced by:
-
a seating the patient in a relaxing, soft, low armchair
-
b a period of unobtrusive observation
-
c examining the patient's gait as he/she takes two or three steps across the room
-
d asking the patient to remove his/her shoes and socks
-
e the use of standardised rating scales.
-
-
2. With reference to tardive dyskinesia:
-
a it is characterised by an absence of distress associated with the orofacial movements
-
b similar ‘spontaneous’ movements are seen in a small proportion of neuroleptic-näve people with early schizophrenia
-
c in individual patients, the nature and severity of the dyskinetic movements tend not to fluctuate over time
-
d the Abnormal Involuntary Movements Scale (AIMS) is the most commonly used rating scale for it in clinical studies
-
e manifestations of orofacial dyskinesia include the ‘bon-bon’ sign and ‘fly-catcher’ tongue movements.
-
-
3. With reference to antipsychotic-induced parkinsonism:
-
a the Neurological Rating Scale for Extrapyramidal Effects is suitable for rating subtle signs of bradykinesia
-
b existing rating scales allow clear differentiation between drug-induced parkinsonism and the negative symptoms of schizophrenia
-
c a handwriting sample may be useful in the assessment of drug-induced parkinsonism
-
d muscle rigidity in the arm can be reduced by asking the subject to move the other arm
-
e sialorrhoea is probably a consequence of less frequent swallowing related to brady- kinesia.
-
-
4. The following may be said about antipsychotic-induced akathisia:
-
a it is primarily a subjective phenomenon, characterised by inner restlessness and unease
-
b the Barnes Scale can be used to rate pseudoakathisia
-
c the diagnosis of akathisia cannot be made in the absence of observable motor restlessness
-
d the subjective sense of restlessness is reliably relieved by lying down and resting
-
e acute akathisia is a transient problem that rarely persists longer than a few days.
-
-
5. With reference to antipsychotic-induced dystonia:
-
a acute dystonia may be mistaken for drug seeking behaviour
-
b acute dystonia does not usually have subjective phenomena
-
c rating scales such as the Dystonia Movement Scale are very useful for detecting acute dystonia
-
d blepharospasm and torticollis are relatively common presentations of tardive dystonia
-
e the movements seen in tardive dystonia are quite distinct from those seen in idiopathic torsion dystonia.
-
MCQ answers
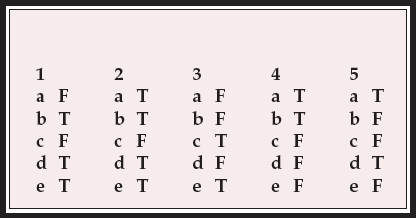
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | T | a | F | a | T | a | T |
| b | T | b | T | b | F | b | T | b | F |
| c | F | c | F | c | T | c | F | c | F |
| d | T | d | T | d | F | d | F | d | T |
| e | T | e | T | e | T | e | F | e | F |



eLetters
No eLetters have been published for this article.