Introduction
This study focuses on the possibilities of a photosynthetic ecology existing on Proxima Centauri b (Anglada-Escudé et al. Reference Anglada-Escudé2016; Ribas et al. Reference Ribas2016; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016), which appears to be a viable habitable planet (Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017; Exoplanet.eu, 2017) despite Proxima Centauri being a very low mass flare star. The temperature issues are dealt with by Rushby et al. (Reference Rushby, Claire, Osborn and Watson2013) and Turbet et al. (Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016). We will concentrate on the suitability of the available irradiance to support photosynthesis as known on the Earth. The habitable zone (HZ) is defined as the circumstellar orbital zone where liquid water could exist on a planet. A planet also needs to be in the HZ long enough for complex life to evolve (see Kasting et al. Reference Kasting, Whitmore and Reynolds1993; Kasting Reference Kasting1997; von Bloh et al. Reference von Bloh, Cunz, Schröder, Bournama and Franck2009, Reference von Bloh, Bounama and Franck2010; Jones & Sleep Reference Jones and Sleep2010; Rushby et al. Reference Rushby, Claire, Osborn and Watson2013; Anglada-Escudé et al. Reference Anglada-Escudé2016; Chopra & Lineweaver Reference Chopra and Lineweaver2016; Gale & Wandel Reference Gale and Wandel2016; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016). Planets of M-type stars could meet these three criteria but M-type stars (red dwarfs) are very different from G-type stars such as the Sun (spectral type G2 V – 5780 K).
M-type stars emit most of their light as infrared (IR) radiation and have very long lifetimes, often greater than current estimates of the age of the Universe. Their total irradiances change only slightly over time when the stars are mature but their spectra are very different to the Sun. While exoplanets of Red Dwarf M stars are considered good potential targets in searches for extraterrestrial life (Kasting et al. Reference Kasting, Whitmore and Reynolds1993; Kasting Reference Kasting1997; Cole & Woolfson Reference Cole and Woolfson2002; Buccino et al. Reference Buccino, Lemarchand and Mauas2007; Lammer Reference Lammer2007; Scalo et al. Reference Scalo2007; Tarter et al. Reference Tarter2007; Lammer et al. Reference Lammer2009; Vogt et al. Reference Vogt, Butler, Rivera, Haghighipour, Henry and Williamson2010; Quintana et al. Reference Quintana2014; Gale & Wandel Reference Gale and Wandel2016), these planets have some fundamental habitability problems arising from the properties of M-type stars; in particular M-type stars are characterized by persistent periodic high-energy flaring [from X-rays to ultraviolet (UV), see Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017] and some currently quiescent stars seemingly more favourable for the development of life on their HZ planets may have been much more active and hence more hostile in the past (Pettersen & Hawley Reference Pettersen and Hawley1989; Audard et al. Reference Audard, Güdel, Drake and Kashyap2000; Buccino et al. Reference Buccino, Mauas, Lemarchand, Norris and Stootman2002, Reference Buccino, Lemarchand and Mauas2007; Segura et al. Reference Segura, Krelove, Kasting, Sommerlatt, Meadows, Crisp, Cohen and Lawler2003, Reference Segura, Walkowicz, Meadows, Kasting and Hawley2010; Jones & Sleep Reference Jones and Sleep2010; Baraffe et al. Reference Baraffe, Homeier, Allard and Chabrier2015; Gale & Wandel Reference Gale and Wandel2016; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017).
The problem of tidal locking of M-type planets is one of the most important limitations for their habitability (Bolmont et al. Reference Bolmont, Raymond, Leconte, Correia, Quintana, Knezevic and Lemaître2014a, Reference Bolmont, Raymond, von Paris, Selsis, Hersant, Quintana and Barclayb). In the Solar System, the Moon and all other moons are tidally locked to their parent planet in a 1 : 1 ratio of orbit to rotation, where one face always faces the planet. Mercury is also tidally locked but in a 3 : 2 orbit/rotation ratio due to the eccentricity of its orbit. The HZ is very close to the parent star in the case of M-stars and so Proxima Centauri b would be tidally locked, but the orbit/rotation ratio is not yet known (see Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017). The case against habitability of planets of M-type stars was raised by Joshi et al. (Reference Joshi, Haberle and Reynolds1997) and these issues have since been extensively discussed but are still controversial (see Chopra & Lineweaver Reference Chopra and Lineweaver2016; Gale & Wandel Reference Gale and Wandel2016; Ribas et al. Reference Ribas2016; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016; Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). In summary, what form of locking occurs has critical multifaceted consequences for the climate of the planet and its habitability. The climate of a 1:1 tidally locked planet would be very severe and only part of the planet would be habitable, but the more insidious consequences are destruction of the planets magnetic field and hence stripping of its atmosphere. Only a spherical frustum of the planet would be habitable in the case of a 1 : 1 tidally locked planet, a planet with a 3 : 2 tidal locking would be much more inhabitable (Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016; Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). Contemporary Proxima Centauri b may be a sterile rock with no remaining atmosphere or water or it could be a habitable world with an atmosphere and surface liquid water (Luger and Barnes, Reference Luger and Barnes2015; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017). The age of red dwarfs, such as Proxima Centauri is difficult to determine but based on its membership in the Alpha Centauri triple system, it is likely to be about 4.8 Gyr old: it might have developed life forms, but has been rendered uninhabitable and so life is now extinct (Rushby et al. Reference Rushby, Claire, Osborn and Watson2013; Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017).
The HZ for planets around a star have often been discussed (Kasting Reference Kasting1993; Kasting et al. Reference Kasting, Whitmore and Reynolds1993; Rushby et al. Reference Rushby, Claire, Osborn and Watson2013; Seager, Reference Seager2014; Chopra & Lineweaver Reference Chopra and Lineweaver2016; Gale & Wandel Reference Gale and Wandel2016; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016). The inner limit for life on a planet is where a runaway green-house effect boils the oceans and the water vapour is lost to space by UV photolysis and the stellar wind (≈0.9 AU for a Sun-like G star). Tidal heating, perhaps generated by an eccentric orbit or other planets, might also render a planet uninhabitable (Heller & Barnes Reference Heller and Barnes2013). Conceivably a planet might have life, but does not occupy the HZ throughout its orbit and at the apogee is well outside the HZ (Williams & Pollard Reference Williams and Pollard2002). The climates even on tidally locked planets with a locking ratio that is not 1 : 1 are nevertheless still likely to be very severe (Edson et al. Reference Edson, Lee, Bannon, Kasting and Pollard2011; Kite et al. Reference Kite, Gaidos and Manga2011), but the polar regions of the Earth are inhabitable: consider life in Siberia where annual variation in temperatures may be −60 to +30°C.
The life absorption properties of photosynthetic organisms are critical for understanding their photosynthesis under different light regimes. Extant terrestrial oxygenic photosynthetic organisms, both algae and land plants, have an array of pigments, which determine their ability to use light of different wavelengths (Falkowski et al. Reference Falkowski, Greene, Kolber, Baker and Bowyer1994; Raven et al. Reference Raven, Kilber and Beardall2000; Bryant & Frigaard Reference Bryant and Frigaard2006; Falkowski & Raven Reference Falkowski and Raven2007; Kiang et al. Reference Kiang, Siefert, Govindjee and Blankenship2007a; Reference Kiang, Segura, Tinetti, Blankenship, Cohen, Siefert, Crisp and Meadowsb; Raven Reference Raven2007; Stomp et al. Reference Stomp, Huisman, Stahl and Matthijs2007; Larkum Reference Larkum and Rengler2008, Reference Larkum2010, Rothschild Reference Rothschild2008; Hohmann-Marriott & Blankenship Reference Hohmann-Marriott and Blankenship2011; Kirk Reference Kirk2011; Ritchie Reference Ritchie2013). Of the six photoautotrophic oxygenic organisms chosen for the present study three were prokaryotic cyanobacteria; Synechococcus R-2 is a conventional cyanobacterium with Chl a, some carotenoids and blue phycocyanin; Prochlorothrix hollandica is an unusual cyanobacterium that has both Chls a and b, carotenoids and limited amounts of phycocyanin. Acaryochloris marina is also a very unusual cyanobacterium with Chl d as its primary photosynthetic pigment together with carotenoids and small amounts of Chl a and phycocyanin (Miyashita et al. Reference Miyashita, Ikemoto, Kurano, Miyachi and Chihara2003; Chen & Scheer Reference Chen and Scheer2013; Schliep et al. Reference Schliep, Cavigliasso, Quinnell, Stranger and Larkum2013). Chlorella vulgaris (Chlorophyta) is a eukaryotic green alga with the same pigment composition as terrestrial plants (Archeogoniophytes) with Chl a + b, carotenoids and no phycobiliproteins, Phaeodactylum tricornutum is a diatom (Bacillariophyta) with Chl a and the antenna pigments Chl c 1&c 2 and high levels of carotenes and xanthophylls (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011). Rhodomonas sp. (Cryptophyta), although a eukaryotic organism, has high levels of red-coloured phycoerythrin, also found in some cyanobacteria, which acts as an antenna photosynthetic pigment. Rhodomonas also has Chl a, Chl c 2 and carotenoids (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011).
Four anoxygenic photosynthetic bacteria were included in this study (Blankenship et al. Reference Blankenship, Madigan and Bauer1995; Hohmann-Marriott & Blankenship Reference Hohmann-Marriott and Blankenship2011; Fischer et al. Reference Fischer, Hemp and Johnson2016): Afifella marina and Rhodopseudomonas palustris are purple non-sulphur bacteria with bacteriochlorophyll a (BChl a) as their primary photosynthetic pigment and carotenoid accessory pigments. They can use metal ions such as Fe2+ and grow photoautotrophically or on organic compounds as electron sources and growing photoheterotrophically. Blastochloris viridis is also a purple non-sulphur bacterium, but has BChl b as its primary photosynthetic pigment and carotenoid accessory pigments. Blastochloris uses metal ions such as Fe2+ and organic compounds as electron sources. One purple sulphur bacterium was included in the study, Thermochromatium tepidum (BChl a + carotenoid pigments). Uses H2S, metal ions and organic carbon as electron sources and like the purple non-sulphur bacteria can grow photoautotrophically or photoheterotrophically. The green sulphur bacterium: Chlorobaculum (Chlorobium) tepidum has large amounts of BChl c acting as an accessory pigment, small amounts of BChl a acting as the primary photosynthetic pigment and various carotenoids. H2S is the typical electron source but can use organic carbon as well.
Primary production by both oxygenic and anoxygenic photosynthesis is not linearly proportional to irradiance because there are saturation effects at quite modest irradiances and photoinhibition occurs at supra-optimal irradiances (Falkowski et al., Reference Falkowski, Greene, Kolber, Baker and Bowyer1994; Falkowski & Raven Reference Falkowski and Raven2007; Jones & Vaughan Reference Jones and Vaughan2010; Ritchie Reference Ritchie2010, Reference Ritchie2013; Kirk Reference Kirk2011; Ritchie & Larkum Reference Ritchie and Larkum2013; Ritchie & Runcie Reference Ritchie and Runcie2013; Ritchie & Mekjinda Reference Ritchie and Mekjinda2015). The shape of photosynthesis versus irradiance curves is discussed in detail in the Appendix.
Estimates of the potential productivities (carbon fixation) of oxygenic and anoxygenic ecosystems based on photosynthetic systems are needed to estimate if oxygenic and or anoxygenic ecology on Proxima Centauri b could be on a scale large enough to be detectable. In the case of oxygenic photosynthesis such calculations are routine (see Appendix) (Ritchie Reference Ritchie2010): nine photons are used to fix one CO2 (quantum number, γ = 9) and the Calvin–Benson cycle is used to fix CO2. In the Appendix, we make estimates of the quantum efficiency values (γ) for RC-2 and RC-1-type photosynthetic bacteria in order to estimate carbon fixation rates from their photosynthetic electron transport rates (ETRs) (Ritchie Reference Ritchie2013; Ritchie & Runcie Reference Ritchie and Runcie2013; Ritchie & Mekjinda Reference Ritchie and Mekjinda2015).
This study consists of several parts. The light regimes of Proxima Centauri b will be assessed in terms of photosynthetically useable irradiance by oxygenic and anoxygenic photosynthetic organisms. The light absorption properties and photosynthetic performance of some representative photosynthetic organisms will be considered based upon their primary and accessory pigmentation (Blankenship et al. Reference Blankenship, Madigan and Bauer1995; Falkowski & Raven Reference Falkowski and Raven2007, Kiang et al. Reference Kiang, Siefert, Govindjee and Blankenship2007a, Reference Kiang, Segura, Tinetti, Blankenship, Cohen, Siefert, Crisp and Meadowsb). We use a primary productivity model for a simple flat sheet of cells (or mat) as the geometrically simplest scenario to assess photosynthetic performance (Kirk Reference Kirk2011). The properties of light attenuation in water will then be taken into account so that photosynthesis on land and in aquatic environments can be compared. The very low irradiance of Proxima Centauri at wavelengths capable of penetrating water is hence a critical issue (Gan et al. Reference Gan, Zhang, Rockwell, Martin, Lagarias and Bryant2014; see Fig. 4 in Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). Finally, the prospects of being able to detect oxygenic and anoxygenic photosynthesis on Proxima Centauri b will be discussed.
Materials and methods
Culturing the oxygenic cells
The green alga C. vulgaris (Beyerinck [Beijerinck]) (Chlorophyta) was from the Phuket Marine Biological Centre, Laem Panwa, Phuket 83000 and the Sydney University Algal Culture Collection. The diatom P. tricornutum (Bohlin) (Bacillariophyta) was from the University of Sydney Algal Culture Collection. Rhodomona sp. (Cryptophyta) was a gift from Professor Pauline Ross (University of Western Sydney – Hawkesbury). P. hollandica PCC9006 (Burger, Weiss, Stal & Mur) and Synechococcus R-2 PCC7942 were from the Pasteur Culture Collection. A. marina MBIC11017 (Miyashita et Chihara) was a gift from Professor A.W.D. Larkum (University of Sydney) and originated from the Marine Biotechnology Institute Culture Collection, Marine Biotechnology Institute, 3-75-1Heita, Kamaishi, Iwate 026-0001, Japan. Chlorella, Synechococcus and Prochlorothrix all grew well in BG-11 medium (Allen Reference Allen and Stein1973). No added vitamins were needed. Acaryochloris, Phaeodactylum and Rhodomonas were grown in seawater supplemented with BG-11 trace elements, 100 mmol m−3 sodium silicate, 200 mmol m−3 KH2PO4 and 1 mol m−3 sodium nitrate with the standard f/2 supplements of B12, Thiamine and Biotin (McLachlan Reference McLachlan and Stein1973).
Chlorella, Synechococcus, Prochlorothrix, Rhodomonas and Phaeodactylum were grown in 250 and 500 ml conical flasks, shaken and stirred daily. Cultures were kept on shelves fitted with overhead fluorescent lights (Panasonic 36 W daylight, colour temperature 6500 K: TIS 956–2533) in continuous light at ≈27°C. The light intensity in the culture room was approximately 100–150 µmol photon m−2 s−1 [photosynthetic photon flux density (PPFD) 400–700 nm], measured using a Li-Cor photon flux meter Model LI-189 (Li-Cor Corp, Lincoln, Nebraska, USA). The irradiance used for culture was fortuitously close to the PPFD irradiance available on Proxima Centauri b.
Culturing the anoxygenic cells
Afifella (Rhodopseudomonas) marina (Imhoff) was isolated from dead pearl oyster shells from a pearl farm located in Phuket, Thailand (Phuket Pearl Industry Co. Ltd., Phuket 83200 Thailand) and grown as described by Ritchie & Runcie (Reference Ritchie and Runcie2013) and Ritchie (Reference Ritchie2013) in BG-11 enriched seawater with 5 mol m−3 NH4Cl as a nitrogen source and 5 mol m−3 acetate as the carbon source. f/2 vitamins were added as described by McLachlan (Reference McLachlan and Stein1973). It was found that growth improved with the addition of para-aminobenzoic acid (1 mg l−1) commonly needed as a vitamin by rhodopseudomonads (Kim & Harwood Reference Kim and Harwood1991). R. palustris (CGA009) is the most well-known strain of the organism and is completely sequenced (Larimer et al. Reference Larimer2004). It was a kind gift from Professor C.S. Harwood (University of Washington, Seattle, Washington State, USA). It was grown in fully-defined simplified PM medium (Kim & Harwood Reference Kim and Harwood1991) with 10 mol m−3 acetate and benzoic acid as carbon sources as described by Ritchie (Reference Ritchie2013). T. tepidum (Madigan, ATCC 43061), B. viridis (Hiraishi) (DSM133) and Chlorobaculum (Chlorobium) tepidum (TLS) were kind gifts from Professor R.E. Blankenship (Washington University, St Louis, Missouri, USA). They were grown in modified PM-media with addition of 2 mol m−3 Na2S and acetate as the only organic carbon source as described by Ritchie & Mekjinda (Reference Ritchie and Mekjinda2015).
Afifella, Rhodopseudomonas, Thermochromatium, Blastochloris and Chlorobaculum cultures were routinely grown in the culture room in capped McCartney bottles and capped 250 ml bottles that were mixed by inversion once a day. All five organisms could be grown in natural sunlight (Ritchie Reference Ritchie2013; Ritchie & Runcie Reference Ritchie and Runcie2013; Ritchie & Mekjinda Reference Ritchie and Mekjinda2015).
Rhodopseudomonas shows considerable photoadaptation to the light intensities under which it is grown (Ritchie Reference Ritchie2013) but Thermochromatium showed some increase in optimum irradiance (E opt) when grown in the laboratory compared with sunlight but no great changes in pigmentation (Ritchie & Mekjinda Reference Ritchie and Mekjinda2015), Afifella saturates at low irradiances whether or not it is grown in high irradiance (Ritchie & Runcie Reference Ritchie and Runcie2013). Blastochloris and Chlorobaculum grown in sunlight showed little chromatic difference compared with cells grown in the laboratory.
Absorbance measurement of oxygenic cell suspensions
Absorbance measurements of algal suspensions were measured using a Taylor Integrating Sphere attachment (ISR-240A) on a Shimadzu UV-2550 UV–visible spectrophotometer (Shimadzu, Kyoto, Japan) at the University of Sydney, NSW, Australia. The Taylor sphere was used to minimize the effects of light scattering. Non-photosynthetic absorptance was allowed for by zeroing and base-lining the spectrophotometer on 750 nm (Cummings & Zimmerman Reference Cummings and Zimmerman2003). For the purposes of the present study, absorbance curves were normalized [Absorbance (A) = 1, A = 2−Log10(%Trans)] onto the blue peak of absorption (Soret Band) for the oxygenic photoorganisms (Ritchie Reference Ritchie2013).
Absorbance measurement of photosynthetic bacterial cell suspensions
No Taylor Integrating sphere was accessible in Thailand at the time. In vivo absorption of Afifella, Rhodopseudomonas, Thermochromatium, Blastochloris and Chlorobaculum (350–1100 nm) were measured on cell suspensions in 60% sucrose using a Shimadzu UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan) based at Prince of Songkla University-Phuket, Thailand (Sojka et al. Reference Sojka, Freeze and Gest1970; Neutzling et al. Reference Neutzling, Imhoff and Trüper1984; Schott et al. Reference Schott, Griffin and Schink2010; Ritchie Reference Ritchie2013; Ritchie & Runcie Reference Ritchie and Runcie2013) or a Spectroquant Pharo 300 (Merck KGaA, Darmstadt, Germany). Neither spectrophotometer could measure absorbances beyond 1100 nm. Sixty per cent sucrose has a refractive index similar to the cytoplasm of microbes and so minimizes scattering of light by the cells. As for oxygenic cells, absorbance curves of photosynthetic bacterial cell suspensions were normalized (absorbance peak scaled to A λ = 1) onto the in vivo blue (Soret band) peak of the anoxygenic photosynthetic organisms as described by Ritchie & Runcie (Reference Ritchie and Runcie2013); Ritchie (Reference Ritchie2013) and Ritchie & Mekjinda (Reference Ritchie and Mekjinda2015). The absorbance curves were zeroed at 1000 nm on 60% sucrose for Afifella, Rhodopseudomonas, Thermochromatium and Chlorobaculum; for Blastochloris the spectrum was zeroed on 900 nm because that was the absorption minima within the wavelength range of the spectrophotometers used in the study because BChl b has substantial in vivo absorbance even at 1100 nm. Absorbance curves for Chlorella and Phaeodactylum measured using the 60% sucrose method were closely comparable with the results using the Taylor sphere.
Total emission spectra of the Sun and Proxima Centauri
Earth surface irradiance/top of atmosphere (TOA) irradiance at each wavelength was based on SMARTS (2011) using the procedure described by Ritchie (Reference Ritchie2010). A total emission spectrum (TES) of Proxima Centauri is now available (Fig. 4 in Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017), and covers a very wide wavelength range from 0.6 to 13 000 nm and has been used to estimate the X-ray/UV environment for Proxima Centauri b. The UV data come from observations using the Space Telescope Imaging Spectrograph (STIS) of the Hubble Space Telescope (HST), as part of the HST Next Generation Spectral Library, and also from HST's Faint Object Spectrograph (FOS). The flux scale of both instruments has been subject to very careful calibration and is expected to be accurate to within a few per cent. The STIS data cover from 180 to 900 nm, while the FOS spectrum covers from 460 to 850 nm, and they show good mutual agreement. A theoretical Phoenix model (Baraffe et al. Reference Baraffe, Homeier, Allard and Chabrier2015) corresponding to an effective temperature of 3000 K was used for wavelengths >900 nm.
Stellar flaring in UV and X-rays could represent a probable limitation for the habitability of the planet Proxima Centauri b (Scalo et al. Reference Scalo2007; Segura et al. Reference Segura, Walkowicz, Meadows, Kasting and Hawley2010; Gale & Wandel Reference Gale and Wandel2016; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017), although its present flaring activity is significantly lower than many other red dwarfs (Pettersen & Hawley Reference Pettersen and Hawley1989; Buccino et al. Reference Buccino, Mauas, Lemarchand, Norris and Stootman2002, Reference Buccino, Lemarchand and Mauas2007; Davenport et al. Reference Davenport, Kipping, Sasselov, Matthews and Cameron2016; Fig. 4 in Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). The flare distribution is in agreement with the analysis of Audard et al. (Reference Audard, Güdel, Drake and Kashyap2000) for the similar star CN Leo and the conclusion is that Proxima Centauri undergoes a major flare (total energy >1032 erg) once in approximately 10 days. It is likely that the UV flux during such strong flares could be up to two orders of magnitude larger than the overall averages shown in Fig. 4 of Ribas et al. (Reference Ribas, Gregg, Boyajian and Bolmont2017) with typical durations of 0.5–1 h (Scalo et al. Reference Scalo2007; Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). Strong flare events would only change the spectrum below about 350 nm (below the photosynthetically useable range).
Planetary atmospheric absorption
Turbet et al. (Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016) discuss many different atmospheric and temperature scenarios for Proxima Centauri b including 1 : 1 and 3 : 2 tidal locking scenarios, which would produce very different environmental conditions on the planet (Edson et al. Reference Edson, Lee, Bannon, Kasting and Pollard2011). Light reaching the surface of Proxima Centauri b with an Earth-like atmosphere could be approximated using atmospheric absorbance ratios of Earth surface irradiance/TOA irradiance at each wavelength based on the SMARTS (2011) data for Earth and models developed by Segura et al. (Reference Segura, Krelove, Kasting, Sommerlatt, Meadows, Crisp, Cohen and Lawler2003, Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005). The SMARTS data shown in Fig. 3 show values for the Earth's extraterrestrial radiation (TOA) and irradiance at the Earth's surface when the Sun was directly overhead at 0.5 or 1 nm intervals over a range that includes all wavelengths used by photosynthetic mechanisms is shown as part of Fig. 4. In the present study, the atmospheric absorbance ratio at each 1 nm interval from 280 to 1200 nm was calculated and used to estimate the on-ground-irradiance for the exoplanet. Passage through the modern (oxic) Earth atmosphere results in the virtual elimination of UV-C and UV-B and nearly all UV-A radiations. There are three H2O absorption bands, one at 810–835 nm and very strong bands at 893–987 nm and 1090–1180 nm (>50% absorption) (Fig. 4 and see Kiang et al. Reference Kiang, Siefert, Govindjee and Blankenship2007a). Within the photosynthetically useful range for photosynthetic bacteria (350–1100 nm) there are minor absorption bands for O2 at 628, 688, 866 and 1068 nm and a very sharp O2 absorption band (A band) at 761 nm. This 761 nm band is the only obvious difference in the range of wavelengths used by photosynthetic organisms (Schindler & Kasting Reference Schindler and Kasting2000; Segura et al. Reference Segura, Krelove, Kasting, Sommerlatt, Meadows, Crisp, Cohen and Lawler2003, Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005; Kiang et al. Reference Kiang, Siefert, Govindjee and Blankenship2007a, Reference Kiang, Segura, Tinetti, Blankenship, Cohen, Siefert, Crisp and Meadowsb). An Earth-like but anoxic atmosphere based on volcanic and hydrothermal outgasing (Ar–H2–N2–CO2–CO–H2O–H2S with traces of CH4 and NH3; Schindler & Kasting Reference Schindler and Kasting2000; Kasting & Howard Reference Kasting and Howard2006; Trail et al. Reference Trail, Watson and Tailby2011; Zahnle et al. Reference Zahnle, Schaefer and Fegley2011) would have very similar absorption properties as the current Earth atmosphere at UV-B, visible, far-red (700–749 nm) and near-IR (NIR) and IR (750–1100 nm) wavelengths. A good approximation of the transmission spectrum of an Earth-like but anoxic atmosphere can be constructed by setting the ozone value to zero in the data files used by the SMARTS software and by setting all the absorbance values in the associated Abs_O2.dat data file to zero. None of the potential replacement gases for O2 (H2–N2–CO2–CO–H2S) have strong absorption bands in the range 350–1100 nm. The absorption data files, showing the calculated proportional absorption of the standard Earth atmosphere from the TOA and ground irradiance and an Earth-like but anoxic atmosphere, are provided as Supplementary Material (Aerobic-Anaerobic Atmospheric Absorption .txt File). The absorption table for an anoxic but Earth-like atmosphere is supplied as part of a Supplementary text file.
Results
Absorption characteristics of selected oxygenic photoautotrophs
To appreciate the spectral properties of irradiance on Proxima Centauri b it is necessary to calculate how extant terrestrial oxygenic photoautotrophs would perform under the spectral signature on Proxima Centauri b. Figure 1 shows a comparison of the in vivo normalized absorption spectrum of the cyanobacteria Synechococcus R-2 PCC7942, P. hollandica and the unusual cyanobacterium A. marina; and also amongst the eukaryotic algae, C. vulgaris, Rhodomonas sp. and the diatom P. tricornutum. All the spectra of these oxygenic photosynthetic organisms have been normalized onto the blue Chl (Soret Band) a or Chl d peak fixed at an Absorbance (A) of 1 [A = 2−log10 (% Trans)]. The in solvent red peak of Chl a is at about 665 nm but in vivo is at about 675–685 nm depending on the organism (Fig. 1). The respective blue and red peaks for the photosynthetic organisms used in the present study were: blue peaks, Rhodomonas, A 437; Phaeodactylum, A 440; Prochlorothrix, A 435, Synechococcus, A 437; Chlorella, A 437; Acaryochloris, A 457, for comparison the red or Qx peaks were Rhodomonas, A 675; Phaeodactylum, A 675; Prochlorothrix, A 676, Synechococcus, A 680; Chlorella, A 681; Acaryochloris, A 705. The spectrum for Chlorella is redrawn from Ritchie & Runcie (Reference Ritchie and Runcie2013).
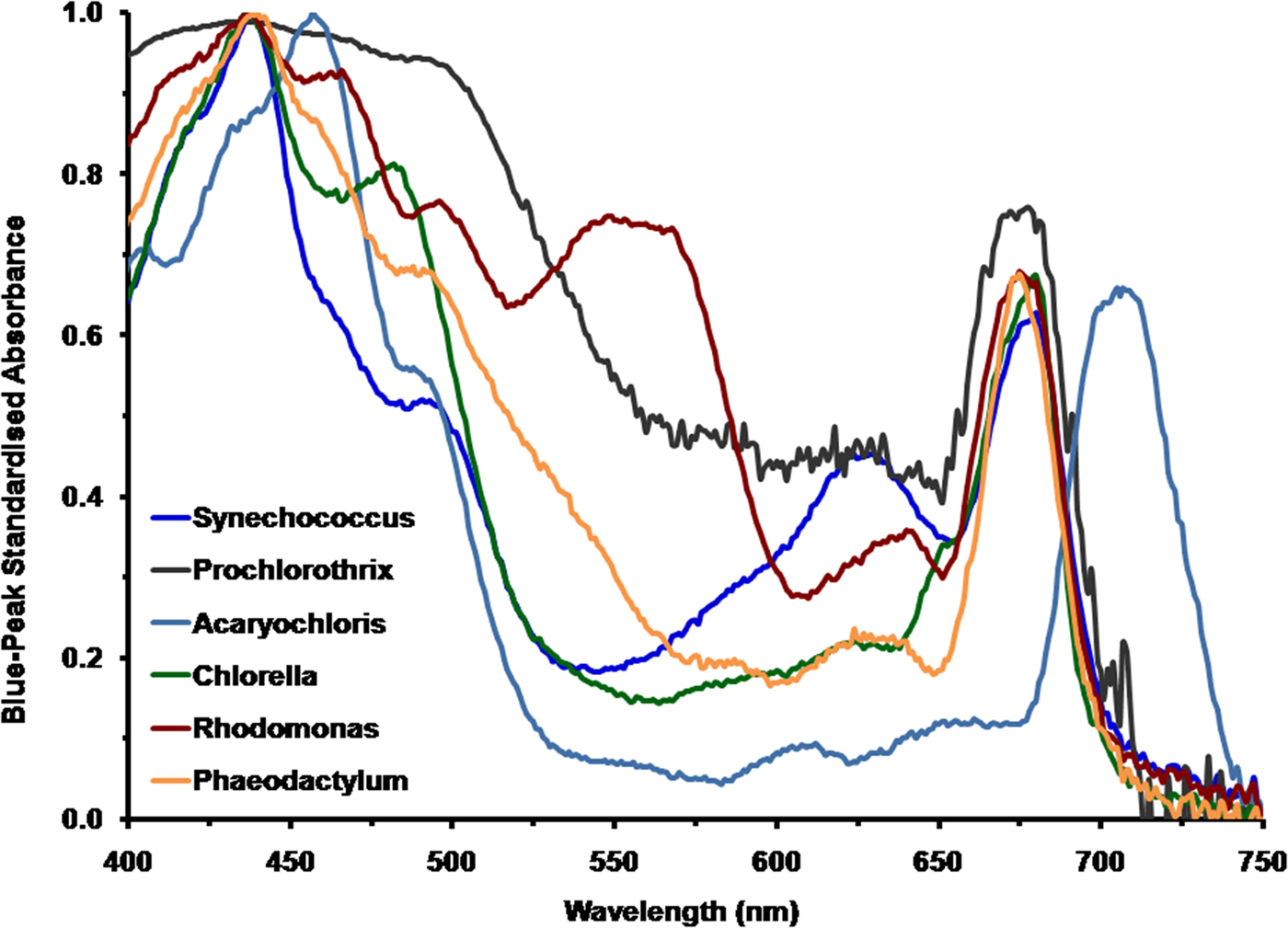
Fig. 1. Comparison of the normalized absorption spectra of selected oxygenic photoautotrophs. These include the cyanobacterium Acaryochloris which uses Chl d as its primary Chl and cyanobacteria Prochlorothrix, Synechococcus and the eukaryotic algae Chlorella (Chlorophyta), Rhodomonas (Cryptophyta) and the diatom Phaeodactylum which use Chl a. All the spectra of these oxygenic photosynthetic organisms have been normalized onto the blue Chl a or d peak (Soret band) fixed at an absorbance of 1 (10% transmission).
All six oxygenic organisms can use blue light (400–500 nm) and the in vivo blue peaks of Chl a and d are very similar. Prochlorothrix, Synechococcus, Acaryochloris and Chlorella cannot effectively utilize green light (500–575 nm) whereas Rhodomonas is able to use green light because of the presence of phycoerythrin (orange/red) light-harvesting pigment. The high levels of carotenoids, especially fucoxanthin, in Phaeodactylum also give it an advantage in green light (500–574 nm). Phycocyanin (blue) pigments of Synechococcus effectively absorb yellow and orange light (575–649 nm); these pigments are also present in both Prochlorothrix and Acaryochloris but in lesser amounts and so are less significant. All the photo-oxygenic organisms, using Chl a as their primary photosynthetic pigment, strongly absorb red light (660–699 nm). The Chl d of Acaryochloris strongly absorbs in the far-red (700–749 nm) and since it also contains some Chl a Acaryochloris also absorbs in the 660–699 nm range (Miyashita et al. Reference Miyashita, Ikemoto, Kurano, Miyachi and Chihara2003). Cyanobacteria that contain Chl f would perform similarly, except that they are able, in addition, to up regulate unique phycobiliproteins that absorb in the far-red/NIR (NIR>750 nm) (Gan et al. Reference Gan, Zhang, Rockwell, Martin, Lagarias and Bryant2014).
Absorption spectra of selected anoxygenic photosynthetic bacteria
Figure 2 shows the spectral absorption properties of a selection of five photosynthetic bacteria with differing pigmentation: three purple non-sulphur bacteria: A. marina, R. palustris and B. viridis; one purple sulphur bacterium: T. tepidum and one green sulphur bacterium: C. (Chlorobium) tepidum. They have been scaled against the in vivo Blue (Soret) band maxima set to an absorbance (A λ) of 1 [10% transmission, A λ = 2-Log10(T λ%)]. The wavelengths of the blue absorption maxima are: Afifella, 370 nm, Rhodopseudomonas, 376 nm, Blastochloris, 400 nm, Thermochromatium, 377 nm and Chlorobaculum, 459 nm. Afifella has a single IR in vivo maximum at 834 nm whereas Rhodopseudomonas has double IR maxima at 807 and 868 nm and Thermochromatium also has double IR maxima at 805 and 872 nm. Blastochloris, which uses BChl b as its primary photosynthetic pigment, has a single IR peak (at 1017 nm) that is more red-shifted than any other known photosynthetic organism (Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005). Chlorobaculum has a prominent peak at 748 nm attributable to BChl c in vivo, which is the most abundant, but accessory, BChl in the cell and a small ‘knee’ at about 810 nm due to BChl a which is the primary BChl even though it is much less abundant. Photosynthetic bacteria, using accessory photosynthetic pigments, are all able to use a substantial proportion of irradiance in the 400–700 nm range and both BChl a and b type photosynthetic bacteria are able to absorb orange/red light at about 600 nm (Fig. 2) due to a secondary Soret absorption band of both BChl a and b (Hellingwerf et al. Reference Hellingwerf, de Vrij and Konings1982). In vivo spectral shape and peaks of BChls are typically very different to in solvent and so in solvent spectra should not be used to deduce photosynthetic behaviour of photosynthetic bacteria (Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005; cf. Komatsu et al. Reference Komatsu, Umemura, Shoji, Kayanuma, Yabana and Shiraishi2015).
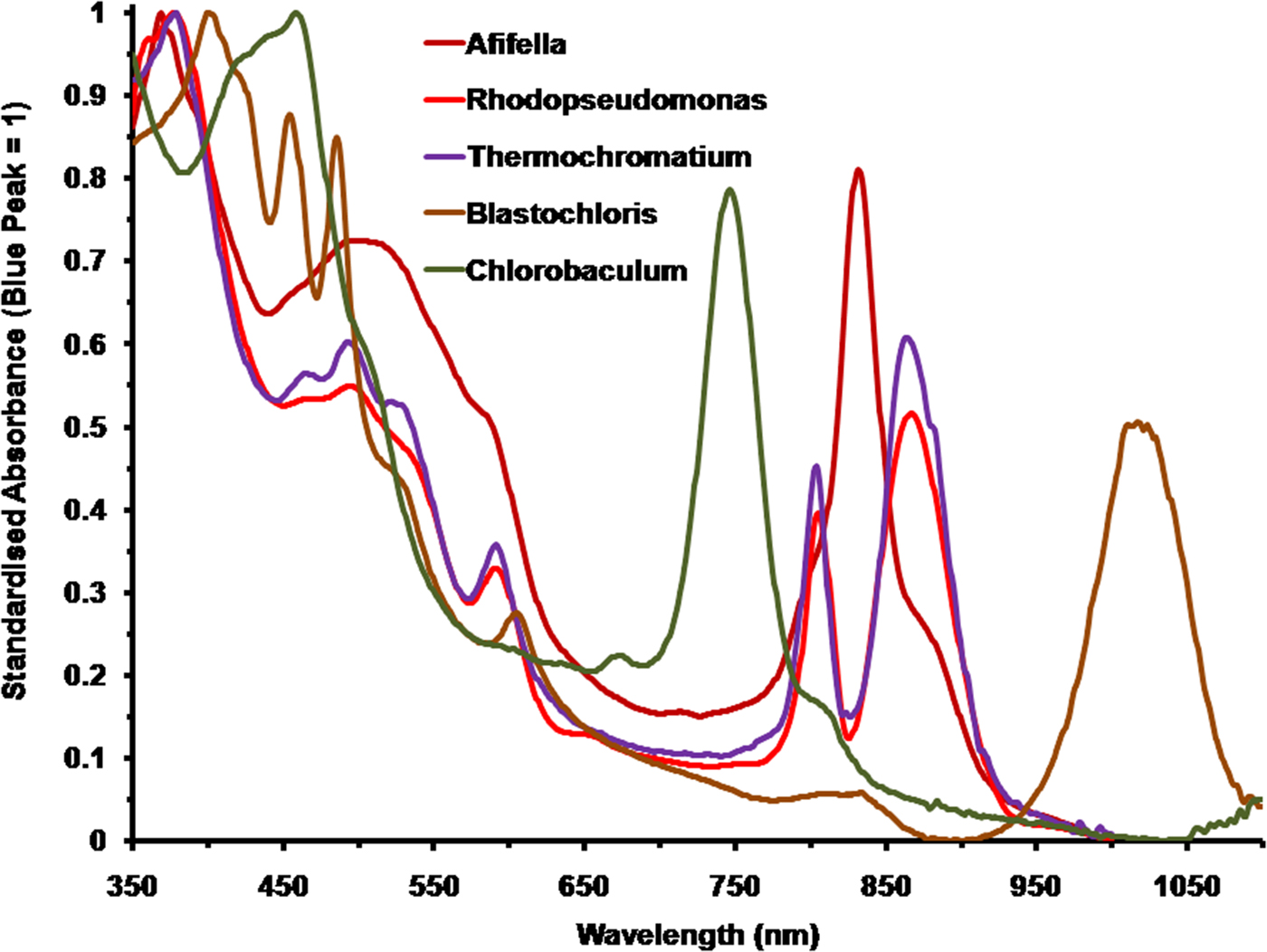
Fig. 2. Comparison of the normalized absorption spectra of the photosynthetic bacteria, Afifella, Rhodopseudomonas, Thermochromatium, Blastochloris and Chlorobaculum. These should be compared with those of the oxygenic photosynthetic organisms in Fig. 3. All the spectra of these anoxygenic photosynthetic organisms have been normalized onto the blue BChl (Soret band) fixed at an absorbance of 1. The spectra are based on scans of laboratory grown cells suspended in 60% sucrose.
TES of the sun and Proxima Centauri b
The irradiance of the Sun at the TOA was calculated using SMARTS (2011) (Fig. 3) and converted into quantum units using Planck's Law. The spectrum for Proxima Centauri has been calculated at the orbit of Proxima Centauri b and is based on the TES of the star. The spectra are very different reflecting the large difference in effective temperature (Sol, Type G2 V – 5780 K) and Proxima Centauri (Type M5.5 V – 3050 K). The stellar atmosphere of Proxima Centauri absorbs light strongly and so the TOA spectrum has much stronger features than the Sun and there are absorption bands of critical importance to photosynthesis. The region where the in vivo red peak of Chl a is located (660–700 nm, see Fig. 1) is strongly depleted and the region near the 761 nm oxygen absorption peak is also strongly depleted. Note that in IR wavelengths (>900 nm) Proxima Centauri bat TOA would experience irradiance higher than Earth.
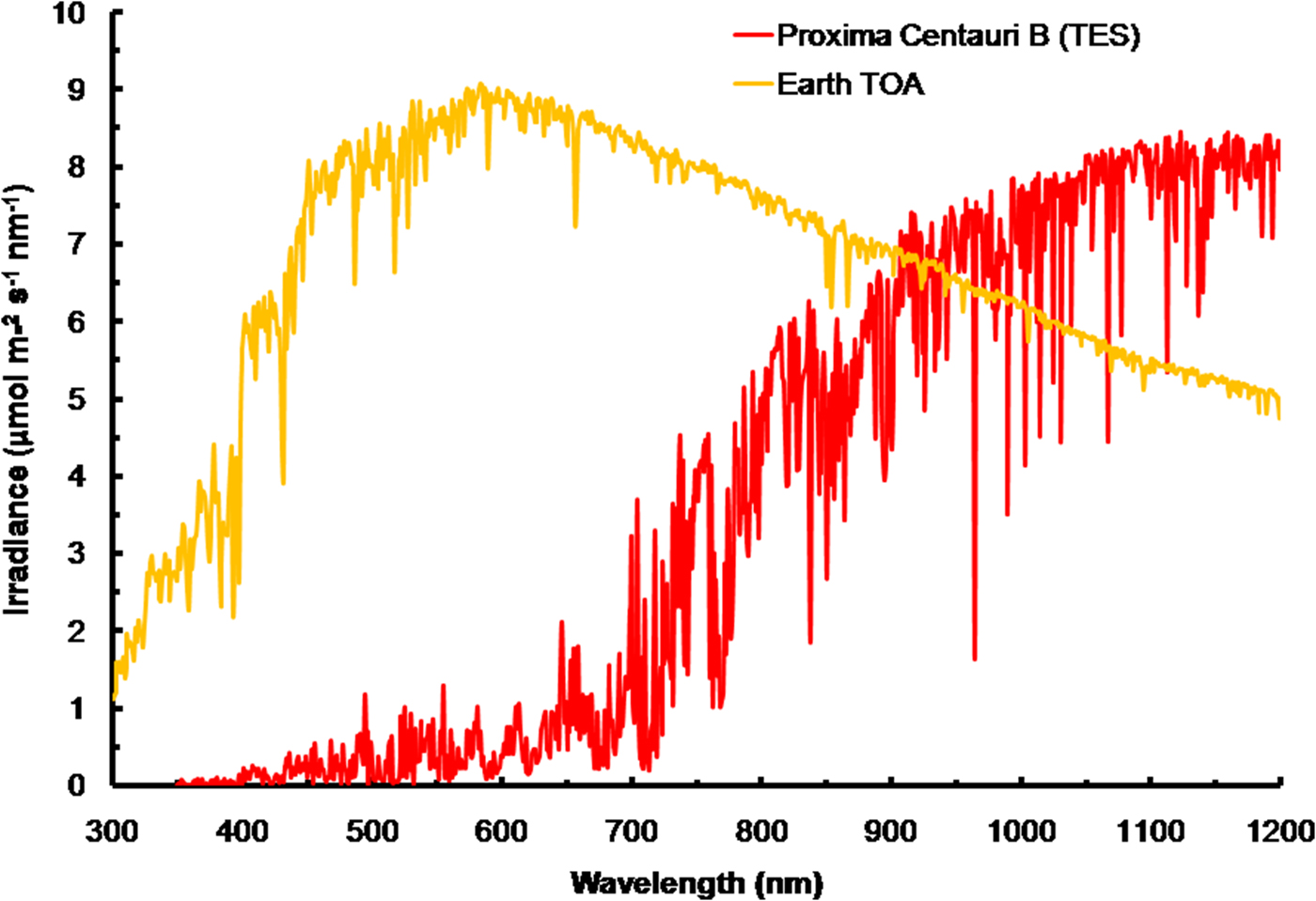
Fig. 3. The total emission spectra of the Sun (in μmol photon m−2 s−1 nm−1) at the top of the atmosphere (TOA) of Earth based on the SMARTS (2011) software for the Equator at noon equinox compared with the recently measured TES of Proxima Centauri b at TOA. Compared to the sun, Proxima Centauri b produces very little visible light (400–749 nm useable by oxygenic photosynthetic organisms but produces comparable irradiance in the far-red IR range. The cool temperature of Proxima Centauri b results in strong absorption bands in its stellar atmosphere. Strong depletion in the range 650 to 699 nm would disadvantage Chl a-based oxygenic photosynthesis.
Planetary atmospheric absorption and the light spectra at the surfaces of Earth and Proxima Centauri b
The solar spectrum reaching the ground was calculated for the Equator at noon equinox (SMARTS 2011) and compared with the TOA at each wavelength in order to calculate an atmospheric absorption ratio for each wavelength (see the Supplementary Atmospheric Absorption File) (Fig. 3, Table 1). When Proxima Centauri is in its usual quiescent state (Anglada-Escudé et al. Reference Anglada-Escudé, Tuomi, Gerlach, Barnes, Heller and Jenkins2013, Reference Anglada-Escudé2016), UV-A and UV-B would be virtually absent from the TOA emission curves for Proxima Centauri b (see Fig. 3) and so the surface of the planets would receive very little UV irradiance regardless of the nature of its atmosphere. Better measurements of frequency and extent of UV flaring are needed to more fully assess the UV-A & UV-B environment for Proxima Centauri b (Davenport et al. Reference Davenport, Kipping, Sasselov, Matthews and Cameron2016; Fig. 4 in Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). Based on current evidence, the episodic flares (every 10 days or so) would increase UB-A & UV-B by about an order of magnitude above current TOA levels of the Earth. For comparison, the irradiance (300–1200 nm) reaching the ground on Earth with the Sun directly overhead is included on Fig. 4 for both an oxic atmosphere and an anoxic but Earth-like atmosphere. Using the absorption properties of an Earth-like oxic and anoxic atmosphere the calculated irradiance on the surface of Proxima Centauri b would have little blue (400–499 nm), green (500–574 nm), orange (575–650 nm), red or far-red light (650–699 & 700–749 nm): overall PPFD irradiance is about 3% that on Earth (Fig. 4, Table 1, PPFD ≈63 µmol photon m−2 s−1). There is more than twice as much useable irradiance for Chl d-type oxygenic organisms able to use 700–749 nm light (132 µmol photon m−2 s−1, Table 1). This would greatly favour Chl d containing organisms and those Chl a organisms able to use far-red light (Fig. 4). The large amounts of far red and NIR (700–799 nm) and IR (I & II) would benefit anoxygenic organisms (Figs. 2 and 4). On the surface of Proxima Centauri b the irradiance potentially usable by oxygenic organisms is very low but total photons potentially usable by anoxygenic photosynthesis approaches that available on Earth and well above the optimum photon fluxes for photosynthetic bacteria (Ritchie Reference Ritchie2013; Ritchie & Runcie Reference Ritchie and Runcie2013; Ritchie & Mekjinda Reference Ritchie and Mekjinda2015). On both planets H2O strongly absorbs irradiance in the range 900–970 and 1100–1150 nm hence the atmospheric IR absorption valleys in Fig. 4 compared with Fig. 3.
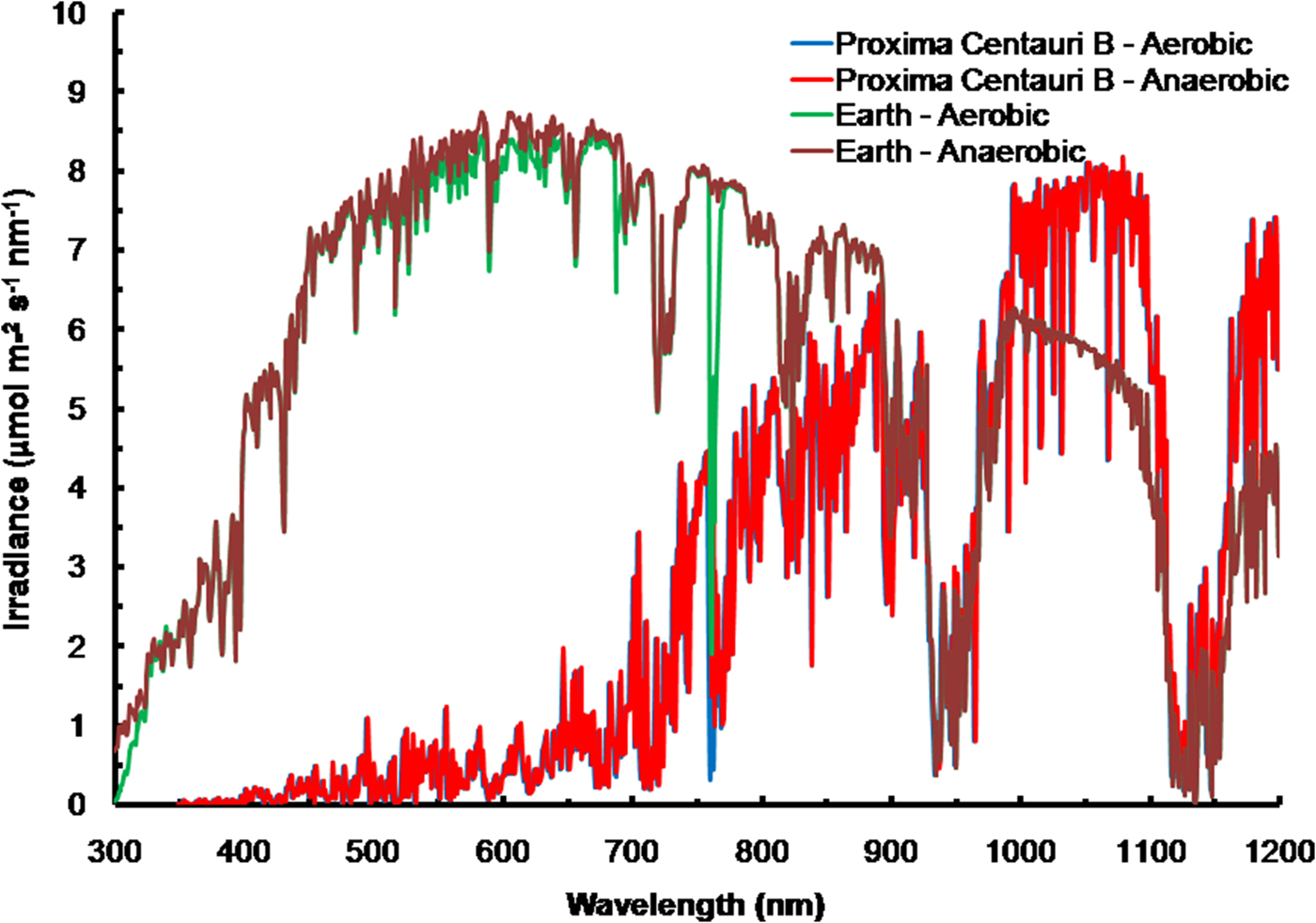
Fig. 4. The ratio of the TOA at each wavelength and the irradiance reaching the ground for the Earth with the current oxic atmosphere and for an Earth-like but anoxic atmosphere were used to calculate the irradiance reaching the surface of Proxima Centauri b with an Earth-like oxygenic or anoxygenic atmosphere. An Earth-like but anoxic atmosphere would have very little effect on the irradiance reaching the Earth's surface in the range of wavelengths useable by oxygenic or anoxygenic organisms. The conspicuous differences are the increase in UV light reaching the Earth's surface and the conspicuous 761 nm O2 absorption band present with an oxygenic atmosphere. In the case of Proxima Centauri b the only conspicuous difference made by an oxic versus anoxic Earth-like atmosphere is the presence of the 761 nm absorption band in the case of an oxic atmosphere.
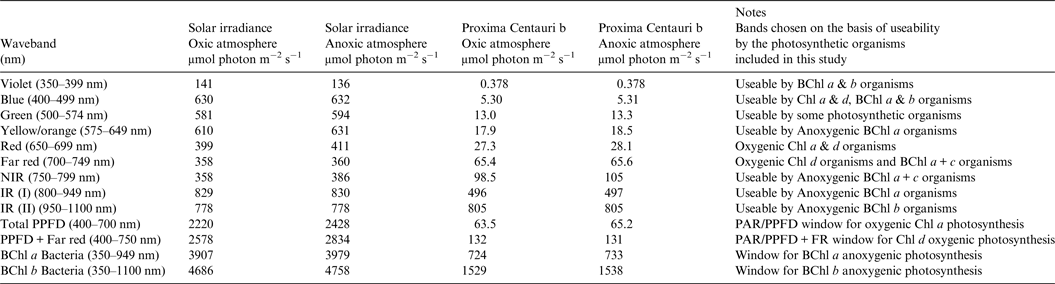
Table 1. Surface irradiance of Earth and Proxima Centauri b. Photon irradiance wavebands of importance for photosynthesis on the Earth and Proxima Centauri b for a planet with Earth-like Oxic Atmosphere and for an Earth-like but Anoxic Atmosphere
Absorption of light by mats of oxygenic photosynthetic organisms on the surface of Earth and predictions for Proxima Centauri b
The Appendix outlines a model for photosynthesis both for mats composed of oxygenic photoautotrophs on the Earth and on Proxima Centauri b. We concentrate on microbial mats in this study because they are the first known photosynthetic structures on Earth (≈3.5 Gyr; Tice & Lowe Reference Tice and Lowe2004; Schopf Reference Schopf2011; Djokic et al. Reference Djokic, Van Kranendonk, Campbell, Walter and Ward2017) and persist to this day and photosynthesis on a surface is straightforward to deal with compared with plankton in water columns, where side and backscattering and progressive absorption by water are major problems for estimating irradiance (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011). Combining the data on the light regime on the surface of the Earth and Proxima Centauri b (Fig. 4, Table 1) with the light absorption characteristics of the oxygenic photosynthetic organisms included in the present study (Fig. 1) provides a comparative estimation of the capacity for growth of these various organisms. The 400–700 nm range is used by all oxygenic photosynthetic organisms with Chl a as their primary photosynthetic pigment and the 400–749 nm range is useable by Acaryochloris which uses the far-red absorbing Chl d as its primary photosynthetic pigment (Miyashita et al. Reference Miyashita, Ikemoto, Kurano, Miyachi and Chihara2003; Larkum Reference Larkum and Rengler2008; Chen & Scheer Reference Chen and Scheer2013; Schliep et al. Reference Schliep, Cavigliasso, Quinnell, Stranger and Larkum2013). To better interpret the importance of various pigments for oxygenic photosynthesis the visible spectrum was divided up into various bands based on what irradiances are most important to known oxygenic photosynthetic organisms: blue (400–499 nm), green (500–574 nm), yellow/orange (575–649 nm) and red (650–699 nm) (Tables 2–5). In addition, far-red (700–749 nm) is useful to Acaryochloris because of the absorption properties of Chl d and a few exotic chlorophytes such as the endolithic Ostreobium, which is able to use 700–749 nm light even though it has only Chl a + b (Wilhelm & Jakob Reference Wilhelm and Jakob2006).
Table 2. Irradiance useable by photooxygenic organisms on the Earth with the modern Oxygenic Atmosphere
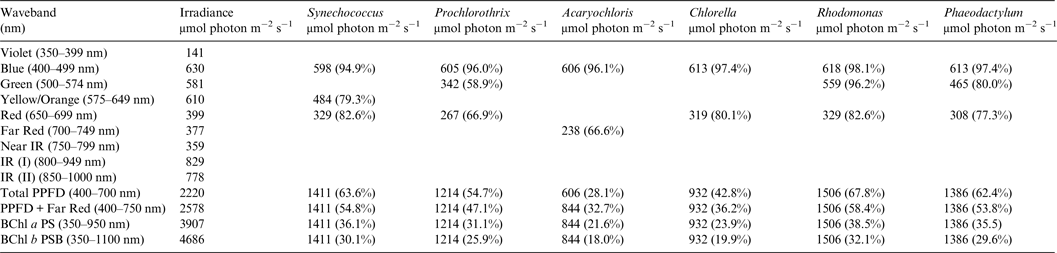
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for solar radiation on Earth with an oxygenated atmosphere. The values are based on the observationally known solar spectrum. The percentage absorption is shown in brackets.
Table 3. Irradiance useable by photooxygenic organisms on an Earth with an Anoxic Atmosphere
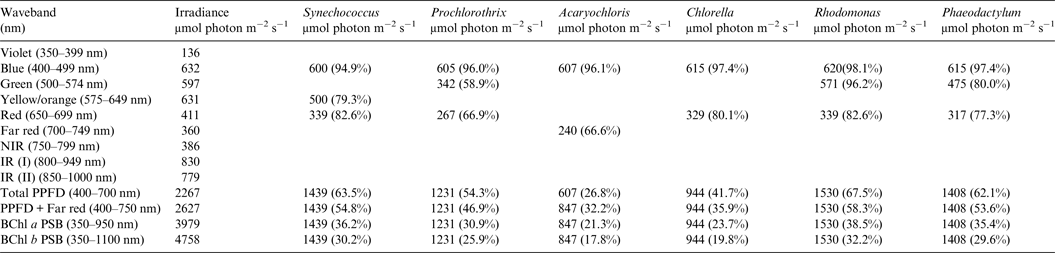
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for solar radiation on Earth with an Anoxic atmosphere. The values are based on the observationally known solar spectrum. The percentage absorption is shown in brackets.
Table 4. Useable irradiance for Photooxygenic organisms on Proxima Centauri b with an Earth-like Oxygenic Atmosphere
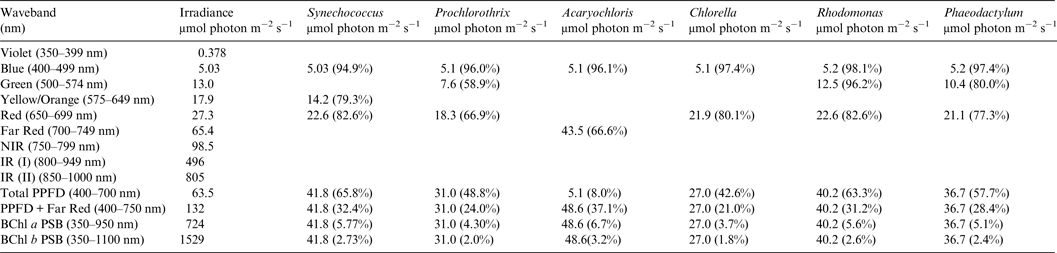
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for stellar radiation on Proxima Centauri b with an oxic atmosphere. The values are based on a detailed TES of Proxima Centauri b. The percentage absorption is shown in brackets.
Table 5. Irradiance useable by Oxygenic organisms on Proxima Centauri b with an Earth-like but Anoxic Atmosphere
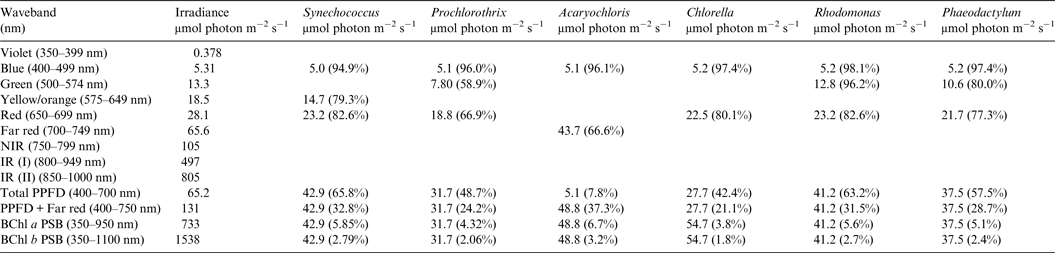
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for stellar radiation on Proxima Centauri b with an anoxic atmosphere. The values are based on a detailed TES of Proxima Centauri b. The percentage absorption is shown in brackets.
Earth
Table 2 shows the in vivo absorption of a mat of each of the six oxygenic photosynthetic organisms with a blue peak absorbance of 2 (1% transmission) of irradiance from the Sun at sea level at the Earth's equator at noon on an equinox. We defined a ‘standard mat’ or idealized model mat as a reference case as having 1% transmission because such a mat would be essentially optically black at the blue peak of Chlorophyll absorption. All six microbial mats would be able to use nearly all blue and red light for photosynthesis but other useable irradiance in the range 500–649 nm depends on their specific accessory pigment composition. Table 2 shows the total absorption for blue (400–499 nm), green (500–574 nm), yellow–orange (575–649 nm), red (650–699 nm) and far-red (700–749 nm) light compared with the number of photons available in each wavelength window for the ground-level irradiance. Where an organism is known not to have a suitable accessory pigment the box is left blank (Falkowski & Raven Reference Falkowski and Raven2007). All the organisms with large amounts of accessory pigments are able to use about 55–69% of the irradiance in the 400–699 nm window usually thought of as the limits of photosynthetically useful radiation for organisms with Chl a as their primary photosynthetic pigment (Synechococcus, Prochlorothrix, Rhodomonas and Phaeodactylum). The cryptophyte, Rhodomonas and the orthodox cyanobacterium, Synechococcus are the most efficient users of 400–749 nm visible light for oxygenic photosynthesis. The green alga, Chlorella, which has the same pigment array as terrestrial vascular plants (Chl a, Chl b + carotenoids), is able to use only about 43% of the 400–699 nm window. The far-red absorption band of Acaryochloris (700–749 nm) enables the alga to use about the same amount of light as the red absorption band of Chlorella and so the irradiance actually photosynthetically useable by Acaryochloris in the range 400–749 nm is about 33%. It turns out that Acaryochloris is the least able to use 400–749 nm light for oxygenic photosynthesis despite its exotic photosynthetic mechanism because it is poorly endowed with blue and green absorbing carotenoids and phycobilin pigments (Fig. 1).
A similar set of calculations was done for an Earth with an Earth-like but anoxic atmosphere (Table 3). There is very little difference between the results for Tables 2 and 3. Aerobic and anaerobic atmospheres are essentially equivalent for oxygenic photosynthetic organisms. The importance of absorption of red/far-red light by Chl a (650–749 nm) and appears to be overestimated. In all the algae used in this study more than 2/3 of the photons that would be absorbed by photosynthetic algal/microbial mats are blue and green light (400–574 nm) not red light (Tables 2 and 3).
Proxima Centauri b
Irradiance is severely depleted on Proxima Centauri b in the blue and green parts of the 400–749 nm range compared with irradiance on the surface of the Earth (Table 1). Tables 4 and 5 summarize the irradiance conditions for Proxima Centauri b for oxygenic organisms on a planet with an Earth-like oxic atmosphere and for an Earth-like but anoxic atmosphere. In the range of wavelengths used by oxygenic photosynthetic organisms there is very little difference in the available irradiance. All the oxygenic photoautotrophs would be able to use nearly all available light in the blue part of the spectrum. Tables 4 and 5 show absorption by layers of oxygenic photoautotrophs with a blue absorption peak of 2 (1% transmission) on Proxima Centauri b. The planet receives only about 3% (≈63 µmol photon m−2 s−1) of the 400–699 nm maximum irradiance experienced on Earth (Tables 1–3) and the spectrum provides very little blue, green and yellow light. Despite the very large difference in total irradiance in the visible range on Earth and Proxima Centauri b the proportion of total light within the 400–749 nm range useful to oxygenic organisms is very close to a constant. Thus, Synechococcus is able to use about 65% of the available light from the Sun and Proxima Centauri, Prochlorothrix 49–55%, Chlorella 43%, Rhodomonas 63–68% and Phaeodactylum 58–62%. The Chl d organism Acaryochloris would be able to use 33–37% of the light available from 400–749 nm. Although light on Proxima Centauri b is depleted of 650–699 nm light because of stellar absorption of light in this part of the spectrum it does not greatly affect the proportion of useable light in the 400–699 nm or 400–749 nm range. The matter of most importance is that the overall amount of useable irradiance in the range useable by oxygenic photosynthetic organisms is very low compared with the Earth (≈3–5%) or 63 to 132 µmol quanta m−2 s−1. Algae are grown routinely in culture rooms under similar irradiance and so the ground-level irradiance on Proxima Centauri b is no barrier to the survival of oxygenic photosynthetic organisms on the planet.
Absorption of light by mats of anoxygenic photosynthetic bacteria on the surfaces of Earth and Proxima Centauri b
Photosynthesis of mats composed of anoxygenic bacteria can be estimated using a similar procedure to that used above for oxygenic photosynthetic organisms on Earth and Proxima Centauri b. Combining the data on the light regime on the surface of the Earth and Proxima Centauri b (Fig. 4, Table 1) with the light absorption characteristics of the anoxygenic oxygenic photosynthetic organisms included in the present study (Fig. 2) provides a comparative estimation of the capacity for growth of these various organisms. Photosynthetic bacteria can use violet light (350–399 nm). The visible range of wavelengths (400–749) nm can also be used for photosynthesis and the defining feature of photosynthetic bacteria is that they have BChls, which enable them to use IR light. In Tables 6–9, the spectrum has been divided up into various bands based on what irradiances are most important to the photosynthetic bacteria selected for the present study: violet (350–399 nm), blue (400–499 nm), green (500–574 nm), yellow/orange (575–649 nm) and red (650–699 nm). Photosynthetic bacteria, using accessory photosynthetic pigments, are all able to use a substantial proportion of irradiance in the 400–700 nm range and both BChl a and b are able to absorb orange/red light at about 600 nm (Figs. 2 and 4) (Hellingwerf et al. Reference Hellingwerf, de Vrij and Konings1982). In addition, far-red (700–749 nm) and NIR (750–799 nm) is useful to Chlorobaculum because of the absorption properties of BChl c. The BChl a containing Chlorobaculum (BChl a + c) and Afifella, Rhodopseudomonas, Thermochromatium can use IR(I) (μmol photon m−2 s−1, 800–949 nm light). Blastochloris has BChl b as its primary photosynthetic pigment and is exceptional: it can use IR light up to 1100 nm (Fig. 2) and so can use a second window unavailable to other photosynthetic bacteria [Fig. 4, IR(II) (μmol photon m−2 s−1, 950–1100 nm].
Table 6. Irradiance useable by photosynthetic bacteria on an Earth with the modern Oxygen Atmosphere
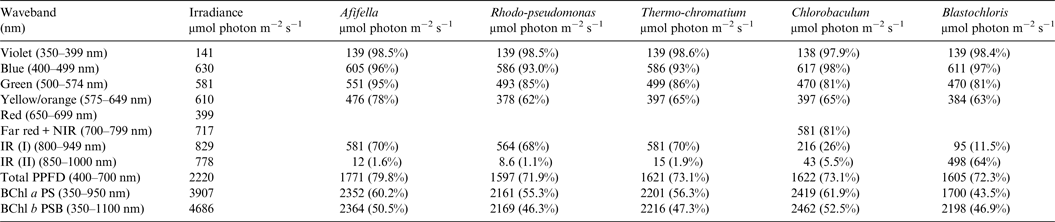
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of photosynthetic bacterial cells with an absorbance of 2.0 (1% transmission) at their Respective Blue Absorbance Peaks for Solar Radiation on Earth with an oxygenated atmosphere. Based on the observationally known solar spectrum. The percentage absorption is shown in brackets.
Table 7. Irradiance useable by photosynthetic bacteria on an Earth with an Anoxic Atmosphere
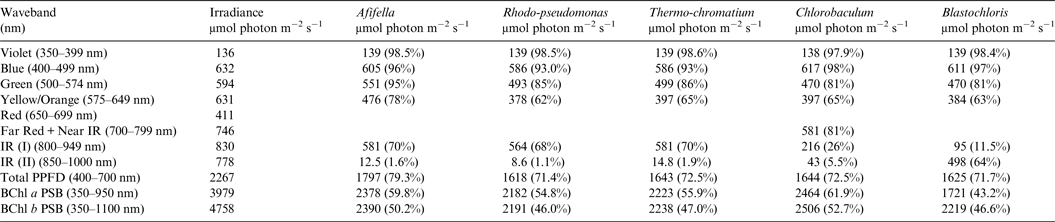
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of photosynthetic bacterial cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for solar radiation on Earth with an oxygenated atmosphere. Based on the observationally known solar spectrum. The percentage absorption is shown in brackets.
Table 8. Irradiance useable by photosynthetic bacteria on Proxima Centauri b with an Oxygen Atmosphere
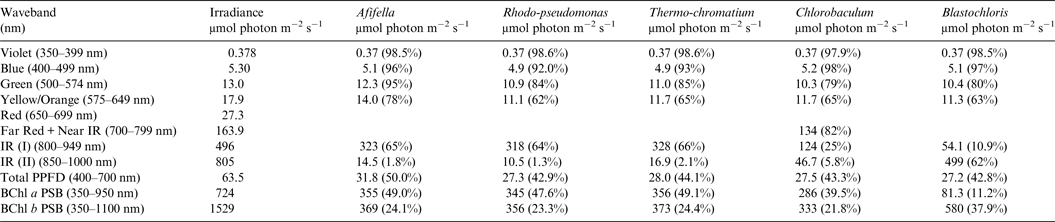
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of photosynthetic bacterial cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for solar radiation on Proxima Centauri b with an oxygenated atmosphere. The values are based on a detailed TES of Proxima Centauri b. The percentage absorption is shown in brackets.
Table 9. Irradiance useable by photosynthetic bacteria on Proxima Centauri b with an Earth-like but Anoxic Atmosphere
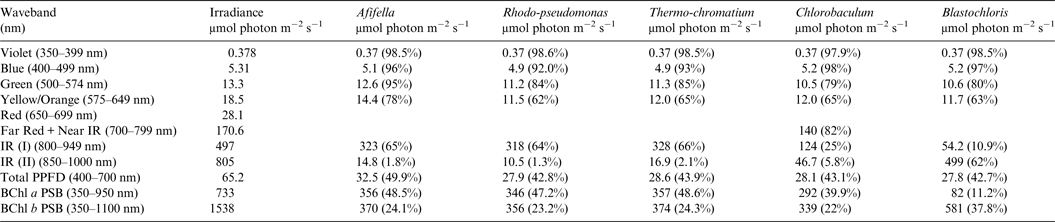
Photosynthetically useable irradiance in different wavebands for a cell layer or thin suspension of photosynthetic bacterial cells with an absorbance of 2.0 (1% transmission) at their respective blue absorbance peaks for solar radiation on Proxima Centauri b with an anoxic atmosphere. The values are based on a detailed TES of Proxima Centauri b. The percentage absorption is shown in brackets.
Tables 6 and 7 show estimates of the useable irradiance for the five anoxygenic photosynthetic bacteria included in the present study for Earth with an oxic and anoxic atmosphere. They are based on the absorption curves in Fig. 2 and the surface irradiance values shown in Fig. 4. Table 6 shows a comparison of absorption of sunlight by layers of a mat of photosynthetic bacteria (Afifella, Rhodopseudomonas, Thermochromatium, Blastochloris and Chlorobaculum) for an oxic Earth atmosphere. Afifella, Rhodopseudomonas, Thermochromatium, Blastochloris and Chlorobaculum could all use violet light (350–399 nm) not generally useable by oxygenic photosynthetic organisms. All the photosynthetic bacteria can use blue (400–499 nm), green (500–574 nm) and orange (575–649 nm) light far better (72 to 80%) than the oxygenic photosynthetic organisms tabulated in Tables 2 and 3. None of the BChl-based photosynthetic bacteria included in this study can use red light (650–699 nm) for photosynthesis. However, in vivo BChl a and BChl b both have a minor peak at about 590 nm (Fig. 2) so some photosynthetic bacteria can use and grow on orange light not useable by most oxygenic organisms (Hellingwerf et al. Reference Hellingwerf, de Vrij and Konings1982). In the case of Chlorobaculum its secondary pigment BChl c strongly absorbs Far Red light (700–749 nm) and NIR light (750–800 nm) with a peak at about 748 nm, but direct absorption by its primary BChl a pigment at about 800 nm is very small. Note that the in vivo absorption peaks for BChl a and BChl b are on opposite sides of the strong atmospheric H2O absorption peak from 930 to 970 nm (Figs. 2 and 4). BChl a organisms can use 55–60% of the light in the range 350–950 nm, Blastochloris can use about 47% of the light available in the range 350–1100 nm. In Table 7, calculations are made for the use of irradiance by photosynthetic bacteria for Earth with an Earth-like but anoxic atmosphere. The results are almost identical as for Table 6. The presence of an oxic or anoxic Earth atmosphere does not significantly affect the irradiance available for anoxygenic photosynthetic bacteria. Photosynthetic bacteria not only have a much wider range of useable irradiance than oxygenic organisms, but also can utilize the 400–749 nm range better than oxygenic organisms.
Table 8 shows useable irradiance for layers of Afifella, Rhodopseudomonas and Thermochromatium, Blastochloris and Chlorobaculum with a blue absorbance peak of 2 (1% transmission, 99% absorption) on Proxima Centauri b with an oxygenic atmosphere. Almost no violet light is available on the surface of Proxima Centauri b (0.38 µmol quanta m−2 s−1) but essentially all would be absorbed by a microbial mat of any of the photosynthetic bacteria included in this study. All five photosynthetic bacteria would also be able to use nearly all (>90%) the blue light (400–499 nm) and large proportions of green and yellow/orange light. The photosynthetic bacteria would be able to use 40–50% of the light in the 400–699 nm range, which is comparable with but perhaps slightly lower than for photosynthetic bacteria under Earth-type irradiance (Tables 6 and 7). Table 9 shows the similar calculations for the scenario where Proxima Centauri b has an anoxic but Earth-like atmosphere. As in the case of oxygenic photosynthetic organisms (Tables 6 and 7) for an oxic versus an anoxic Earth there are no significant differences in the photosynthetic quality of light available for anoxygenic photosynthetic bacteria on Proxima Centauri b whether or not the atmosphere contains oxygen.
The critical difference between Earth and Proxima Centauri b is that the irradiance available in the 400–699 and 400–749 nm wavelength ranges is only 3–5% that found on Earth. In sharp contrast, far-red/NIR (700–799 nm), IR(I) and IR(II) available on Proxima Centauri b are much more comparable with that found on Earth (Table 1). Photosynthetic bacteria with BChl a-based mechanisms would be use about 40–50% of total irradiance in the range 350–950 nm on Proxima Centauri b (≈700–749 µmol quanta m−2 s−1). Blastochloris with its BChl b-based photosynthetic mechanism can use 38% of light in the range 350–1100 nm (≈1550 µmol quanta m−2 s−1). In terms of photic resources available photosynthetic bacteria are at a clear advantage over oxygenic photosynthetic organisms on the surface of Proxima Centauri b.
Irradiance under water
As is well documented, most red and far-red light is eliminated in the first 1 m of water (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011), but it is less well known that UV-A, UV-B and UV-C also disappear with depth, with absorbance coefficients of ≈0.1–1 m−1 for wavelengths from 400 to 280 nm (and higher than 1 m−1 for UV-C <280 nm). Absorbance data for 280–300 nm (Smith & Baker Reference Smith and Baker1981) and 300–749 nm consensus absorbance data from Ritchie (Reference Ritchie2013) were used to calculate irradiance at different depths of water on the various planets in this study. As a result of red and violet–blue light absorption in very deep clear water (100 m) the irradiance peak is at about 400 nm. Figure 5 shows the absorption of solar irradiance useable by oxygenic photosynthesis (400–699 and 400–749 nm) with depth in deep, very transparent, water on an oxic Earth. In shallow water the longer wavelengths are rapidly eliminated (Fig. 5) and wavelengths shorter than 400 nm are also removed with the shorter wavelengths removed first. As the irradiance becomes more monochromatic, with a peak at about 400 nm, the attenuation of irradiance versus depth approaches Beer's law. In the case of Proxima Centauri b the irradiance at the planet surface is already very low (Fig. 6) and heavily red-shifted and so the irradiance reaching the ground on Proxima Centauri b has poor water penetrating properties (400–699 nm, 63 µmol quanta m−2 s−1, 400–749 nm, 132 µmol quanta m−2 s−1. The oxygenic photosynthetic compensation point for Earth (net photosynthesis above respiration is zero) is at about 10 µmol quanta m−2 s−1 in oceanic water or at about 200 m (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011): the equivalent compensation point would be reached on Proxima Centauri b at only about 10 m depth (Fig. 7). Oxic or anoxic atmospheric conditions would make no significant effect upon these results and the compensation depth for Chl d containing organisms is little different to that of Chl a organisms because the far-red (700–749 nm) is so rapidly eliminated by passage through water (Figs. 5–7). An ocean on Proxima Centauri b, as proposed by Turbet et al. (Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016), would be able to support very limited oxygenic photosynthesis because the surface irradiance is so low and the oxygenic euphotic zone would be only a maximum of about 10 m thick.
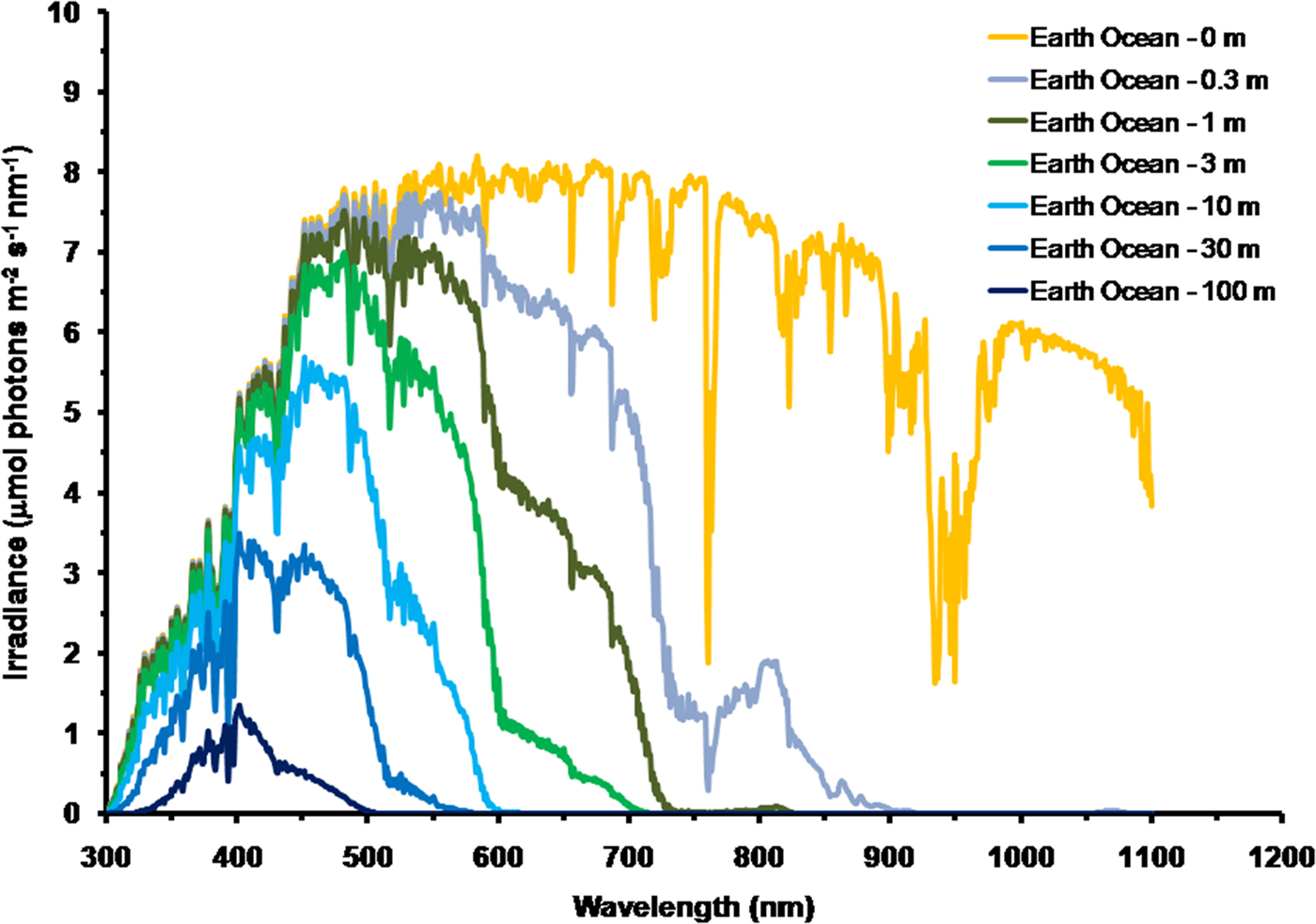
Fig. 5. Decrease in irradiance versus depth in deep water for Earth with an oxic atmosphere. For an anoxic atmosphere the results are little different except that on an Earth with an anoxic atmosphere there would be no strong absorption band at 761 nm. Near IR (750–799 nm) and IR (I) (800–949 nm) and IR II (950–1100 nm) is largely eliminated in less than 0.3 m of water and completely eliminated in 1 m of water. Most far-red light and then red light is absorbed in very shallow water and so the irradiance spectrum changes rapidly with depth. As depth increases longer wavelengths are progressively eliminated: IR (II) → IR (I) → near IR → far red → red → orange → green. Violet light is also progressively eliminated in deeper water leaving blue light centred around a wavelength of 400 nm.
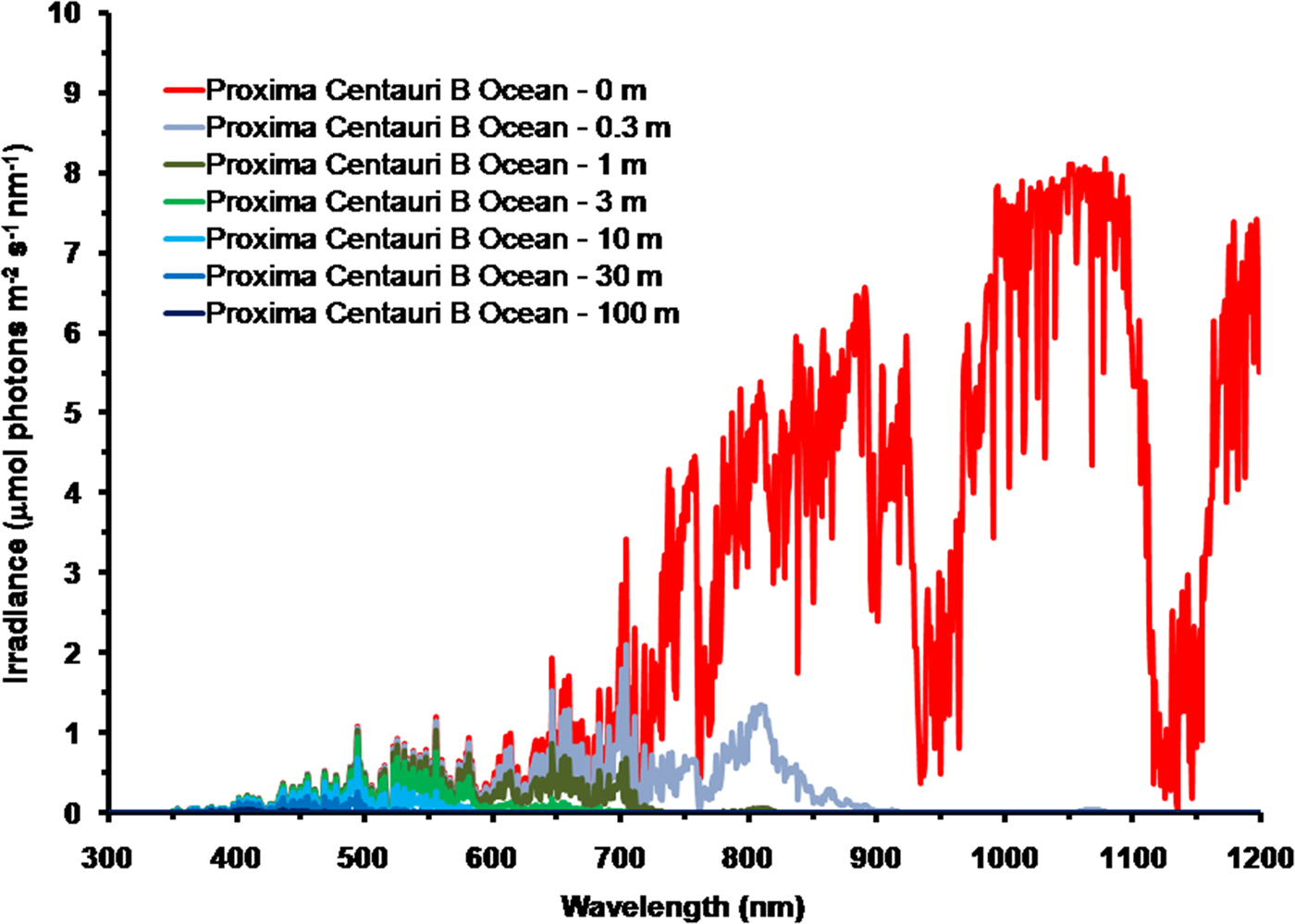
Fig. 6. Absorption of photosynthetic irradiance with depth in deep very transparent water on Proxima Centauri b. As in the case for Earth (Fig. 5) for Proxima Centauri b an anoxic atmosphere the results are little different except that for an oxic atmosphere there is a strong O2 absorption band at 761 nm. Compared with Earth there is very little light in the range useable by oxygenic photosynthesis (400 to 749 nm) at the surface of the planet. IR light (NIR, 750–799, IR(I), 800 to 949 nm and IR(II), 950 to 1100 nm) are all eliminated or severely limited under only 0.3 m of water. By 1 m all NIR, red and far-red light absorbable by Chl a and BChls has disappeared. Photosynthetically useable irradiance progressively disappears with depth from the red end of the spectrum and the irradiance peak moves towards the blue end of the spectrum. UV-A and UV-B also disappear with depth starting at the shortest wavelengths. As a result of these two processes total photosynthetically useable irradiance for oxygenic organisms is reduced to a blue peak with less than 10 µmol quanta m−2 s−1 in only about 10 m of water.
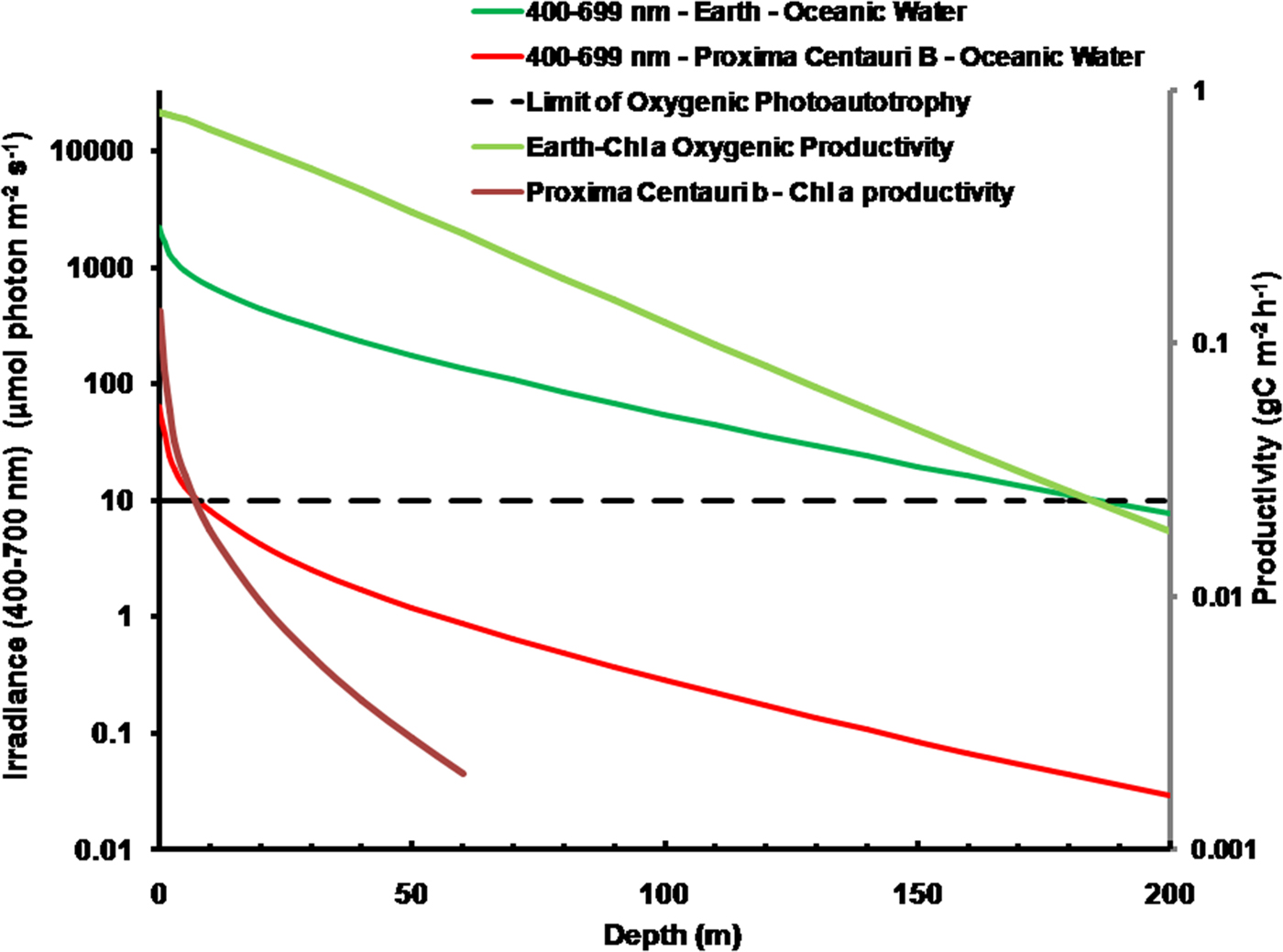
Fig. 7. Absorption of solar irradiance useable by oxygenic photosynthesis (400–699 and 400–749 nm) with depth in deep very transparent water on an oxic Earth. Fig. 7 also shows estimates of oxygenic photosynthesis of algal mats on Earth and on Proxima Centauri b with depth of clear water using an average proportion of useable irradiance from Tables 10 and 12 for Earth and Proxima Centauri b respectively. In shallow water the longer wavelengths are rapidly eliminated (Fig. 5) and wavelengths shorter than 400 nm are removed with the shorter wavelengths removed first. As the irradiance becomes more monochromatic with a peak at about 400 nm the attenuation of irradiance versus depth approaches Beer's law. On Proxima Centauri b the irradiance at the planet surface is already very low (Fig. 6) and heavily red-shifted. The irradiance reaching the ground on Proxima Centauri b has poor water penetrating properties. Oxic or anoxic atmospheric conditions would make no significant effect upon these results. Primary productivity of an algal mat was calculated using equation (10). The oxygenic photosynthetic compensation point for Earth is at about 10 µmol quanta m−2 s−1 in oceanic water or at about 200 m: the equivalent compensation point would be reached on Proxima Centauri b at only about 10 m depth and productivity is very low underwater. The photic zone reaches down to about 200 m on Earth and the plot of productivity versus irradiance slowly intercepts with Irradiance versus depth because photosynthesis is not directly proportional to irradiance over most of the range of depth.
Table 10. Estimates of possible primary production by oxygenic photosynthesis as mg C m−2 s−1 and per h on the surface of Proxima Centauri b compared with Earth using the same model
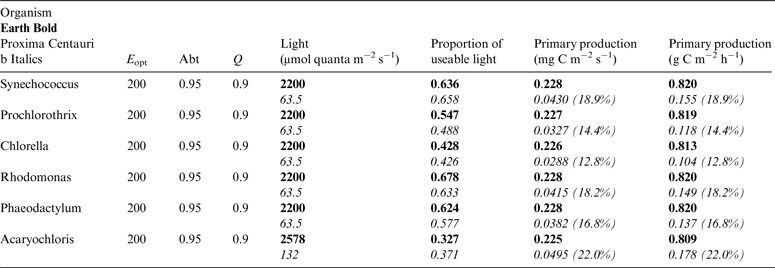
E opt is the optimum irradiance, Abt is the absorptance of the algal mat, Q is the quantum efficiency. Primary production estimates are shown for Earth and Proxima Centauri b and the percentage of the production found for a mat on earth is expressed as a percentage.
It is possible that Proxima Centauri b has an anoxic ecology similar to the early Earth. Figure 8 compares the absorption of irradiance useable by photosynthetic bacteria (350–1100 nm) with depth in deep very transparent water on an anoxic Earth and anoxic Proxima Centauri b. Most NIR, IR(I) and IR(II) light are eliminated in the first 0.3 m of water (Fig. 6). By 1 m all NIR, red and far-red light absorbable by BChls has disappeared and with increasing depth the spectrum becomes more and more monochromatic with a peak at about 400 nm. Photosynthetic bacteria can grow at much lower irradiances than oxygenic organisms and so the compensation irradiance has been taken as 1 µmol quanta m−2 s−1 (Parkin & Brock Reference Parkin and Brock1980; Burke & Burton Reference Burke and Burton1988) Photosynthetic bacteria should be able to exist photoautotrophically on Earth at oceanic depths as much as 400 m (although this appears to be undocumented). On Proxima Centauri b the 1 µmol quanta m−2 s−1 euphotic zone threshold would be reached in only 60 m of water.
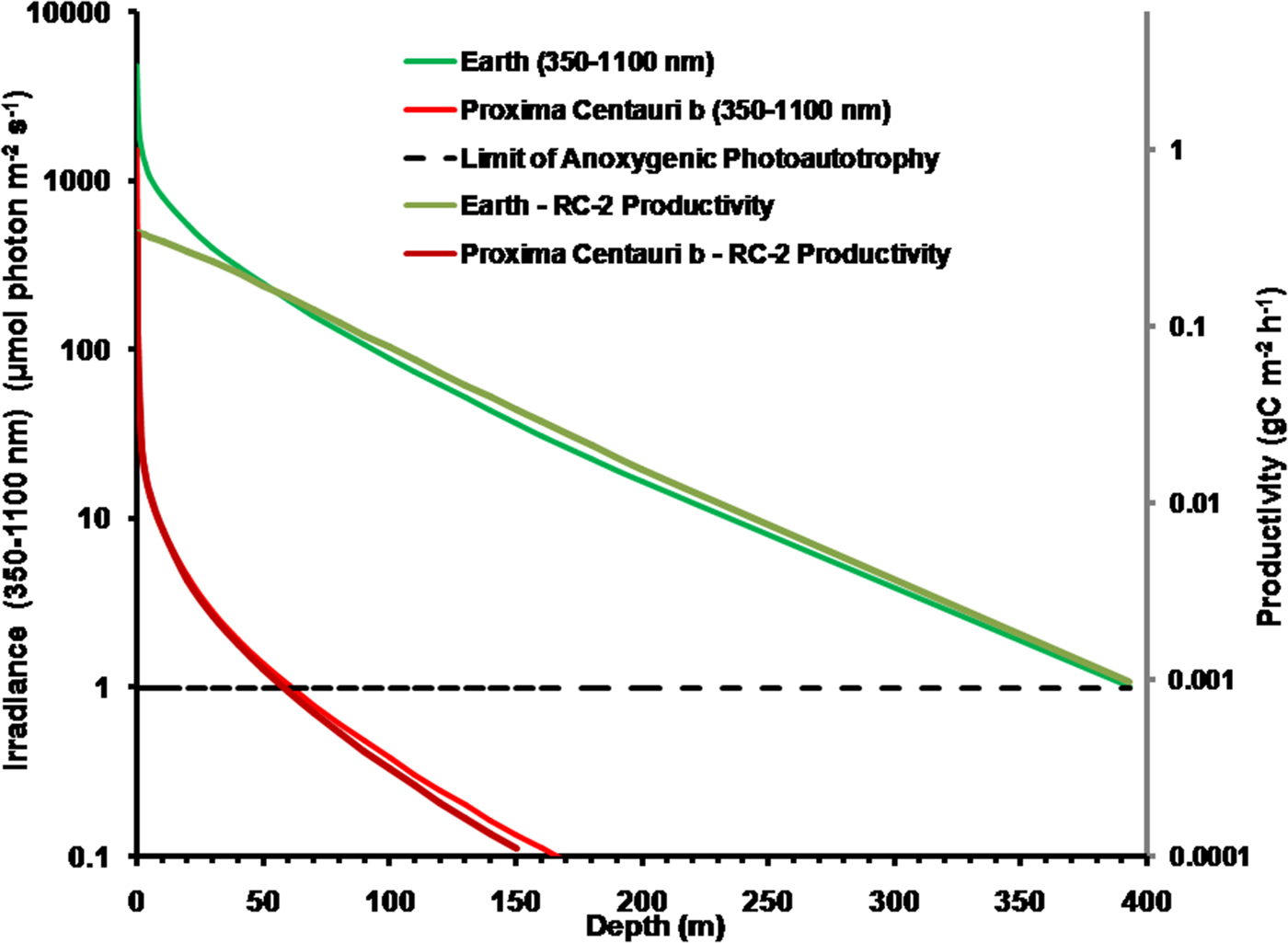
Fig. 8. Absorption of irradiance useable by photosynthetic bacteria (350–1100 nm) with depth in deep very transparent water on an anoxic Earth and anoxic Proxima Centauri b. Fig. 8 also shows estimates of anoxygenic primary production for a mat of photosynthetic bacteria with an RC-2 photosystem at various depths of clear water on Earth and on Proxima Centauri b. By 1 m all NIR, red and far-red light absorbable by BChls has disappeared. The irradiance on the surface of Proxima Centauri b is of very poor quality with regards to its ability to penetrate water NIR, IR(I) and IR(II) irradiance are quickly eliminated in 0.3 to 1 m of water and the amount of deeply penetrating Violet (350–399 nm) and Blue (400–499 nm) light available at the surface is very low (Table 1, Fig. 6). Primary production was estimated using equation (11) using parameters from Tables 11 and 13, including on a mean value for the proportion of useable irradiance by the four RC-2 organisms. Photosynthetic bacteria can grow at much lower irradiances than oxygenic organisms and so the compensation irradiance has been taken as 1 µmol quanta m−2 s−1. On that criterion, photosynthetic bacteria should be able to exist photoautotrophically on Earth at depths as much as 400 m but below ≈50 m depth productivity is essentially directly proportional to irradiance. Productivity on Proxima Centauri b is limited by light: the compensation depth is only at about 60 m and production is directly proportional to irradiance except at the near surface.
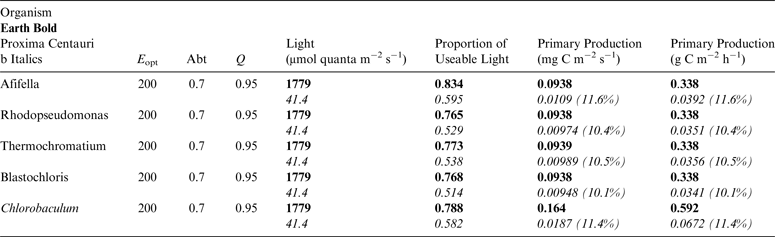
Table 11. Estimates of possible primary production by anoxygenic photosynthesis as mg C m−2 s−1 and per h on the surface of Proxima Centauri b compared with Earth using the same model as for Table 10
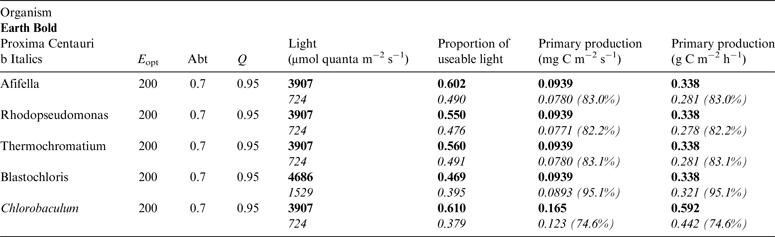
E opt is the optimum irradiance, Abt is the absorptance of the algal mat, Q is the quantum efficiency. Primary production estimates are shown for Earth and Proxima Centauri b and the percentage of the production found for a mat on earth is expressed as a percentage.
Photosynthesis under shallow water
Figures 5 and 6 show the elimination of IR irradiance with depth in shallow water for the Earth and for Proxima Centauri b. By 1 m all NIR, red and far-red light absorbable by Chl a and BChls has disappeared on both Earth and Proxima Centauri b. Violet light is also progressively eliminated with depth but not as drastically as far-red and IR light and there is no significant attenuation of violet and blue light under only 1 m of water. Table 10 is an analysis of the different photosynthetically useable wavebands of light on Earth under 1 m of water for oxygenic photosynthetic organisms. These values should be compared with surface irradiance data shown Figs. 4–6, Table 1). On the Earth surface 400–699 nm light totals about 2200 µmol photons m−2 s−1, but under 1 m of water this is reduced to 1616 µmol photons m−2 s−1, however nearly all of this loss is as red light (650–699 nm) and far-red light (700–749 nm). There is very little loss of blue or green light. Synechococcus, Prochloroxthrix, Rhodomonas and Phaeodactylum are all able to use most of the available light, Chlorella and Acaryochloris are more dependent on red/far-red light than the other oxygenic photosynthetic organisms and so are at some disadvantage underwater.
Table 11 shows an analysis of the different photosynthetically useable wavebands of light on Proxima Centauri b under 1 m of water for oxygenic photosynthesis (compare with surface irradiance Figs. 4 and 6, Table 1). On the planet surface 400–700 nm light totals only about 64 µmol photons m−2 s−1 but under 1 m of water this is reduced to 36 µmol photons m−2 s−1. Nearly all of this loss (≈50%) is as red light (650–699 nm) and far-red light (700–749 nm). There is very little loss of the small amounts of blue or green light available on the planet. All the oxygenic organisms are disadvantaged by the loss of much of the red and far-red light because it is a much higher proportion of photosynthetically useable irradiance on Proxima Centauri b than on Earth. Chlorella and Acaryochloris are more dependent on red/far-red light than the other oxygenic photosynthetic organisms and so are disadvantaged underwater because much of the light available from a red dwarf is easily attenuated by water.
Tables 12 and 13 show that 1 m of water effectively eliminates the far-red, IR(I) and IR(II) radiation useable by anoxygenic photosynthetic organisms on both Earth and Proxima Centauri b. This effectively leaves them only the visible light resources also used by oxygenic organisms although they can also use violet light. On Earth photosynthetic bacteria can use well over 80% of the light available under 1 m of water and the total irradiance is high (1616 µmol photons m−2 s−1) (Table 12). This irradiance is well above the saturation point for photosynthetic bacteria and so effectively under 1 m of water they are not light limited. On the surface of Proxima Centauri b only about 63–65 µmol photons m−2 s−1 is available in the wavelength range 350–699 nm but 700 (350–949 nm) to 1500 (350–1100 nm) μmol photons m−2 s−1 is available to photosynthetic bacteria depending on whether they use BChl a or b as their primary photosynthetic pigment. One meter of water eliminates at least 90% of the available photons for anoxygenic photosynthesis leaving only 37 to 41 µmol photons m−2 s−1 available for photosynthesis. Photosynthetic bacteria would be disadvantaged underwater on Proxima Centauri b.
Table 12. Estimates of possible primary production by oxygenic photosynthesis as mg C m−2 s−1 and per h under 1 m of water on Proxima Centauri b compared with Earth using the same model
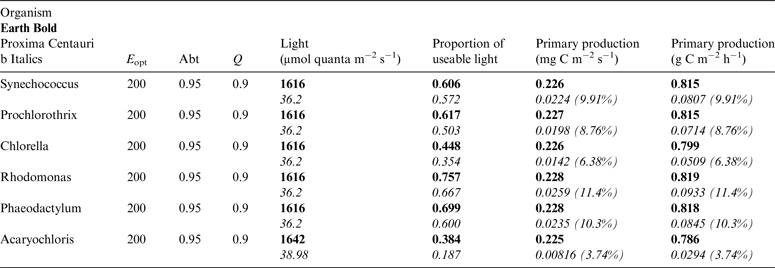
E opt is the optimum irradiance, Abt is the absorptance of the algal mat, Q is the quantum efficiency. Primary production estimates are shown for Earth and Proxima Centauri b and the percentage of the production found for a mat on earth is expressed as a percentage.
Table 13. Estimates of possible primary production by anoxygenic photosynthesis as mg C m−2 s−1 and per h under 1 m of water on Proxima Centauri b compared with Earth using the same model as for Table 12
E opt is the optimum irradiance, Abt is the absorptance of the algal mat, Q is the quantum efficiency. Primary production estimates are shown for Earth and Proxima Centauri b and the percentage of the production found for a mat on earth is expressed as a percentage.
Estimations of potential primary production
Equations (10)–(12) in the Appendix have been used to make estimates of potential primary productivity for oxygenic photosynthetic organisms [quantum number (γ) = 9], photosynthetic bacteria using RC-2 with a γ ≈ 17 and Chlorobium-type photosynthetic bacteria with a γ ≈ 9.7 respectively (see Appendix). These equations make minimal assumptions about the photosynthetic mechanisms and only require estimates of the optimum irradiance (E opt), the absorptance (Abs) of the algal or microbial mat and the quantum efficiency (Q) (McCree Reference McCree1972) and calculations of the proportion of useable light in the photosynthetically useable range. Total photosynthesis of an opaque algal or microbial mat that is thick enough to absorb virtually all incident light is a slowly saturating exponential curve.
Table 10 shows estimates of potential primary activity of oxygenic algal mats using equation (10) on Earth and on Proxima Centauri b on the surfaces of the planets. A representative E opt value of 200 µmol photons m−2 s−1 was used based upon Falkowski et al. (Reference Falkowski, Greene, Kolber, Baker and Bowyer1994); Falkowski & Raven (Reference Falkowski and Raven2007) and Ritchie (Reference Ritchie2008). Absorptances are taken as 0.95, based on reflectance–absorptance–transmission (RAT) measurements of algae filtered onto glass fibre discs (Ritchie Reference Ritchie2014; Ritchie & Runcie Reference Ritchie and Runcie2014). Quantum yield (Q) is taken as 0.9 (McCree Reference McCree1972). Estimates of useable irradiance in the 400–700 nm windows for Synechococcus, Prochlorothrix, Chlorella, Rhodomonas and Phaeodactylum and useable irradiance for Acaryochloris (400–750 nm) are taken from Tables 2–5). Estimates of primary production of mats on Earth of the representative oxygenic organisms included in the present study cover only a small range (in gC m−2 h−1) from 0.809 (Acaryochloris) to 0.820 (Synechococcus, Rhodomonas and Phaeodactylum). Table 10 also includes similar calculations for Proxima Centauri b (in Italics). The 400–699 nm and 400–749 nm light available is very low (≈ 3%) that found on Earth but the non-linear response of photosynthesis to irradiance results in much higher potential productivity than might be expected. Productivity estimates (gC m−2 h−1) vary from 0.10 (Chlorella) to 0.149 (Rhodomonas) and Acaryochloris has the highest estimate 0.178 due to the higher amount of light available because Chl d allows it to use far-red light. Overall productivity would be about 17% that found on Earth.
Table 11 shows estimates of potential primary activity of photosynthetic bacterial mats using equations (11) and (12) on Earth (in Bold) and on Proxima Centauri b (in Italics). A representative E opt value of 200 µmol photons m−2 s−1 and absorptance of 0.7 were used which was based on RAT measurements of algae filtered onto glass fibre discs (Ritchie Reference Ritchie2013; Ritchie & Runcie Reference Ritchie and Runcie2013; Ritchie & Mekjinda Reference Ritchie and Mekjinda2015). The quantum efficiency (Q) of rhodopseudomonads is known to be 0.95 or higher (see Ritchie Reference Ritchie2013; Ritchie & Runcie Reference Ritchie and Runcie2013; Ritchie & Mekjinda Reference Ritchie and Mekjinda2015). Estimates of the proportion of useable irradiance in the BChl a window (350–949 nm) for Afifella, Rhodopseudomonas, Thermochromatium and Chlorobaculum were taken from and useable irradiance for the BChl b organism, Blastochloris (350–1100 nm) are all taken from Tables 6–9. On the surface of the Earth much higher amounts of irradiance are available to organisms that can use violet, visible and IR light than oxygenic photosynthetic organism. Estimates of primary production of mats of BChl a – containing anoxygenic organisms included in the present study (Afifella, Rhodopseudomonas and Thermochromatium) are all about 0.338 gC m−2 h−1. The productivity of Blastochloris is also about 0.338 gC m−2 h−1 despite the wider range of wavelengths useable by Blastochloris. Chlorobaculum would be capable of much higher productivities (0.592 gC m−2 h−1, approaching those of oxygenic organisms) because Chlorobaculum uses 9.7 photons to fix one CO2 compared with ≈17 required by RC-2 photosynthetic bacteria. Table 11 also includes similar calculations for Proxima Centauri b (in Italics). The 350–949 nm and 350–1100 nm irradiance is lower than on Earth (≈19%), but is much higher than the optimum irradiance of the photosynthetic bacteria (≈200 µmol photon m−2 s−1). Productivity estimates (gC m−2 h−1) vary from 0.278 (Rhodopseudomonas) to 0.321 (Blastochloris) for RC-2 type photosynthesizers. Chlorobaculum has the highest estimate (0.442 gC m−2 h−1) because it uses the RC-1 type photosynthetic mechanism. Productivity of photosynthetic bacteria on the surface of Proxima Centauri b would be nearly as high (≈85%) as those found on Earth and importantly would be greater than achievable by oxygenic photosynthesis (≈0.14 gC m−2 h−1).
Underwater, the red and far-red light useable by oxygenic organisms is rapidly attenuated (Figs. 5 and 6) and so it is useful to estimate productivity under a standardized aquatic condition (1 m) and compare them with productivity estimates on the surface of Earth and Proxima Centauri b. Using the data in Tables 10 and 11, it is possible to estimate productivity under 1 m of water. Table 12 compares productivity under 1 m of water on Earth (in Bold) and on Proxima Centauri b (in Italics) making the same assumptions about E opt, Abt and Q as for Table 10 but using the values for total potential useable irradiance and proportion of useable irradiance taking pigment absorption into account. Under 1 m of water on Earth about 25% of 400–699 nm and 400–749 nm light is lost, the bulk of it red and far-red light, losses are heavier (≈50%) on Proxima Centauri b because of the low temperature of the star and the red shift in its emission maxima. On Earth primary productivity under 1 m of water is barely affected by the small loss of total irradiance or the change in spectral properties of the incident light (Table 10: Earth Surface ≈0.82 gC m−2 h−1versus Table 12: 1 m Underwater ≈0.81 gC m−2 h−1). On Proxima Centauri b loss of light is more severe and the effects of attenuation of red and far-red light have more serious consequences (Table 5: Proxima Centauri b Surface ≈0.14 gC m−2 h−1versus Table 12: 1 m Underwater ≈0.068 gC m−2 h−1). Aquatic conditions adversely affect oxygenic primary productivity far more severely (≈50%) on Proxima Centauri b than on Earth.
In Table 13, the consequences of aquatic conditions upon productivity of anoxygenic photosynthetic conditions using similar reasoning as used to prepare Table 12. One (1) m of water on Proxima Centauri b is sufficient to completely eliminate infra-red light (Fig. 6) and so would be expected to have severe effects on anoxygenic primary productivity. Surprisingly, on Earth anoxygenic primary productivity by RC-2 organisms under 1 m of water is unaffected by the complete loss of IR light or the large change in spectral properties of the incident light (Table 11: Earth Surface ≈0.338 gC m−2 h−1 versus Table 13: 1 m Underwater ≈0.338 gC m−2 h−1). The same result is found for Chlorobaculum, which uses an RC-1 mechanism (Table 11: Earth Surface ≈0.592 gC m−2 h−1versus Table 13: 1 m Underwater ≈0.592 gC m−2 h−1). The reason is that the total useable irradiance available 1 m underwater is still well above the optimum irradiance (E opt) [equations (11) and (12)]. This reflects the photonic nature of photosynthesis: a blue photon can do no more photochemistry than an infra-red photon. In contrast, on Proxima Centauri b only 1 m of water drastically reduces the number of photons available for anoxygenic photosynthesis (by ≈95%). Available light fell from well above optimum irradiance to well below optimum irradiance. On Proxima Centauri b anoxygenic primary productivity by RC-2 organisms under 1 m of water is drastically affected by the complete loss of IR light (Table 11: Earth Surface ≈0.29 gC m−2 h−1versus Table 13: 1 m Underwater ≈0.0369 gC m−2 h−1). The same result is found for Chlorobaculum, which uses an RC-1 mechanism (Table 11: Earth surface ≈0.442 gC m−2 h−1versus Table 13: 1 m Underwater ≈0.0672 gC m−2 h−1).
Primary production under water
Figure 7 also shows estimates of oxygenic photosynthesis of algal mats on Earth and on Proxima Centauri b with depth using an average proportion of useable irradiance from Tables 2 and 3 for Earth and Tables 4 and 5 for Proxima Centauri b. The photic zone reaches about 200 m on Earth and the plot of productivity versus irradiance slowly intercepts with Irradiance versus depth because photosynthesis is not directly proportional to irradiance over most of the range of depth. The current record depth is 268 m (Littler et al. Reference Littler, Littler, Blair and Norris1985, Reference Littler, Littler, Blair and Norris1986). Finds are common to depths of 200 m. Questions arose whether oxygenic photosynthesis could actually occur at such depths but Runcie et al. (Reference Runcie, Gurgel and Mcdermid2008) proved that algae are photosynthetically active in situ at such depths. Productivity on Proxima Centauri b underwater is limited by light. The compensation depth is only at about 10 m and production is both low and falls off very quickly with depth.
Figure 8 includes a similar calculation for mats made up of RC-2-type anoxygenic photosynthetic organisms based on a mean value for the proportion of useable irradiance by the four RC-2 organisms (Tables 6–9). On Earth below about 50 m the irradiance is essentially monochromatic blue light and productivity is directly proportional to irradiance down to a theoretical compensation depth of nearly 400 m. On Proxima Centauri b large amounts of irradiance is available at the surface but in water the IR light of benefit to photosynthetic bacteria is eliminated in the first 1 m of water. The compensation depth is at about 60 m and because useable irradiance is so low underwater on Proxima Centauri b. Anoxygenic photosynthesis is directly proportional to irradiance at depths below 1 m. Productivity by anoxygenic bacteria in aquatic environments on Proxima Centauri b will be very low compared with anoxygenic photosynthesis on the planet surface and lower than oxygenic photosynthetic organisms underwater (Fig. 8, Table 13): 1 m Underwater ≈0.0684 gC m−2 h−1).
Discussion
Oxygenic photosynthesis on Earth evolved over 3.5 Gyr BP (Anbar et al. Reference Anbar2007; Schopf Reference Schopf2011). Previously, there was a widespread opinion that oxygenic photosynthesis did not evolve for at least another 0.5 Gyr (Crowe et al. Reference Crowe, Døssing, Beukes, Bau, Kruger, Frei and Canfield2013) and did not accumulate in the atmosphere in significant amounts (>1%) until the Great Oxidation Event (GOE) at ~2.45 Gyr (Catling et al. Reference Catling, Glein, Zahnle and McKay2005; Anbar et al. Reference Anbar2007; Johnston et al. Reference Johnston, Wolfe-Simon, Pearson and Knoll2009; Blank & Sanchez-Baracaldo Reference Blank and Sanchez-Baracaldo2010; Schopf Reference Schopf2011; Fischer et al. Reference Fischer, Hemp and Johnson2016). Several lines of evidence now point to trace amounts of oxygen well before this time (≈3 Gyr; Stüeken et al. Reference Stüeken, Catling and Buick2012; Crowe et al. Reference Crowe, Døssing, Beukes, Bau, Kruger, Frei and Canfield2013; Cardona Reference Cardona2015, Reference Cardona2016). Eukaryotic organisms evolved about 1 Gyr after the GOE; and it was another 1 Gyr before complex plant life invaded the land (Catling et al. Reference Catling, Glein, Zahnle and McKay2005).
Oxygenic photosynthetic pigments
On the Earth the pigmentation of terrestrial plants seems to be a consequence of land plants evolving from a line of green eukaryotic algae, a group which recruited a green primary plastid with Chl a + b (Larkum Reference Larkum and Rengler2008). Proxima Centauri b could have terrestrial vegetation of a variety of pigmentations (Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005; Raven Reference Raven2007; Stomp et al. Reference Stomp, Huisman, Stahl and Matthijs2007; Larkum Reference Larkum and Rengler2008; Rothschild Reference Rothschild2008; Hohmann-Marriott & Blankenship Reference Hohmann-Marriott and Blankenship2011) but the popular conception [Kiang Reference Kiang2008; Plants under alien Suns (http://www.solstation.com/life/eur-life.htm)] that they would necessarily be greatly different to that found on Earth might be misleading.
The most obvious difference between the Sun and Proxima Centauri b lies in the shape of their spectra (Ribas et a l. Reference Ribas, Gregg, Boyajian and Bolmont2017); blue light (400–499 nm) that can be used directly by Chl a, b, c 1, c 2 and d drops off dramatically the cooler the star (Figs. 3 and 4). In the case of the Sun, 29% of the irradiance can be directly absorbed by the blue (Soret) absorption peaks of Chls a and d (Figs. 1 and 4). In the case of Proxima Centauri b, the blue part of the spectrum makes up only 8.3% of the total PPFD (Table 1). Irradiance in the range 500–650 nm (green, yellow and orange light) is absorbed poorly by chlorophylls (Chls) and oxygenic photoautotrophs depend on accessory pigments to be able to utilize such wavelengths (Fig. 1). In terms of light directly useable by Chls (the sum of blue + red light) about 47% of total PPFD is useable directly in Earth sunlight. For Proxima Centauri b about 51% of total PPFD would be readily useable or about the same proportion of total PPFD as for a planet of a G-star. Thus, the relative proportions of useable blue to red light decreases as the stellar temperature decreases, but the proportion of PPFD light directly useable by Chls (the sum of red + blue absorption bands) remains about 47–51% of total PPFD for both G or a cold M-type star like Proxima Centauri b. It can be inferred that accessory pigments play an essentially constant role regardless of the stellar temperature and hence the spectrum of the starlight. The absorption of red light by the stellar atmosphere of Proxima Centauri turns out to be less of a problem for oxygenic photosynthetic organisms on the planet Proxima Centauri b than would be thought at first sight.
The oxygenic photoautotrophs used in the present study were grown under fluorescent lights with a colour temperature of 6500 K. Only small differences were found in their ability to use PPFD in the present study regardless of the spectrum of the star (Tables 2–5). Chl d organisms would have some advantage on Proxima Centauri b because the photosphere of the star would strongly absorb at the red in vivo peak of Chl a (Figs. 1 and 4) cutting out much of the red light useable by Chl a oxygenic photosynthetic organisms (Chen & Scheer Reference Chen and Scheer2013). Not only would a much larger amount of light be available for Chl d-based photosynthesis but a Chl d organism with both Chl a + d (like Acaryochloris) would have access to 74% of the 400–749 nm light on Proxima Centauri b. On Earth the gain from being able to use 700–749 nm light for oxygenic photosynthesis is not as great (Tables 1–5).
Importance of the sum of blue and red light for oxygenic photosynthesis
For the sun and Proxima Centauri b, the differences in stellar spectra (Fig. 4) do not confer conspicuous advantages upon any one of the different types of oxygenic photosynthetic organisms (Fig. 1) or anoxygenic photosynthetic organisms examined in this study (Fig. 2). This result was not anticipated and is contrary to popular ideas about what terrestrial plants/algae and other oxygenic photoautotrophs would look like on exoplanets (Kiang Reference Kiang2008) and is also contrary to the widely held chromatic adaptation theory: i.e. that changes in the spectral properties of light with depth in aquatic environments is an important evolutionary selection pressure, which favours certain types of eukaryotic algae and other oxygenic photoautotrophs over others with increasing depth. On Earth so many exceptions to the chromatic adaptation theory are found that it should be viewed very cautiously (Larkum & Barrett Reference Larkum and Barrett1983; Falkowski & Raven Reference Falkowski and Raven2007; Raven Reference Raven2007; Stomp et al. Reference Stomp, Huisman, Stahl and Matthijs2007; Runcie et al. Reference Runcie, Gurgel and Mcdermid2008; Kirk Reference Kirk2011). No exotic pigmentation or photosystems (for example, a double Z-scheme PS I + II + III) need to be invoked for oxygenic photosynthetic organisms to grow on Proxima Centauri b (Wolstencroft & Raven Reference Wolstencroft and Raven2002; Raven & Cockell Reference Raven and Cockell2006; Tinetti et al. Reference Tinetti, Rashby and Yung2006). Acaryochloris has a useable spectral range from 400 to 749 nm because it has both Chl a and d. Such pigmentation would offer considerable advantage under the red, far-red and NIR-dominated light environment of planets of M-stars such as Proxima Centauri b (Figs. 1–4).
Light attenuation in water and oxygenic photosynthesis
Irradiance decreases with depth, but the spectrum also changes with depth becoming progressively monochromatic with a peak at about 400 nm. The shorter visible wavelengths can actually favour growth at depth: for example, an oxygenic photosynthetic organism might have extreme difficulty in surviving in 20 µmol photon m−2 s−1 white light but might survive well on the same irradiance if it is in a narrow 350–500 nm waveband. Figure 5 affirms that oxygenic photosynthetic organisms on Earth can photosynthesize in situ under 200 m or more of water as confirmed experimentally by Runcie et al. (Reference Runcie, Gurgel and Mcdermid2008).
The red and far-red peaks of Chls a and d play no significant role in photosynthesis on Earth below about 10 m depth of even very clear water. The photosynthetic compensation depth (where net photosynthesis is greater than zero) on Proxima Centauri b is not very deep by Earth standards (Falkowski & Raven Reference Falkowski and Raven2007; Runcie et al. Reference Runcie, Gurgel and Mcdermid2008; Kirk Reference Kirk2011) because of the very low visible irradiance at the planet surface. Proxima Centauri b has a much lower proportion of its total irradiance as blue and green light (Fig. 6) and the euphotic zone would be very thin compared with that on Earth for both oxygenic and anoxygenic photosynthetic organisms (Figs. 7 and 8) because useable irradiance would fall off more rapidly from an already low value where the stellar spectrum is severely depleted of blue and green wavebands compared with solar radiation. Proxima Centauri b experiences a maximum 400–699 nm irradiance of about 64 µmol photon m−2 s−1 (PPFD) or about 3% that of the Earth. The spectrum is different from that of Earth (Figs. 4 and 7) but terrestrial type oxygenic photosynthetic organisms would be able to grow under up to 10 m of water.
UV light
The current consensus is that UV flaring is probably not as major an obstacle to life taking hold on planets of M-type stars as once thought (Kasting Reference Kasting1993; Buccino et al. Reference Buccino, Mauas, Lemarchand, Norris and Stootman2002, Reference Buccino, Lemarchand and Mauas2007; Scalo et al. Reference Scalo2007; Tarter et al. Reference Tarter2007; Lammer et al. Reference Lammer2009; Anglada-Escudé et al. Reference Anglada-Escudé2016; Gale & Wandel Reference Gale and Wandel2016; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016). Deep water (≈10 m) is a very efficient UV-shield (Gale & Wandel Reference Gale and Wandel2016). A few metres of water will effectively shield organisms from UV damage while providing adequate irradiance for oxygenic and anoxygenic photosynthesis (Figs. 5 and 6) and there are plenty of other refugia from UV-radiation such a cryptophytic algae growing in crystalline rocks. Furthermore, it takes only small amounts of oxygen to be produced by oxygenic photosynthetic organisms or from other non-biological sources for an effective ozone shield to develop in the atmosphere (Tarter et al. Reference Tarter2007; Haqq-Misra et al. Reference Haqq-Misra, Kasting and Lee2010; Léger et al. Reference Léger, Fontecave, Labeyrie, Samuel, Demangeon and Valencias2010) and this would allow periodic recolonization of terrestrial and shallow water environments. The anoxygenic form of photosynthesis on the Earth occurred on stromatolites in shallow aquatic environments as far back as 3.5 Gyr (Tice & Lowe Reference Tice and Lowe2004; Schopf Reference Schopf2011), long before there was any significant atmospheric O2. Stromatolites would not have formed if the intertidal zone was uninhabitable because of UV radiation. The same argument applies to the biota of terrestrial hot spring environments (Djokic et al. Reference Djokic, Van Kranendonk, Campbell, Walter and Ward2017). UV irradiance on the surface of the anoxic early Earth was not fatal to organisms existing at the time that are known to live only in shallow water. Domagal-Goldman et al. (Reference Domagal-Goldman, Kasting, Johnston and Farquhar2008) point out that the early earth most likely had a considerable organic haze or photochemical smog that would also have acted as a potent UV shield.
Photosynthesis under ice
Oxygenic photosynthesis under sea ice and in seasonally frozen lakes and ponds is well known (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011). In Antarctica, there are many permanently ice-covered lakes that have thriving algal communities, including large stromatolites as well as photosynthetic bacteria. These lakes are capped by 3–6 m of ice but sufficient light (heavily blue-shifted by the absorption properties of ice) penetrates to support large biomasses (Burke & Burton Reference Burke and Burton1988; Vincent et al. Reference Vincent, Rae, Laurion, Howard-Williams and Priscu1998; Vopel & Hawes Reference Vopel and Hawes2006; Anderson et al. Reference Anderson, Sumner, Hawes, Webster-Brown and McKay2011). Environments comparable with Lake Untersee in Antarctica (Anderson et al. Reference Anderson, Sumner, Hawes, Webster-Brown and McKay2011) are likely to be found on cold extrasolar planets.
Low-light environments
Light is often the lower limiting factor of photosynthesis, for example, photosynthetic photoautotrophs growing in deep water and in heavy shade in crystalline rocks and in caves (Falkowski et al. Reference Falkowski, Greene, Kolber, Baker and Bowyer1994; Kühl & Fenchel Reference Kühl and Fenchel2000; Raven et al. Reference Raven, Kilber and Beardall2000; Thomas Reference Thomas2005; Raven & Cockell Reference Raven and Cockell2006; Wilhelm & Jakob Reference Wilhelm and Jakob2006; Falkowski & Raven Reference Falkowski and Raven2007; Runcie et al. Reference Runcie, Gurgel and Mcdermid2008; Cockell et al. Reference Cockell, Kaltenegger and Raven2009a; Hubas et al. Reference Hubas, Jesus, Passarelli and Jeanthon2011; Kirk Reference Kirk2011). Irradiance on Proxima Centauri b has been shown here to be sufficient for substantial photosynthesis even under 10 m of water. Primary Productivity on Proxima Centauri b: We can make some cautious estimates of the potential photosynthetic productivity of terrestrial and aquatic ecologies of extrasolar planets taking into account the documented limitations of estimates of global productivity (Falkowski & Raven Reference Falkowski and Raven2007; Jones & Vaughan Reference Jones and Vaughan2010; Ritchie Reference Ritchie2010; Kirk Reference Kirk2011; Ritchie & Larkum Reference Ritchie and Larkum2013). Oxygenic photosynthesis is based on absorption of quanta of light and so a quantum of 430 nm light can do no more than a quantum of 680 nm light even though the blue quanta have 680/430 = 1.58 more energy (Planck's Law). In the case of anoxygenic photosynthetic bacteria the difference in energy between useable photons at the blue and IR absorption bands for BChl a are even more extreme (Rhodopseudomonas, blue peak 376 nm, IR peak 866 nm, difference in energy 866/376 = 2.30). In both oxygenic and anoxygenic photosynthesis for any quanta of shorter wavelength than the maximum useable wavelength, significant amounts of energy are lost per quantum in doing the same photochemistry. Optimistic estimates of photosynthetic thermodynamic efficiencies for green algae and terrestrial plants in sunlight are around 6% and more usual values are 2–3% (Larkum Reference Larkum2010; Ritchie Reference Ritchie2010). In all types of photosynthetic organism, some incident light is reflected or transmitted without absorption, some is re-emitted as fluorescent light.
Table 5 shows that algal mats on Earth would be expected to be capable of fixing about 0.82 gC m−2 h−1. Modelling a mat is easier than a water column of phytoplankton (Falkowski & Raven Reference Falkowski and Raven2007; Kirk Reference Kirk2011). The generalized model compares well to experimentally-based estimates of gross photosynthesis in habitats such as coral reefs, rainforests and sugarcane crops of about 10–12 gC m−2 d−1 (Ritchie Reference Ritchie2010). Acaryochloris has a photosynthetic potential of about 0.809 gC m−2 h−1 (Table 5) because it is not well endowed with accessory pigments even though it can use a wider range of wavelengths of light (400–749 nm) but it has a low optimum irradiance (Ritchie Reference Ritchie2008).
The efficiency of oxygenic photosynthesis for various ecosystems and communities on the Earth has been well established (Falkowski & Raven Reference Falkowski and Raven2007; Jones & Vaughan Reference Jones and Vaughan2010; Ritchie Reference Ritchie2010; Ritchie & Larkum Reference Ritchie and Larkum2013). However, estimates of absorbed light by thick films of photosynthetic cells do not readily convert to primary production values (Ritchie Reference Ritchie2010) because of variable characteristics of saturation and photoinhibition have to be taken into account. Rough estimates of potential gross oxygenic photosynthesis at solar or stellar midday (directly overhead) can be made based on using equations (10)–(12) (Tables 5–8) for Earth and Proxima Centauri b, given estimates of optimum irradiance (E opt), absorptance (Abt) and quantum efficiency (Q) and the proportion of the spectrum that is useable by the photosynthetic mechanism.
The potential oxygenic photosynthesis calculated for Proxima Centauri b show that gross photosynthesis (P g) of about 0.14 gC m−2 h−1 is possible on the surface of Proxima Centauri b (Table 6) or about 17% of the rates found on Earth even though PAR irradiance is only ≈3% of PAR on Earth. Such results are a good demonstration of the non-linear characteristics of photosynthesis versus irradiance curves. The Chl d organism (Acaryochloris) does better than the Chl a organisms on Proxima Centauri b (Table 6) because the red Chl a absorption band at 650–700 nm unfortunately coincides with the strong absorption bands found in the spectra of red dwarf stars because of the chemical compounds in their stellar atmospheres (Fig. 4 in Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). The 700–749 nm window useable by Acaryochloris is less affected (Fig. 1) and it can adjust its Chl a/d ratio in different light conditions (Gloag et al. Reference Gloag, Ritchie, Chen, Larkum and Quinnell2007; Duxbury et al. Reference Duxbury, Schliep, Ritchie, Larkum and Min Chen2009). A P g of 0.14 gC m−2 h−1 is higher than much of the terrestrial grasslands and much higher than the open ocean on Earth (Falkowski & Raven Reference Falkowski and Raven2007; Jones & Vaughan Reference Jones and Vaughan2010).
More than 10 times more PAR plus IR light is available on the surface of Proxima Centauri b (350–949 nm, 724 µmol quanta m−2 s−1, 350–1100 nm, 1529 µmol quanta m−2 s−1) than PAR light (400–700 nm for Chl a organisms, 400–750 nm for Chl d organisms). Therefore, one might expect that anoxygenic photosynthesis might be more or less as successful as oxygenic photosynthesis on Proxima Centauri b. Calculations shown in Table 6 compared with Table 5 for estimates of the productivity on the surface of Proxima Centauri b bear this out. Anoxygenic photosynthesis by a microbial mat under Earth conditions on the surface could reach ≈0.34 gC m−2 h−1 for the RC-2-type anoxygenic photosynthetic organisms (Afifella, Rhodopseudomonas, Thermochromatium and Blastochloris) and as high as ≈0.59 gC m−2 h−1 for the RC-1 BChl a + c organism Chlorobaculum or about 85% of the primary production such organisms are capable of on Earth (Table 6). Anoxygenic photosynthesis is known to be able to support substantial ecologies (Lyons & Reinhard Reference Lyons and Reinhard2009; Klepac-Ceraj et al. Reference Klepac-Ceraj, Hayes, Gilhooly, Lyons, Kolter and Pearson2012).
Detection of oxygenic photosynthesis on Proxima Centauri b – ozone and oxygen
Oxygen has strong absorption bands at 688 and 761 nm (Wolstencroft & Raven Reference Wolstencroft and Raven2002; Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005; Kiang et al. Reference Kiang, Siefert, Govindjee and Blankenship2007a, Reference Kiang, Segura, Tinetti, Blankenship, Cohen, Siefert, Crisp and Meadowsb, Stomp et al. Reference Stomp, Huisman, Stahl and Matthijs2007; Kaltenegger & Traub Reference Kaltenegger and Traub2009; Kaltenegger et al. Reference Kaltenegger2010; von Paris et al. Reference von Paris, Hedelt, Selsis, Schreier and Trautmann2013; Seager & Bains Reference Seager and Bains2015) but the absence of detectable O2 does not necessarily mean absence of oxygenic photoautotrophs, nor does the presence of O2 conclusively show the presence of oxygenic photosynthetic organisms. Abiotic generation of oxygen could reach extreme levels under some conditions generating a thick O2 atmosphere, which would show strong atmospheric O2 and ozone (O3) signatures in the absence of oxygenic photosynthesis on the planet (Luger & Barnes Reference Luger and Barnes2015). O3 is produced from O2 in the upper atmosphere of the Earth but O3 (up to several ppm) can be formed from O2 that in itself is not of biogenic origin (Haqq-Misra et al. Reference Haqq-Misra, Kasting and Lee2010; Léger et al. Reference Léger, Fontecave, Labeyrie, Samuel, Demangeon and Valencias2010; Luger & Barnes Reference Luger and Barnes2015). Detection or non-detection of O2 or O3 are both actually ambiguous on the issue of whether or not oxygenic photosynthetic organisms are present on an extrasolar planet.
Detection of photosynthesis on Proxima Centauri b – the red edge and the IR edge
The ‘red edge’ at about 700 nm (Fig. 4), characteristic of oxygenic plants is caused by strong absorption of Chl a (in vivo maximum at ≈680 nm) and strong reflectance in the far-red and NIR causing a large increase in reflectance from 680 to 730 nm (Seager et al. Reference Seager, Turner, Schafer and Ford2005, Figs. 2 and 4; Tinetti et al. Reference Tinetti, Rashby and Yung2006; Kiang et al. Reference Kiang, Siefert, Govindjee and Blankenship2007a; Cockell et al. Reference Cockell, Kaltenegger and Raven2009a, Reference Cockell, Raven, Kaltenegger and Loganb; Jones & Vaughan Reference Jones and Vaughan2010; Howard Reference Howard2013; Seager Reference Seager2014; Seager & Bains Reference Seager and Bains2015; Gale & Wandel Reference Gale and Wandel2016; Seager et al. Reference Seager, Bains and Petkowski2016). Oxygenic vegetation on an extrasolar planet might be detectable on land, however, the red edge is not readily measureable in oceanic phytoplankton populations because of the lower concentration of Chl a (Seager et al. Reference Seager, Turner, Schafer and Ford2005, Fig. 4; Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005) and the light absorption properties of water (Figs. 5 and 6). Both IR reflectance and fluorescence of BChls of surface mats would be difficult to detect due to IR absorption by water in the atmosphere of planets (Schwieterman et al. Reference Schwieterman, Cockell and Meadows2015). An ‘IR edge’ of anoxygenic organisms, although measureable in the laboratory, is unlikely to be detectable by remote sensing of extrasolar planets because of the absorption bands of atmospheric and liquid water (Figs. 2–4).
Photosynthetic bacteria
BChls absorb blue light strongly (Fig. 2) just like oxygenic photosynthetic organisms, which use Chl a or d as their primary photosynthetic pigment. Tables 6–9 demonstrate that photosynthetic bacteria can not only utilize a much broader range of irradiance wavelengths than oxygenic organisms. BChls and carotenoids of photosynthetic bacteria strongly absorb violet, blue and green light (Fig. 2) and so have a conspicuous advantage over oxygenic photosynthetic organisms in aquatic environments (Tables 6, 7, 8 and 9, Figs. 5 and 6). Comparison of Figs. 7 and 8 shows that photosynthetic bacteria can survive at much greater depths than oxygenic photosynthetic organisms. Large biomasses of photosynthetic bacteria are found under thick layers of Antarctic ice where only dim blue light is available (Vincent et al. Reference Vincent, Rae, Laurion, Howard-Williams and Priscu1998; Anderson et al. Reference Anderson, Sumner, Hawes, Webster-Brown and McKay2011).
Irradiance resources in aquatic environments on Proxima Centauri b
Figures 5 and 6 show that under only 1 m of water there is no IR light to support anoxygenic photosynthesis. On the surface of Proxima Centauri b photosynthetic bacteria would benefit from the IR light available, oxygenic photosynthetic organisms would have to depend on the very limited amount of 400–749 nm light available (Tables 1, 4, 5, 8 and 9; Figs. 6–8). Anoxygenic photosynthetic bacteria on an anoxic red dwarf planet could have potential carbon fixation rates comparable in magnitude to what is potentially possible in anaerobic environments on Earth (Lyons & Reinhard Reference Lyons and Reinhard2009; Raven Reference Raven2009) but this optimistic view applies only to anoxygenic organisms growing on the surface of the planet not in aquatic environments (Tables 10–13; Figs. 7 and 8). Under more than a few cm depth (<30 cm) of water oxygenic and anoxygenic photosynthetic organisms use essentially the same photon resources (violet, blue and green light) because both red and NIR light is eliminated so rapidly with depth (Figs. 7 and 8).
Atmospheric absorption and planetary surface temperatures
A critical reconsideration of temperature issues is needed here because our study shows that minimal but adequate light would be available on Proxima Centauri b. The average current global surface temperature of Earth is about +15°C and so the atmosphere and hydrosphere have a +30 K effect on the Planck equilibrium global temperature (Selsis et al. Reference Selsis, Kasting, Levrard, Paillet, Ribase and Delfosse2007). Selsis et al. (Reference Selsis, Kasting, Levrard, Paillet, Ribase and Delfosse2007) places the upper T Planck temperature limit at about 273 K or 0°C, arguing that a planet with a T Planck above this limit would experience runaway greenhouse effects and end up an ersatz-Venus. Solar flux calculations lead to similar conclusions (Fig. 8 in Kopparapu et al. Reference Kopparapu, Ramirez, Kasting, Eymet, Robinson, Mahadevan, Terrien, Domagal-Goldman, Meadows and Deshpande2013). The HZ most likely lies between T Planck temperatures of about −60 to 0°C and not 0 and 100°C (Selsis et al. Reference Selsis, Kasting, Levrard, Paillet, Ribase and Delfosse2007; Vogt et al. Reference Vogt, Butler, Rivera, Haghighipour, Henry and Williamson2010; Kopparapu et al. Reference Kopparapu, Ramirez, Kasting, Eymet, Robinson, Mahadevan, Terrien, Domagal-Goldman, Meadows and Deshpande2013). Proxima Centauri b is most likely to be a cold world (T Planck ≈ 240 K; Anglada-Escudé, et al. Reference Anglada-Escudé2016; Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016) but would be easily habitable if it had enough of an atmosphere to generate a strong greenhouse effect (CO2 0.1–1 bar) (Ramirez et al., Reference Ramirez, Kopparapu, Zuggers, Robinson, Freedman and Kasting2013; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016).
Planets need to be habitable for long enough for complex life to evolve (≈2–3 Gyr, Catling et al. Reference Catling, Glein, Zahnle and McKay2005; Jones & Sleep Reference Jones and Sleep2010; Rushby et al. Reference Rushby, Claire, Osborn and Watson2013; Chopra & Lineweaver Reference Chopra and Lineweaver2016): this might be a major limiting factor for the contemporary Proxima Centauri b because its early history may have rendered it permanently uninhabitable by atmospheric stripping either before or after life gained a foothold (Ribas et al. Reference Ribas2016, Reference Ribas, Gregg, Boyajian and Bolmont2017). If the planet has an intact atmosphere, biological effects of periodic UV-flaring might very well periodically render its surface uninhabitable but organisms would be able to recolonize rapidly any land and shallow waters after flares. UV-protectant compounds are well known in microbes on Earth and avoidance and protective measures would quickly evolve and oxygenic and anoxygenic photosynthetic organisms would be well protected under 10 m of water.
Detection of a photosynthetic bacterial ecology on Proxima Centauri b
Photosynthetic bacteria are often incorrectly thought of as cryptic organisms that are not conspicuous in oxic environments. Blooms of photosynthetic bacteria (commonly Chromatium, Rhodopseudomonas or Thiocapsa) do sometimes appear on the surface of the modern earth despite the oxic atmosphere (intertidal sand flats: Herbert Reference Herbert1985; Hubas et al. Reference Hubas, Jesus, Passarelli and Jeanthon2011; dry valley seeps in Antarctica: Mikucki & Priscu Reference Mikucki and Priscu2007; some lakes (commonly Chromatium sp.): Cohen et al. Reference Cohen, Krumbein and Shilo1977; Madigan Reference Madigan2003; Klepac-Ceraj et al. Reference Klepac-Ceraj, Hayes, Gilhooly, Lyons, Kolter and Pearson2012; Schwieterman et al. Reference Schwieterman, Cockell and Meadows2015; sewage ponds: Gitelson et al. Reference Gitelson, Stark, Oron and Dor1997, Reference Gitelson, Stark, Dor, Michielson and Yacobi1999). Photosynthetic bacteria are also major components of oceanic plankton in the oxygenic photic zone on Earth (Kolber et al. Reference Kolber, Van Dover, Niederman and Falkowski2000).
Blooms of anoxygenic photosynthetic bacteria in pondages on earth can be large enough to be clearly visible using remote sensing (Gitelson et al. Reference Gitelson, Stark, Oron and Dor1997, Reference Gitelson, Stark, Dor, Michielson and Yacobi1999; Schwieterman et al. Reference Schwieterman, Cockell and Meadows2015). Algorthims developed for monitoring terrestrial photosynthetic bacterial blooms would improve our chances of being able to identify the presence of anoxygenic photosynthesis on extrasolar planets (Tinetti et al. Reference Tinetti, Rashby and Yung2006; Scalo et al. Reference Scalo2007). The chemical signature of an earth-like, but anoxic atmosphere is still not well defined (Seager et al. Reference Seager, Bains and Petkowski2016).
Conclusions
The light available on Proxima Centauri b for photosynthesis is very limited, but would be able to support both oxygenic and anoxygenic photosynthesis by organisms with pigmentation like that found in photosynthetic organisms on the Earth. There is no need to invoke exotic pigmentation or photosynthetic mechanisms (cf. Wolstencroft & Raven Reference Wolstencroft and Raven2002). Would oxygenic photosynthesis be detectable on Proxima Centauri b? The problem is that the oxygenic photosynthetic biomass is likely to be very low and the overall ecology would be most likely dominated by carbon fixation by anoxygenic photosynthesis as was the case on the early Earth before the great oxidation event about 2.5 Gyr ago. If Proxima Centauri b has an ocean (Ribas et al. Reference Ribas2016; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016) its primary productivity potential for oxygenic photosynthesis would be very low because the euphotic zone could be no more than about 10 m deep. Oxygenic photosynthesis could in principle be identified on Proxima Centauri b using three criteria: (a) blue light absorbing pigments, (b) a ‘red edge’ and (c) O2 and O3; however, these turn out to be more ambiguous indicators than they are usually thought to be. Criteria (a) and (b) are both consequences of a Chl or Chl-like compound such as BChl c being the primary light absorbing pigment. No known oxygenic photosynthetic mechanism on the Earth uses BChls as primary absorption pigments. Terrestrial vegetation or oxygenic photosynthetic microbial mats might be detectable based on criteria (a) and (b), but the ‘red edge’ (b) cannot be detected in phytoplankton (Seager et al. Reference Seager, Turner, Schafer and Ford2005) and see Fig. 4 and so would not be detectable if Proxima Centauri b was a water world. Other biosignature compounds should also be considered (Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005; Scalo et al. Reference Scalo2007; Seager Reference Seager2014; Schwieterman et al. Reference Schwieterman, Cockell and Meadows2015; Seager & Bains Reference Seager and Bains2015; Seager et al. Reference Seager, Bains and Petkowski2016).
For biogenic oxygen to build up in the atmosphere there needs to be substantial burial of carbon and this is likely to occur only if there is a substantial carbon fixation rate (Raven Reference Raven2009). Known photosynthetic organisms on Earth would be able to live and grow under the light regime found on Proxima Centauri b if it is a warm wet planet (Falkowski et al. Reference Falkowski, Greene, Kolber, Baker and Bowyer1994; Raven et al. Reference Raven, Kilber and Beardall2000; Wolstencroft & Raven Reference Wolstencroft and Raven2002; Thomas Reference Thomas2005; Raven & Cockell Reference Raven and Cockell2006; Cockell et al. Reference Cockell, Raven, Kaltenegger and Logan2009b) and photosynthesis on the planet fixed enough carbon to be able to support substantial growth. In turn, such primary production could support complex ecologies. The stellar spectra of such stars would seem to favour oxygenic Chl d organisms over other types of oxygenic organisms because the stellar atmospheres of red dwarfs strongly absorb 650–699 nm light, but calculations in Table 7 show that the advantage is minimal.
An anoxygenic photosynthetic ecology is likely be more difficult to detect and the very question of how to detect it has not been given proper attention in astrobiology (Hubas et al. Reference Hubas, Jesus, Passarelli and Jeanthon2011; von Paris et al. Reference von Paris, Gebauer, Godolt, Rauer and Stracke2011, Reference von Paris, Hedelt, Selsis, Schreier and Trautmann2013). An anoxygenic ecology should modify a primordial atmosphere sufficiently for biological activity to be detectable such as high levels of methane, SO2 and H2S, photochemical smog and evidence for sequestering of CO2, but all of these criteria could have an abiotic origin and so cannot be considered definitive even in combination (Segura et al. Reference Segura, Kasting, Meadows, Cohen, Scalo, Crisp, Butler and Tinetti2005; Domagal-Goldman et al. Reference Domagal-Goldman, Kasting, Johnston and Farquhar2008; Seager et al. Reference Seager, Bains and Petkowski2016). BChls have strong blue absorption peaks like Chls, but it is not a simple matter to distinguish Chl and BChls based on their blue peaks (Figs. 1 and 2). The corresponding ‘IR edge’ of in vivo BChls a and b would be unlikely to be detectable because their ‘IR edge’ is at wavelengths overlapping where both atmospheric and liquid water strongly absorbs IR light (Figs. 1, 2, 4–6). BChl-based photosynthesis able to use IR light has conspicuous advantages in land environments or in extremely shallow water (a few cm) but in aquatic environments at depths greater than about 30 cm they would only have access to the same blue, green and orange light accessible to oxygenic photosynthetic organisms (Fig. 6). Absorption of IR radiation by a capping layer of ice would have the same effect. We know that anoxygenic photosynthesis predated oxygenic photosynthesis on the Earth and so it is likely to be common on extrasolar planets, perhaps more common than oxygenic photosynthesis, simply because we know that anoxygenic photosynthesis predates oxygenic photosynthesis and predates it in the evolutionary sense as well (Hohmann-Marriott & Blankenship Reference Hohmann-Marriott and Blankenship2011; Crowe et al. Reference Crowe, Døssing, Beukes, Bau, Kruger, Frei and Canfield2013). Anoxygenic photosynthesis on Proxima Centauri b would be limited in aquatic environments because of the severe lack of water penetrating blue light, but surface environments would be favourable for photosynthetic bacteria. If Proxima Centauri b has a large landmass and does not have a large ocean perhaps an equivalent of a GOE might never occur on the planet. The biomass of the planet could be very low difficult not only because of low irradiance, making detection more difficult. In terrestrial environments it is usually water stress not light that limits photosynthesis (Raven Reference Raven1977). A primitive land-surface microbial crust ecology whether oxygenic or anoxygenic on Proxima Centauri b would be limited by water availability (Lange et al. Reference Lange, Kidron, Budel, Meyer, Kilian and Abeliovich1992; Thomas Reference Thomas2005; Cockell et al. Reference Cockell, Kaltenegger and Raven2009a; Ritchie Reference Ritchie2014).
Since Proxima Centauri b is our closest extrasolar planet it is an obvious target for projects that aim at observing extrasolar planets by direct imaging seeking to detect biogenic atmospheric and surface signatures (Turnbull et al. Reference Turnbull, Glassman, Roberge, Cash, Noecker, Lo, Mason, Oakley and Bally2012; Seager Reference Seager2014; Turbet et al. Reference Turbet, Leconte, Selsis, Bolmont, Forget, Ribas, Raymond and Anglada-Escudé2016). Stellar transits of Proxima Centauri b would greatly help in characterizing the planet if we are fortunate enough that they do occur (Kaltenegger & Traub Reference Kaltenegger and Traub2009; van Belle & von Braun Reference van Belle and von Braun2009; Jones & Sleep Reference Jones and Sleep2010; Kaltenegger et al. Reference Kaltenegger2010; Turnbull et al. Reference Turnbull, Glassman, Roberge, Cash, Noecker, Lo, Mason, Oakley and Bally2012; Bailey Reference Bailey2014).
We suspect the planets of red dwarf stars might never evolve oxygenic photosynthesis because it confers little competitive advantage. Furthermore, atmospheric loss might be a general factor, which severely limits the habitability lifetimes of the planets of red dwarfs (Rushby et al. Reference Rushby, Claire, Osborn and Watson2013; Ribas et al. Reference Ribas, Gregg, Boyajian and Bolmont2017). Few may ever reach the switchover from an anoxic ecology to an oxic ecology but many evolutionary scenarios are possible in the many extra solar systems that exist (von Bloh et al. Reference von Bloh, Bounama and Franck2010; Traub Reference Traub2012).
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S1473550417000167
Acknowledgements
The authors gratefully acknowledge Professor Franck SELSIS (Laboratoire d'Astrophysique de Bordeaux, University of Bordeau, France) for his interest and encouragement in this study. We also greatly appreciate the interest and helpful comments made by Professor J.A. Raven (University of Dundee, Scotland, UK) on earlier versions of this study. Dr John Runcie (Aquation Ltd) helped us with his first-hand experience in a submersible measuring photosynthesis in very deep water.
Disclosure Statement
The author has no competing financial interests.
Appendix
Theoretical development of a primary productivity model
Quantum requirements for carbon fixation in photosynthesis
Estimates of the potential productivities (carbon fixation) of oxygenic and anoxygenic ecosystems based on photosynthetic systems are needed to estimate if oxygenic and or anoxygenic ecology on Proxima Centauri b could be on a scale large enough to be detectable. In the case of oxygenic photosynthesis such calculations are routine (Ritchie Reference Ritchie2010): nine photons are used to fix one CO2 (quantum number, γ = 9) and the Calvin–Benson cycle is used to fix CO2. Such calculations are not straightforward in the case of anoxygenic photosynthesis because the quantum efficiency of these organisms has not been given great attention in modern times: most information available is over 50 years old when the carbon fixation pathways of photosynthesis were not well understood and technology limited the accuracy of irradiance measurements. Anoxygenic photosynthesis, particularly by Rhodopseudomonads is much more widespread than generally realized, generally they are more tolerant of oxygen than usually supposed and so are not restricted to anoxic refugia (Blankenship et al. Reference Blankenship, Madigan and Bauer1995).
Rhodopseudomonads such as Rhodopseudomonas and Afifella are known to use the Calvin–Benson cycle (Photosynthetic Carbon Reduction Cycle, PCRC) to fix CO2 (Larimer et al. Reference Larimer2004) but there is a problem: the Calvin–Benson cycle requires both 2NADPH2 (reduced form of nicotinamide adenine diphosphate) and 3ATP (adenosine triphosphate) to fix one CO2 but the RC-2 photosystem of these photosynthetic bacteria is a cyclic photophosphorylation mechanism only producing ATP (Blankenship et al. Reference Blankenship, Madigan and Bauer1995; Raven Reference Raven2009; Hohmann-Marriott and Blankenship Reference Hohmann-Marriott and Blankenship2011; Fischer et al. Reference Fischer, Hemp and Johnson2016). The required reducing equivalents (NADH2 and/or NADPH2) need to be made using an indirect method (reverse electron flow, Raven Reference Raven2009). Hence, without a quantum efficiency value (γ) it is very difficult to estimate carbon fixation rates from photosynthetic ETRs in the case of photosynthetic bacteria with an RC-2 photosystem (Ritchie Reference Ritchie2013; Ritchie and Runcie Reference Ritchie and Runcie2013; Ritchie and Mekjinda Reference Ritchie and Mekjinda2015).
French (Reference French1937a, Reference Frenchb) obtained quanta/fixed CO2 (γ) values of 11 and 17 on a purple photosynthetic bacterium ‘Streptococcus varians C11’ that is now known as Rhodobacter capsulatus (ATCC 11166) (Syn. Rhodopseudomonas capsulatus). Larsen et al. (Reference Larsen, Yocum and van Niel1952) calculated a quantum number (γ) of 9.7 ± 0.4 for the RC-1 (FeS reaction centre) photosynthetic bacterium Chlorobium thiosulfatophilum. Chlorobium produces NADH2 as its terminal electron acceptor but does not make ATP (Blankenship et al. Reference Blankenship, Madigan and Bauer1995; Bryant and Frigaard Reference Bryant and Frigaard2006). In addition, Chlorobium fixes carbon using the reverse citric acid cycle and not the Calvin–Benson cycle. Using the reverse citric acid cycle, 1ATP and 4NADH2 equivalents (3NADH2 + 1FADH2) are required to fix two carbons per turn of the cycle and so one carbon requires 1/2ATP + 2NADH2 equivalents.
A rough and provisional estimate of how many photons are used to fix one CO2 can be calculated based on bioenergetic considerations. Since RC-2 is similar to photosystem II (PSII) and the transmembrane cytochrome chain is also comparable with that found in oxygenic photosynthetic organisms it can be estimated that it takes five photons to produce three ATP or 5/3 photons per ATP [one electron per photon: four electrons passing through the electron transport chain from PSII + one photon being used to drive a recycled electron through the electron transport chain from photosystem I (PSI)]. The ∆G of ATP can be taken as ∆G o ≈50 kJ mol−1. In an anoxic environment, photosynthetic bacteria are using H2S, acetate and Fe2 + as electron donors not water. To make NADPH2 under anaerobic conditions a more realistic ∆G o would be about 120 kJ mol−1 rather than the 220–250 kJ mol−1 in the case of the H2O/O2 couple found in oxygenic photosynthesis. Hence, optimistically, it would take three ATP to make one NADPH2 and hence 3 × 2 × 5/3 = 10 photons to make 2NADPH2 and therefore 10 + 5 = 15 photons to make enough ATP + NADPH2 to fix one carbon using the Calvin–Benson cycle. Alternatively, based on growth of Rhodopseudomonas capsulata and R. acidophila, Gobel (Reference Gobel, Feher, Okamura, Clayton and Sistrom1978) estimated that 1.5 (possibly 2) photons were needed to make one ATP (Table 3, p 921): hence 3ATP would require 4.5 (or 6?) photons and the 2NADPH2 manufactured using ATP would require six ATP or 1.5 (2?) × 3 × 2 = 9 or 12 photons and hence 13.5–16.5 photons overall. These results differ little from the estimate of γ = 17 made by French (Reference French1937a).
Thermodynamic considerations make a quantum number (γ) value of ≈17 for rhodopseudomonads seem plausible; anoxygenic photosynthetic bacteria have only one photosystem compared with the two in oxygenic photosynthesis. However, carbon fixation by a two-stage system is not necessarily more efficient and it largely depends on whether it is compared with a type RC-I or RC-II photosystem. Comparisons can be made for the two mechanisms for the Q Y absorption peaks in the red and IR parts of the spectrum where thermodynamic efficiency would be highest in monochromatic light and asymptotically low irradiance. Similar overall photosynthetic equations can be written for oxygenic and anoxygenic organisms for the synthesis of one mole of glucose (Van Niel Reference van Niel1944; Ritchie Reference Ritchie2010; Raven and Donnelly Reference Raven, Donnelly, De Vera and Seckbach2013).
Calvin Cycle Oxygenic Photosynthesis (nine photons per carbon fixed: PSII/PSI)
Calvin Cycle Anoxygenic Photosynthesis (17 photons per carbon fixed: RC-2)
Reverse Krebs Cycle in Chlorobium and Chlorobaculum (9.7 photons per carbon fixed: RC-1)
In equations (2) and (3), H2A is the alternative source of electrons and protons used by the photosynthetic bacterium such as organic compounds or H2S or various organic compounds. The heat of formation of glucose is 2803 kJ mol−1. The energy in the moles of quanta of the specified wavelength used by the photosynthetic mechanism can be calculated as N A hc/λ, where N A is Avogadro's number, h is Planck's constant, c is the speed of light, and λ is the wavelength of the monochromatic light. For oxygenic photosynthesis, where nine photons would be able to make 3ATP and 2NADPH2 to fix one CO2, a total of 9 × 6 = 54 photons would be needed to synthesize one glucose (C6H12O6) (Walker Reference Walker1990; Ritchie Reference Ritchie2010). From Planck's law, 54 moles of photons at 680 nm would have 9509 kJ of total energy and hence the theoretical maximum thermodynamic efficiency would be 2803/9509 × 100 or 29%. This theoretical thermodynamic efficiency of oxygenic photosynthesis is very high. In the case of anoxygenic photosynthesis the farthest IR absorption peak of R. palustris is at 866 nm. One mole of 866 nm photons has 138 kJ mol−1 of energy. Since 17 × 6 = 102 moles of photons would provide 14076 kJ of energy the theoretical efficiency of photosynthesis would be 2803/14076 × 100 = 19.9%. For the BChl b organism B. viridis the in vivo BChl b peak is at 1020 nm giving 117.3 kJ mol−1 of energy: using the same calculations as for Rhodopseudomonas the Calvin cycle would have a theoretical efficiency of 2803/11963 × 100 = 23.4%. For a BChl c organism such as Chlorobium or C. tepidum, the in vivo BChl c peak is at about 748 nm and so photons would have an energy of 160 kJ mol−1 and hence 58.2 photons would supply 9308 kJ giving a theoretical efficiency of 2803/9308 × 100 = 30% efficiency for carbon fixation using the reverse Krebs cycle.
In a microbial or algal mat or in a battery of leaves photosynthetic cells shade cells beneath them and so light decreases approximately logarithmically with depth. Total photosynthesis is the integral of photosynthesis of all layers of the mat or battery of leaves each layer obeying the waiting-in-line equation (Ritchie Reference Ritchie2010). At high irradiances the top layers shade those below and so the shape of the photosynthesis of a serial battery of photosynthetic cells usually does not show photoinhibition as a whole unit but instead a slowly saturating curve.
The shape of photosynthesis versus irradiance curves of both oxygenic and anoxygenic photosynthetic organisms are basically limited by the photochemical yield (Y) of the photosynthetic apparatus (see Appendix) (Ritchie Reference Ritchie2010; Ritchie Reference Ritchie2013; Ritchie and Larkum Reference Ritchie and Larkum2013; Ritchie and Runcie Reference Ritchie and Runcie2013). It has a maximum efficiency at asymptotically low irradiance (E→0) and is usually observed to decrease exponentially with irradiance (Y = Y maxe−kE) (Ritchie Reference Ritchie2010; Ritchie and Larkum Reference Ritchie and Larkum2013). Photosynthesis of a simple layer of cells is proportional to the product of the yield and the irradiance leading to an equation of the form y = xe−x known as the waiting-in-line equation (Ritchie Reference Ritchie2008; Ritchie Reference Ritchie2013; Ritchie and Larkum Reference Ritchie and Larkum2013; Ritchie and Mekjinda Reference Ritchie and Mekjinda2015). At low irradiances photosynthesis is proportional to irradiance, but as irradiance increases inhibition sets in until a saturating irradiance is reached. At supraoptimal irradiance the rate of photosynthesis decreases but more slowly than the rate of photosynthesis increases as irradiance increases at sub-optimal irradiances: the curve is therefore asymmetric in shape around the optimum irradiance. Such light curves are characteristic of both oxygenic and anoxygenic photosynthetic organisms (Ritchie Reference Ritchie2008; Ritchie Reference Ritchie2013; Ritchie and Larkum Reference Ritchie and Larkum2013; Ritchie and Runcie Reference Ritchie and Runcie2013; Ritchie and Mekjinda Reference Ritchie and Mekjinda2015).
A Simple model for primary production:
Photosynthetic Light Saturation
Quantum yield
where, Y is the quantum yield (Y),
Y max is the maximum yield at asymptotic zero irradiance, E is the irradiance, and k is a constant.
The ETR can be defined as the flow of electrons through the photosystems of both oxygenic and anoxygenic photosynthetic organisms. The ETR is proportional to the product of the yield (Y, defined as having a range 0 → 1) and the irradiance (μmol quanta m−2 s−1).
Since yield is of the form y = e−x , then since photosynthesis is proportional to the product of the yield and irradiance then an appropriate model for photosynthesis is of the form y = xe−x (Ritchie Reference Ritchie2008). The equation y = xe−x has a maximum at x = 1 and the slope of the line at x = 0 is 1 and there is a point of inflection (d 2 y/d 2 x = 0) at x = 2. A form suitable for modelling photosynthesis with experimentally determinable constants that are easily recognizable on a graphical representation of the data (Ritchie Reference Ritchie2012; Ritchie and Larkum Reference Ritchie and Larkum2013) is,
where ETR is ETR as a measure of gross photosynthesis,
E is the irradiance [μmol m−2 s−1 400–700 nm PPFD],
E opt is the optimum irradiance,
ETRmax is the maximum gross photosynthesis.
The maximum photosynthetic efficiency (α0) is the initial slope of the curve at E = 0
(α = ETRmax × e/E opt). It can be shown by analysis of equation (5) that the half-maximum photosynthesis (ETRhalf-max) occurs at 0.231961 × E opt and that photosynthesis is also inhibited by 50% at 2.67341 × E opt. The asymptotic photosynthetic efficiency at zero irradiance is theoretically useful but perhaps a more useful expression for productivity studies is the photosynthetic efficiency at optimum irradiance (α × E opt). It can be shown that α × E opt is equivalent to αEopt = α0/e. For example, if α0 = 0.2 then the photosynthetic efficiency at optimum irradiance is 7.4%.
The waiting-in-line equation applies to a photosynthetic surface rather than a translucent lamina of cells such as in leaves or in a plankton population in a column of water or a mat of photoorganisms (Ritchie Reference Ritchie2010; Ritchie Reference Ritchie2013; Ritchie and Larkum Reference Ritchie and Larkum2013). For a cell suspension or mat of cells an integration procedure is needed to estimate the photosynthetic capacity of the 3D (three-dimensional) object. If transmission decreases exponentially with depth, the integrated total photosynthesis of the algal film or battery of leaves is approximately described by a slowly saturating exponential curve,
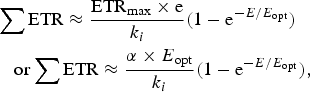 $$\eqalign{& \sum {{\rm ETR}} {\rm} \approx {\rm} \displaystyle{{{\rm ET}{\rm R}_{{\rm max}}{\rm} \times {\rm e}} \over {k_i}}{\rm (1} - {\rm e}^{ - E{\rm /}E_{{\rm opt}}}{\rm )} \cr & \quad {\rm or} \sum {{\rm ETR}} {\rm} \approx {\rm} \displaystyle{{{\rm \alpha} \times E_{{\rm opt}}} \over {k_i}}{\rm (1} - {\rm e}^{ - E{\rm /}E_{{\rm opt}}}{\rm ),}} $$
$$\eqalign{& \sum {{\rm ETR}} {\rm} \approx {\rm} \displaystyle{{{\rm ET}{\rm R}_{{\rm max}}{\rm} \times {\rm e}} \over {k_i}}{\rm (1} - {\rm e}^{ - E{\rm /}E_{{\rm opt}}}{\rm )} \cr & \quad {\rm or} \sum {{\rm ETR}} {\rm} \approx {\rm} \displaystyle{{{\rm \alpha} \times E_{{\rm opt}}} \over {k_i}}{\rm (1} - {\rm e}^{ - E{\rm /}E_{{\rm opt}}}{\rm ),}} $$
where k i is the exponential constant for the decrease in irradiance through the translucent photosynthetic material. For terrestrial vegetation with a leaf area index of 4.7 the value of k i is 1.127 and so approximates unity (k i ≈ 1) (Ritchie Reference Ritchie2010). The irradiance which results in 50% of the maximum ETR (ETR50% max) is equal to −ln(0.5) × E opt or 0.6931 × E opt.
It has been found experimentally that the waiting-in-line model [equation (5)] and its integrated-by-depth form [equation (6)] can be applied to both oxygenic and anoxygenic organisms (Ritchie Reference Ritchie2008; Ritchie Reference Ritchie2010; Ritchie Reference Ritchie2012; Ritchie Reference Ritchie2013; Ritchie and Larkum Reference Ritchie and Larkum2013; Ritchie and Runcie Reference Ritchie and Runcie2013; Ritchie Reference Ritchie2014). In the case of oxygenic photosynthesis, gross photosynthesis (P g) can be calculated as ETR/4 on the basis that four electrons and one O2 are derived from each 2H2O used as electrons sources in oxygenic photosynthesis. For anoxygenic photosynthesis the allocation factor is unity rather than 0.5 because there is only one photosystem: the potential gross photosynthesis (P g) of anoxygenic purple sulphur bacteria has been estimated to be about 17 in the present study so P g ≈ ETR/17. In primary productivity studies, it is convention to express photosynthesis in terms of gC m−2 s−1 or per h.
Gross photosynthesis (gC m−2 s−1) of oxygenic photosynthesis
Gross photosynthesis (gC m−2 s−1) of anoxygenic photosynthesis using the Calvin Cycle
Gross photosynthesis (gC m−2 s−1) of anoxygenic photosynthesis using the reverse Krebs cycle such as in Chlorobium,
where P g is gross photosynthesis, α is the photosynthetic efficiency based on an allocation factor of 0.5 in the case of oxygenic photosynthesis and 1 in the case of anoxygenic photosynthesis, E opt is the optimum irradiance, and E is the irradiance. The quantum number (γ, electrons/carbon fixed) is taken as 9 in oxygenic organisms, 17 in the case of anoxygenic photosynthetic organisms using RC-2 and 9.7 for anoxygenic photosynthetic organism using RC-1.
An estimation of the potential total gross photosynthesis of a lamina of photosynthetic cells can also be made from the total absorption (A) of a laminated layer of cells, which absorbs nearly all photosynthetically useable light, the absorptance of the lamina of cells (Abt), the quantum efficiency (Q), the Irradiance (E) and the optimum irradiance of a thin layer of the cells (E opt). Absorptance (Abt) needs to be measured experimentally using an integrating sphere spectrophotometer or a RAT machine (McCree Reference McCree1972 Ritchie Reference Ritchie2014; Ritchie and Runcie Reference Ritchie and Runcie2014): typical values for Abt at blue wavelengths are about 0.8–0.95 and similar values are found for red light and IR light in the case of photosynthetic bacteria. In oxygenic photosynthesis nine (9) photons are needed to produce enough ATP and NADPH2 to fix one mole of CO2 using the Calvin–Benson cycle [quantum number (γ) ≈9] (Walker Reference Walker1990; Rothschild Reference Rothschild2008; Hohmann-Marriott and Blankenship Reference Hohmann-Marriott and Blankenship2011); one mole of carbon weighs 12 g and so expressed in mol carbon m−2 s−1 the potential total gross photosynthesis (Ritchie Reference Ritchie2010) is,
The purple sulphur and purple non-sulphur bacteria use a single photosystem (RC-2) to generate the ATP and NADPH2 needed to drive the Calvin–Benson cycle. Equation (9) can be adapted for use with anoxygenic photobacteria. An estimation of the total gross photosynthesis of a lamina of rhodopseudomonad photosynthetic cells can be estimated from the total absorption (A) of a lamina of cells which absorbs nearly all photosynthetically useable light, the absorptance of the lamina of cells (Abt), the quantum efficiency (Q), the Irradiance (E) and the optimum irradiance of a thin layer of the cells (E opt). Seventeen (17) photons are estimated to be needed to produce enough ATP and to indirectly synthesize enough NADPH2 to fix one mole of CO2 (quantum number (γ) ≈17). One mole of carbon weighs 12 g and so expressed in mol carbon m−2 s−1 the potential total anoxygenic gross photosynthesis in a Rhodopseudomonad would be,
For a BChl a + c organism such as Chlorobium, which uses RC-1 the quantum number is about 9.7 and so the appropriate model would be:
Equations (10)–(12) will be used in the present study to estimate the potential primary production of Proxima Centauri b because of the minimal assumptions that need to be made about photosynthesis of an optically black algal or microbial mat.























