Introduction
Schizophrenia (SCZ) and schizoaffective disorders (SAD) are relatively heterogeneous psychiatric disorders characterized by an array of symptoms including delusions, hallucinations, psychomotor, social, and cognitive impairment [Reference Heilbronner, Samara, Leucht, Falkai and Schulze1]. SCZ affects approximately 1% of the population [Reference Heilbronner, Samara, Leucht, Falkai and Schulze1], whereas SAD has a lifetime prevalence of approximately 0.3% [Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä and Pirkola2]. SCZ and SAD are among the most debilitating mental disorders. However, our comprehension of their pathophysiological underpinnings remains inadequate. Indeed, they have complex pathogenesis that involves the interaction of multiple biological, genetic, and environmental factors [Reference Kircher, Wöhr, Nenadic, Schwarting, Schratt and Alferink3]. SCZ and SAD both demonstrate high heritability according to family, twin, and adoption studies [Reference Kety4, Reference Cardno, Marshall, Coid, Macdonald, Ribchester and Davies5], indicating a major genetic contribution to the illness risk. In fact, in SCZ, genome-wide association studies (GWASs) have successfully identified genetic variants contributing to the risk of developing SCZ, with 287 distinct genomic loci associated with the disorder [Reference Trubetskoy, Pardiñas, Qi, Panagiotaropoulou, Awasthi and Bigdeli6].
In this context, the brain-derived neurotrophic factor (BDNF) seemingly plays a relevant role [Reference Duman and Monteggia7]. BDNF is the most prevalent and extensively studied neurotrophin in the human central nervous system (CNS), and is able to cross the blood–brain barrier [Reference Pan, Banks, Fasold, Bluth and Kastin8]. BDNF is a key regulator of a wide range of neurophysiological processes including neurogenesis, neuronal differentiation [Reference Altar, Cai, Bliven, Juhasz, Conner and Acheson9], synaptogenesis, and long-term potentiation [Reference Bramham and Messaoudi10]. Like other neurotrophins, BDNF is synthesized as a precursor form, prepro-BDNF, which is further cleaved to produce mature BDNF either intracellularly or extracellularly [Reference Matsumoto, Rauskolb, Polack, Klose, Kolbeck and Korte11].
Even though a strong correlation between peripheral BDNF and BDNF levels in the CNS has been reported [Reference Sartorius, Hellweg, Litzke, Vogt, Dormann and Vollmayr12], studies measuring BDNF levels in the serum as a potential biomarker of SCZ have yielded controversial results. Several studies have found decreased peripheral levels of BDNF in SCZ patients including first-episode psychosis patients [13–15] and chronic patients that have been medicated for a substantial period of time [Reference Ikeda, Yahata, Ito, Nagano, Toyota and Yoshikawa16, Reference Xiu, Hui, Dang, De Hou, Zhang and Zheng17]. Nevertheless, some studies found no difference between serum BDNF levels of SCZ patients and those of healthy controls [Reference Huang and Lee18, Reference Shimizu, Hashimoto, Watanabe, Komatsu, Okamura and Koike19] and some even found an increase [Reference Gama, Andreazza, Kunz, Berk, Belmonte-de-Abreu and Kapczinski20]. Meta-analytical findings demonstrated that there was a substantial decrease in BDNF serum and plasma levels of SCZ patients in acute episodes, suggesting that decreased peripheral BDNF levels can be considered as a biomarker of disease activity [Reference Fernandes, Berk, Turck, Steiner and Gonçalves21].
The finding of a decline in BDNF levels of SCZ patients is consistently observed. However, little is known about the temporal trajectory, and the modulators, of this decline. Indeed, several factors, including genetic, treatment, and clinical moderators, might affect the peripheral levels of BDNF in SCZ patients. BDNF levels appear to be affected by the Val66Met (rs6265) polymorphism, a single nucleotide polymorphism (SNP) in the BDNF gene leading to valine (Val) for methionine (Met) substitution at codon 66 [Reference Terracciano, Martin, Ansari, Tanaka, Ferrucci and Maudsley22]. The Val66Met polymorphism has been associated with intracellular trafficking and activity-dependent secretion of mature BDNF as well as neurocognitive deficits [23–25]. While some studies have found an association between the functional Val66Met variant and peripheral BDNF levels [Reference Aas, Haukvik, Djurovic, Tesli, Athanasiu and Bjella26, Reference Ozan, Okur, Eker, Eker, Gönül and Akarsu27], others have not [Reference Terracciano, Martin, Ansari, Tanaka, Ferrucci and Maudsley22, Reference Chen, Lee, Chang, Chen, Chu and Wang28]. Consistently, two meta-analyses looking at the association between BDNF Val66Met polymorphism and several neurocognitive phenotypes found no significant difference between carriers of Met allele and Val/Val homozygotes [Reference Ahmed, Mantini, Fridberg and Buckley29, Reference Mandelman and Grigorenko30].
Peripheral BDNF levels might also be affected by treatment factors, and psychopathological and cognitive changes. Cognitive impairments are widely observed in patients with SCZ, and together with symptom severity, they are central to the prediction of the clinical and functional outcomes in SCZ [Reference Fervaha, Foussias, Agid and Remington31, Reference Green, Kern and Heaton32]. Several studies have shown the strong correlation between reduced peripheral BDNF levels and impaired neurocognitive and psychopathology test scores [Reference Carlino, Leone, Di Cola, Baj, Marin and Dinelli33, Reference Pillai, Kale, Joshi, Naphade, Raju and Nasrallah34].
Overall, the analysis of peripheral BDNF levels and their relationship with clinical and treatment factors in SCZ spectrum disorders has provided inconsistent results. These discrepancies assume even higher significance in consideration of the possible clinical relevance of using BDNF as diagnostic/prognostic marker in psychiatric disorders. For instance, it is conceivable that patients with a more severe course of illness or with higher genetic predisposition for SCZ might have lower levels of BDNF compared with those with less severe presentation (or with less genetic loading). The present study sought to investigate the longitudinal variation of serum BDNF levels in a 24-month observational cohort study named Longitudinal Assessment of BDNF in Sardinian Psychotic patients (LABSP) [Reference Primavera, Manchia, Deriu, Tusconi, Collu and Scherma35]. Several aims were tested, primarily the assessment of the variation of BDNF serum levels over time and its relationship with psychopathological changes, cognitive function, and social functioning. We hypothesized that BDNF serum levels would decrease in association with longer and more severe clinical course as well as in association with other possible factors such as cognitive decline. Furthermore, we also examined if genetic variation (four tag SNPs) within the BDNF gene could moderate these relationships. Finally, as an aside, we performed discriminatory analysis of SCZ and SAD using Receiver Operating Characteristic (ROC) curve, expecting that BDNF serum levels could differentiate individuals affected by the two disorders.
Methods and Materials
Sample
An a priori power analysis was conducted using repeated measures and sample size (RMASS; Center for Health Statistics, Chicago, IL, USA) software and the results indicated that the sample size of 59 individuals was sufficient to achieve 90% statistical power to detect significant difference at α = 0.05. Our sample was comprised of 105 patients with psychosis treated at the community mental health center of the Unit of Psychiatry of the Department of Medical Science and Public Health, University of Cagliari and University of Cagliari Health Agency, Cagliari, Italy. A Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P) [Reference First, Spitzer, Gibbon and Williams36] was administered by trained mental health professionals to confirm the diagnosis of SCZ or SAD. To be considered eligible for participation in the LABSP study, patients had to be between 18 and 65 years old; diagnosed with SCZ or SAD according to DSM-IV-TR, and with absence of acute psychopathological manifestations for the past 6 months before recruitment. Patients were excluded from the study if they refused to provide consent; had acute psychopathological symptoms, major unstable medical illness, severe mental retardation, major neurological disorder or a previous head injury, current drug and alcohol dependence, or severe illness-related cognitive impairment which affected their ability to participate in the study. The study was approved by the University of Cagliari Health Agency Ethics Committee and the protocol followed the principles of the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Assessment procedures
Recruited patients were assessed and evaluated using various measures at five different waves. Clinical, cognitive and social performance measures, and blood samples of the patients were collected at the baseline (T0), and at four consecutive time points: 6 months (T1), 12 months (T2), 18 months (T3), and 24 months (T4). The details of the assessment and evaluation process including used measures and materials [Reference Primavera, Manchia, Deriu, Tusconi, Collu and Scherma35], as well as preliminary findings [Reference Manchia, Diego, Deriu, Caboni, Iaselli and Sundas37], have been previously published. General psychopathology, the severity of positive and negative symptoms, and clinical status of the patients were assessed using the original 30-item Positive and Negative Syndrome Scale (PANSS) [Reference Kay, Fiszbein and Opler38] and Clinical Global Impression Scale for Schizophrenia (CGI-SCH) [Reference Haro, Kamath, Ochoa, Novick, Rele and Fargas39]. In addition, we applied the consensus five-factor model of PANSS (PANSS-FCTcr) [Reference Wallwork, Fortgang, Hashimoto, Weinberger and Dickinson40] consisting of 20 items that are categorized into Positive, Negative, Disorganized/Concrete, Excited, and Depressed factors, because previous studies have shown that PANSS-FCTcr better characterizes the structure of PANSS data [Reference Wallwork, Fortgang, Hashimoto, Weinberger and Dickinson40, Reference Pinna, Bosia, Cavallaro and Carpiniello41]. The Brief Assessment of Cognition in Schizophrenia (BACS) scale [Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour42] was used to evaluate changes in cognitive domains including verbal memory, working memory, reasoning, and processing speed. The evaluation of social functioning was carried out using Personal and Social Performance (PSP) scale [Reference Goldman, Skodol and Lave43] that has shown to be a valid and reliable measure for patients with SCZ [Reference Nasrallah, Morosini and Gagnon44, Reference Vauth, Carpiniello, Turczyński, Ivanov, Cherubin and Lahaye45].
Sample collection and measurement of BDNF
For the assessment of BDNF serum levels, the blood from each patient was drawn at the same time of the day (between 8:00 and 10:00 AM) at each visit. Collected blood samples were kept at room temperature for approximately 4 h to allow for clotting, after which they were centrifuged at approximately 1,000 × g for 15 min. All samples were immediately stored in small aliquots at −20°C until analyzed. Then, the serum BDNF levels were determined using a commercial human enzyme-linked immunoassay (ELISA) kit (Booster Immunoleader, CA, USA; Cat. N° EK0307) following the manufacturer’s instructions. This kit is used for the quantitative detection of human BDNF in cell culture supernatants, serum, and plasma with a high sensitivity of <2 pg/mL, the measuring interval of 31.2–2,000 pg/mL, and no detectable cross-reactivity with other relevant proteins. The absorbance was measured using a microplate reader (Thermo Scientific Multiskan FC MA, USA) set at 450 nm within 30 min after the final step of the kit procedure.
Genetic analysis
We used the Tagger program implemented in the Haploview v4.2 to select SNPs in linkage disequilibrium (LD) (r 2 ≥ 0.8) and with a minor allele frequency threshold of 0.01. The genotyping of SNPs rs1519480, rs11030104, rs6265 (Val66Met), and rs7934165 was performed using TaqMan probes on demand (C_11592757_20, C_1751792_10, C_11592758_10, C_1197567_10, ThermoFisher Scientific) on a StepOne Plus instrument (ThermoFisher Scientific). The reaction mixture was prepared in a final volume of 10 μL consisting of 5 μL of MasterMix (2 times), 0.5 μL of Custom TaqMan® SNP Genotyping Assay (20 times) containing primers marked as VIC and FAM to discriminate between alleles, 1 μL of cDNA, and 3.5 μL of RNase-free water. Polymerase chain reaction was performed with the following conditions: 30 s 60°C, 10 min 90°C, and 40 cycles at 95°C for 15 s and 60°C for 1 min.
Statistical analysis
We analyzed the longitudinal data using mixed-effects linear regression models (MLRM). MLRM was used particularly because it allows the observation of the effects and interaction of multiple independent variables on a dependent variable while considering repeated measures across participants [Reference Koerner and Zhang46]. To test our hypotheses, we regressed our predictor variables and fixed effects on our log-transformed serum BDNF data. In a preliminary step, PANSS, CGI-SCH, BACS, and PSP scale scores of each subject at each time point were separately regressed on BDNF data to analyze the relationship between them. We fitted regression models while adjusting for age and sex and checked for linearity and homoscedasticity by examining plots of residuals against fitted values. Lastly, BDNF gene polymorphisms were added to the MLRM as a covariate to examine the possible moderating effect. The longitudinal data were analyzed using the statistical programming language R [47]. All regression models were fitted using “lme4” package [Reference Bates, Mächler, Bolker and Walker48]. A significance level of p < 0.05 was considered after Holm–Bonferroni corrections for multiple comparisons. ROC analysis, with sensitivity, specificity, and predictive value analysis of the ability to discriminate between SCZ and SAD in relation to longitudinal BDNF levels was also applied.
Results
Sample characteristics
Table 1 summarizes the demographic and clinical characteristics of the patients that participated in this study. The sample consisted of 105 patients including 64 with a diagnosis of SCZ and 41 with SAD. The mean age of the sample at the baseline was 48.85 ± 10.45 years.
Table 1. Main demographic and clinical characteristics of LABSP sample.
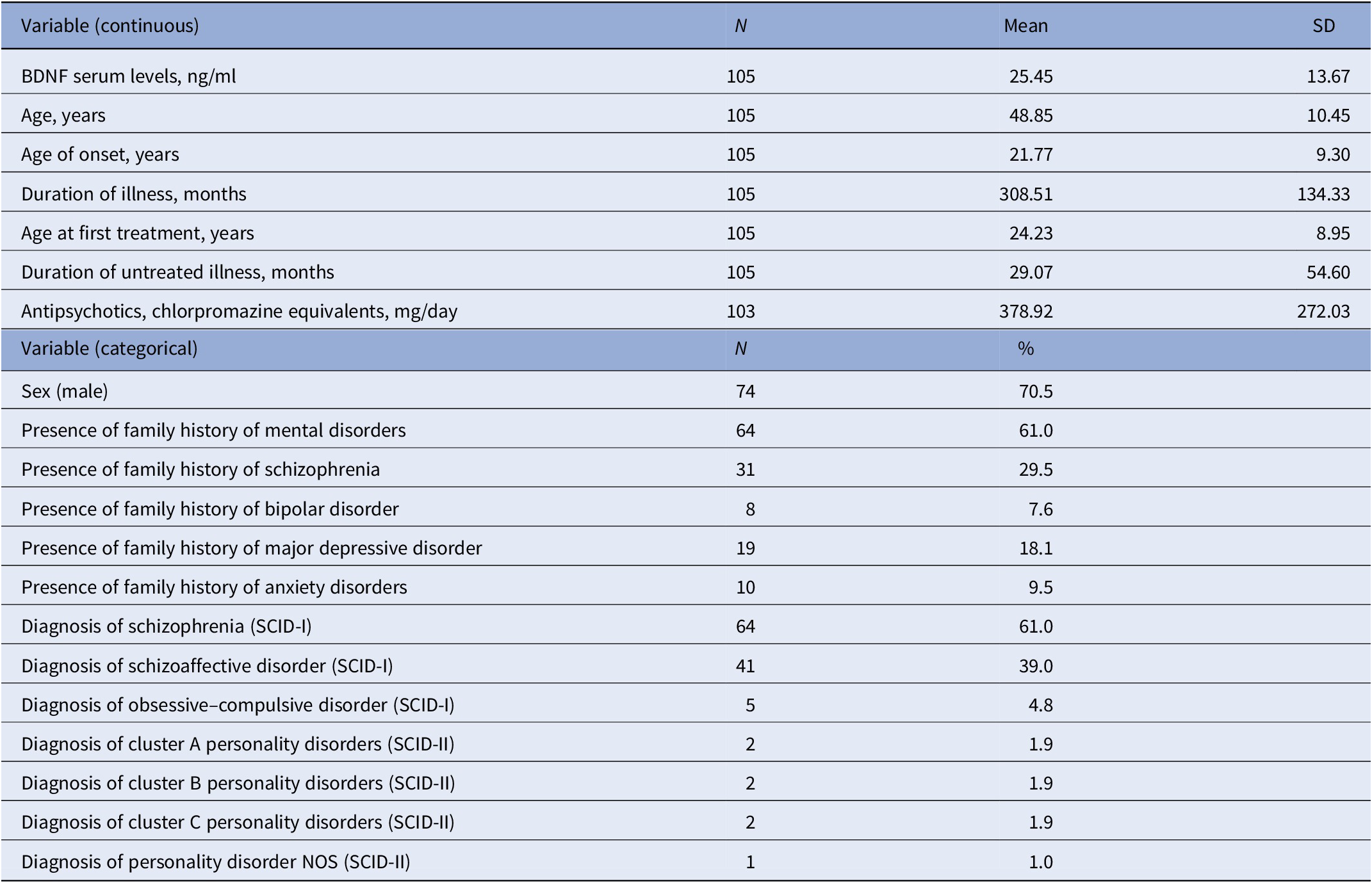
Abbreviations: BDNF, brain-derived neurotrophic factor; LABSP, Longitudinal Assessment of BDNF in Sardinian Psychotic patients; SCID-I, Structured Clinical Interview for DSM-IV Axis I Disorders; SCID-II, Structured Clinical Interview for DSM-IV Axis II Disorders; SD, standard deviation.
Associations between psychopathological symptoms and serum BDNF levels
The MLRM analysis showed a statistically significant decline in BDNF levels over time (Z = −4.9, p = 9.02 × 10−7). As shown in Table 2, analysis of the relationship between scores of original three-subscale PANSS and longitudinal BDNF levels yielded no significant association. However, when we examined the association between serum BDNF levels and five-factor PANSS scores we found a significant relationship between longitudinal BDNF levels and negative factor (Z = −2.245, p = 0.025). MLRM found a significant relationship between CGI depressive symptoms and BDNF levels (Z = −2.796, p = 0.005). This association remained significant when we added age and sex as covariates (Z = −2.819, p = 0.009). The results also showed a significant association between CGI negative symptoms and serum BDNF levels (Z = −2.057, p = 0.039). In terms of serum BDNF levels and scores of positive symptoms subscale of CGI-SCH, the results did not show a significant relationship between them.
Table 2. Results of mixed effects linear regression models.
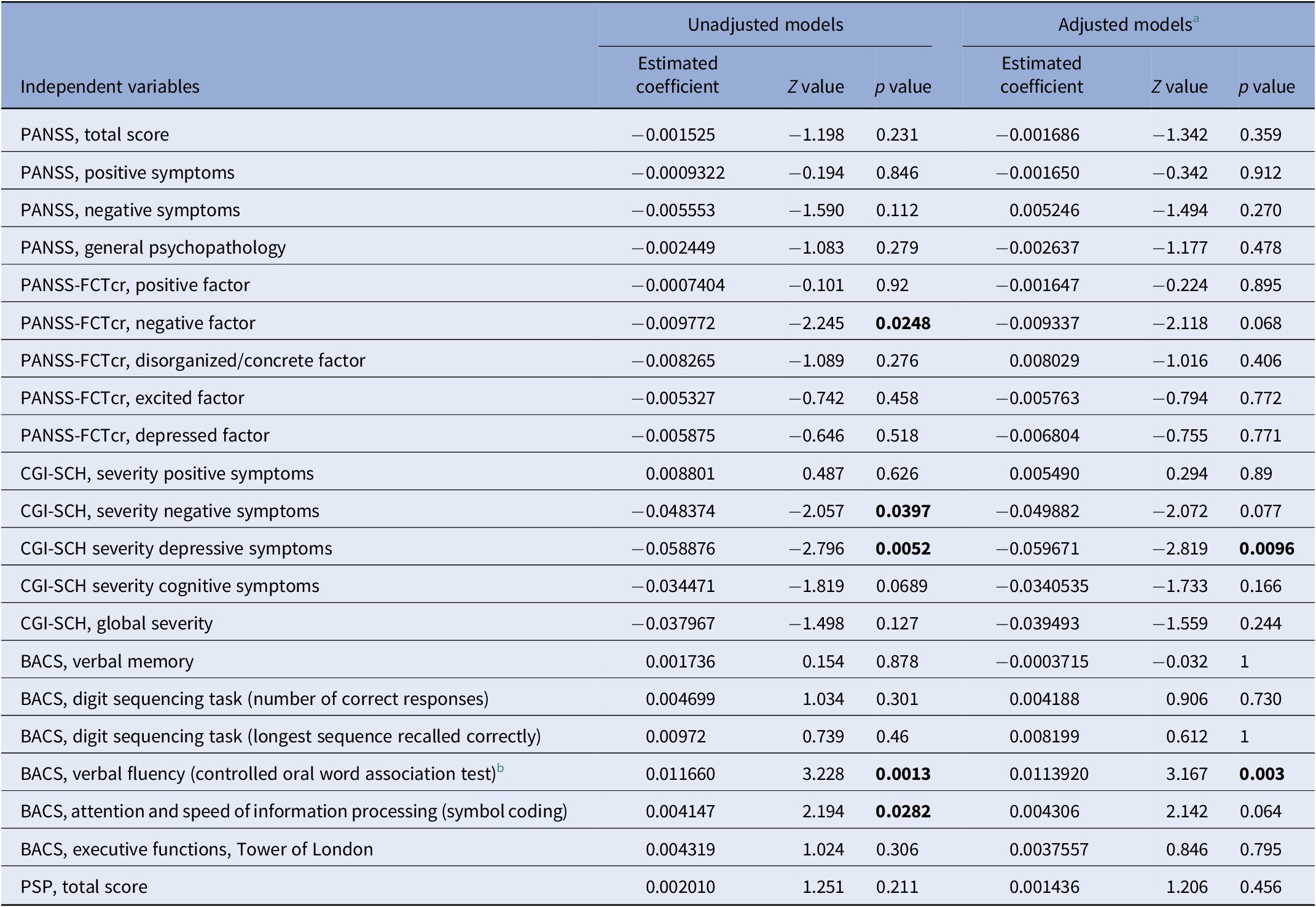
Note: Significant p values in bold.
Abbreviations: BACS, Brief Assessment of Cognitive in Schizophrenia scale; CGI-SCH, Clinical Global Impression Scale for Schizophrenia; PANSS, Positive and Negative Syndrome Scale; PANSS-FCTcr, consensus five-factor model of PANSS; PSP, Personal and Social Performance scale.
a Adjusted for age and sex.
b BACS, verbal fluency (category instances) variable was not included in the table as there were no sufficient observations to support the model.
Associations between cognition, social functioning, and serum BDNF levels
The analyses showed that the BDNF serum levels decreased in patients scoring lower on symbol coding (Z = 2.194, p = 0.028) and semantic fluency (Z = 3.228, p = 0.001) subscales of BACS. The association between semantic fluency and BDNF levels remained significant after correcting for age and sex (Z = 3.167, p = 0.003). An examination of the relationship between the measure of social functioning and longitudinal BDNF levels yielded no significant associations.
Moderating effect of genetic variation
The moderating effect of the SNPs within BDNF gene was analyzed using genotypic and allelic effect models including additive, dominant, and recessive models (Supplementary Table 2). We found a significant moderating effect of rs7934165 on the relationship between BACS subscale for semantic fluency and serum BDNF levels when analyzed using the recessive model (Z = −2.359, p = 0.0367). This interaction effect remained significant after adjusting for age and sex (Z = −2.339, p = 0.0466).
ROC curve
We performed ROC analysis (Supplementary Figure 1) to examine the ability of our model to discriminate between SCZ and SAD in relation to longitudinal BDNF levels. The area under the curve (AUC) was calculated to evaluate the overall accuracy of the diagnostic test in discriminating between SCZ and SAD patients. Optimal diagnostic cut-off value was 2.8303 with a sensitivity 65.2% and specificity of 50.4%. Algorithm of this model had an AUC of 57.1% (95% CI: 0.5183–0.624), which indicates poor diagnostic performance.
Discussion
In this study, we sought to clarify whether longitudinal BDNF serum levels of psychotic patients were correlated with treatment-related, psychopathological, cognitive, and social changes. Previous analysis of LABSP data examined the impact of antipsychotics on BDNF serum levels and found a significant longitudinal increase in those treated with depot/long-acting injectables, but not oral antipsychotics [Reference Manchia, Diego, Deriu, Caboni, Iaselli and Sundas37]. Our current study had several main findings. First, we found an overall decline in the trajectory of serum BDNF levels over time (Supplementary Figure 3). The results revealed that this decline was more pronounced in patients with more severe depressive and negative symptoms. In addition, BDNF serum levels were more declined in patients with lower scores in two cognitive domains including speed of processing and verbal fluency. Finally, when we examined the possible moderating effect of genetic polymorphisms within BDNF gene on these statistically significant associations, we found that rs7934165 polymorphism had a significant moderating effect on the association between verbal fluency and BDNF serum levels.
As mentioned before, the reason for the temporal decline in BDNF serum levels is unknown, but it might be hastened by disease progression or associated with other factors such as drug treatments and severity of clinical symptoms. A meta-analytic study by Fernandes et al. [Reference Fernandes, Steiner, Berk, Molendijk, Gonzalez-Pinto and Turck49] established that peripheral BDNF levels of SCZ patients were moderately decreased in comparison to healthy controls and the decline was associated with the temporal course of the disease. Results of a recent meta-analysis by Rodrigues-Amorim et al. [Reference Rodrigues-Amorim, Rivera-Baltanás, Bessa, Sousa, de Carmen Vallejo-Curto and Rodríguez-Jamardo50] showed that BDNF levels of both drug-naïve and medicated SCZ patients were reduced throughout the disease course. Indeed, the reduced BDNF expression have also been associated with neuroinflammation [Reference Lima Giacobbo, Doorduin, Klein, Dierckx, Bromberg and de Vries51], increased cortisol levels [Reference Mondelli, Cattaneo, Murri, Forti, Handley and Hepgul52], while enriched environment has been shown to increase BDNF levels in psychiatric and neurodegenerative disorders [Reference Miranda, Morici, Zanoni and Bekinschtein53]. Nevertheless, the interaction between these factors should be better understood.
We did not find an association between BDNF levels and psychopathological symptoms when measured by 30-item three-subscale PANSS. Consistent with our results, some studies also did not observe any significant association between PANSS scores and peripheral BDNF levels [Reference Man, Lv, Du, Yin, Zhu and Zhang15, Reference Huang and Lee18, Reference Pırıldar, Gönül, Taneli and Akdeniz54]. Considering that five-factor PANSS has shown to be better at representing the dimensional structure of PANSS data, we utilized this model for our analysis as well. Surprisingly, we found a significant relationship between negative factor of the five-factor scale and reduced serum BDNF levels. This is a rather interesting outcome, as we also found a significant association between the severity of depressive and negative symptoms and reduced BDNF serum levels when we regressed CGI-SCH subscales on BDNF data. To our best knowledge, this is the first longitudinal study assessing the relationship between BDNF levels of psychotic patients and the severity of psychopathological symptoms using CGI-SCH. In their recent clinical study, Fang et al. [Reference Fang, Chen, Wang, Ren and Zhang55] observed a significant association between reduced plasma BDNF levels and depressive symptoms in SCZ patients. Similar results were observed in another study where a significant negative association between depressive symptoms and BDNF serum levels of chronic SCZ patients was found [Reference Wysokiński56].
A possible explanation for the observed correlation between negative and depressive symptoms and peripheral BDNF levels with CGI-SCH and not PANSS might be because CGI-SCH could be a more reliable measure than the 30-item PANSS for monitoring the longitudinal course of psychopathology in SCZ and SAD. [Reference Pinna, Deriu, Diana, Perra, Randaccio and Sanna57] The five-factor/20-item PANSS model have also demonstrated to be a better fitting model for the symptoms of SCZ [Reference Pinna, Bosia, Cavallaro and Carpiniello41, Reference Rodriguez-Jimenez, Bagney, Mezquita, Martinez-Gras, Sanchez-Morla and Mesa58, Reference Stefanovics, Elkis, Zhening, Zhang and Rosenheck59]. which could explain the reason the association between negative factor and serum BDNF levels was detected by this model and not the entire 30-item PANSS scale.
Cognitive impairment is a core symptom of SCZ and a number of studies have found an association between neurocognitive deficits and peripheral BDNF levels [Reference Carlino, Leone, Di Cola, Baj, Marin and Dinelli33, Reference Vinogradov, Fisher, Holland, Shelly, Wolkowitz and Mellon60, Reference Wu, Shi, Chen, Chen, Xiu and Zhang61]. We found a significant correlation between BDNF levels and two cognitive domains including symbol coding and semantic fluency as measured by BACS. A recent meta-analysis examining the relationship between neurocognitive deficits and BDNF levels revealed that higher peripheral BDNF levels were associated with better performance on reasoning and problem-solving tasks in people with SCZ [Reference Ahmed, Mantini, Fridberg and Buckley29]. There is growing evidence associating BDNF to cognitive dysfunctions in psychotic patients at different stages of the disease. According to these findings, it can be assumed that peripheral BDNF levels can be considered as a potential biomarker for neurocognitive deficits in psychosis.
This study did not show any significant association between serum BDNF levels and Val66Met polymorphism within the BDNF gene. Our findings are in agreement with the results of meta-analysis and GWAS analysis of the Sardinian sample conducted by Terracciano et al. [Reference Terracciano, Piras, Lobina, Mulas, Meirelles and Sutin62], where no association was found between Val66Met polymorphism and serum BDNF levels.
We first observed a significant moderating effect of Val66Met on the relationship between BDNF levels and BACS subscale for symbol coding, as well as BDNF levels and CGI depressive symptoms. However, these associations did not survive correction for multiple comparisons. Nevertheless, we found that, when using recessive model, rs7934165 polymorphism within the BDNF gene moderated the significant association between serum BDNF levels and verbal fluency cognitive domain. SNPs in the BDNF gene have been previously linked to peripheral BDNF levels as well as psychopathological and cognitive aspects of SCZ [Reference Aas, Haukvik, Djurovic, Tesli, Athanasiu and Bjella26, Reference Ozan, Okur, Eker, Eker, Gönül and Akarsu27, Reference Xiu and Zhang63, Reference Zhang, Chen, Xiu, Haile, Luo and Xu64]. One possible interpretation of these findings is that the genetic variants are to some extent incorporating the effect of the predictors. This would be consistent with the putative influence exerted by genetic variation on BDNF levels.
Our results should be interpreted considering several limitations. A major limitation of this study is the lack of control group. In addition, the sample size for this study is moderate, especially regarding the analysis of genetic variations of the BDNF gene. However, this is compensated by the longitudinal design and the presence of five time points for assessment. Moreover, the sample was rather heterogeneous regarding the duration and stage of illness. Indeed, some patients were in the early years of their illness course while others had in some instances decades of clinical history. Hence, we tested the interaction effect of duration of untreated psychosis and duration of illness on BDNF levels but did not find any significant effect (Supplementary Table 3). Likewise, in a recent meta-analysis by Rodrigues-Amorim et al. [Reference Rodrigues-Amorim, Rivera-Baltanás, Bessa, Sousa, de Carmen Vallejo-Curto and Rodríguez-Jamardo50], they regressed the duration of illness on serum BDNF levels and did not find a significant effect. Nonetheless, larger sample size and more homogenous sample will be required for future studies to overcome these limitations. The relatively small sample size did not make possible to perform subgroup analyses and led to the inclusion of a limited number of covariates in MLRM models to prevent saturation. Even though there is a substantial genetic overlap between SCZ and SAD [Reference Cardno, Marshall, Coid, Macdonald, Ribchester and Davies5], further research should be undertaken to explore the differences between these subgroups. In addition to the latter point, not all confounding variables could be added to the same model to avoid overfitting and saturation. Finally, even considering the longitudinal design, and the MLRM modeling applied, it is not possible to exclude that some of the time-varying variables were not entirely captured by our analysis.
Another limitation of our study is that only serum BDNF levels of the patients were collected, whereas plasma BDNF levels were not assessed. We are not sure about the extent to which serum BDNF reflects the processes in CNS. While some authors have proposed that plasma BDNF is a more reliable proxy of what happens in CNS [Reference Fernandes, Steiner, Berk, Molendijk, Gonzalez-Pinto and Turck49], others consider serum BDNF to be a better correlate of cortical BDNF levels [Reference Tsuchimine, Sugawara, Ishioka and Yasui-Furukori65]. Finally, the inability of ELISA kits to distinguish between pro and mature BDNF is another limitation of our study. Unlike mature BDNF, pro-BDNF plays role in inducing apoptosis, reducing dendritic spines, and other processes that may contribute to long-time depression [Reference Kowiański, Lietzau, Czuba, Waśkow, Steliga and Moryś66]. Being able to measure BDNF by differentiating between these two isoforms is essential as they may have opposing effects, and future studies should consider using newly developed specific mBDNF and pro-BDNF ELISA assays [Reference Lim, Zhong and Zhou67] when investigating the proposed associations.
Conclusion
Even considering these limitations, our study identified a longitudinal trajectory of decline of BDNF levels associated with decline in some cognitive domains and higher severity of depressive and negative symptoms in patients affected by SCZ and SAD. These findings in a real-world patient sample suggest a plausible role of peripheral BDNF levels as a marker of illness burden in SCZ spectrum disorders.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2022.2333.
Data Availability Statement
The data that support the findings of this study are available from the authors upon request in anonymized form.
Acknowledgements
We gratefully acknowledge the clinical staff, the nurses, and the residents at the Section of Psychiatry of the University of Cagliari for the constant commitment to the care of people with lived experience of mental illness.
Author Contributions
Conceptualization: P.F., A.S., W.F., B.C.; Data curation: D.P., L.D., E.C., N.I., P.P., M.S., A.M., D.C.; Data collection: D.P., C.P., M.T.; Formal analysis: U.I., M.M.; Methodology: U.I., M.M., R.C., M.T., F.P., D.P., M.S., D.C., A.S., P.F.; Resources: B.C.; Supervision: F.P.,P.F.,B,C.,M.M.; Writing—original draft: U.I., M.M.; Writing—review and editing: R.C., F.P., P.P., M.S., C.P., A.S., W.F., P.F., B.C.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
All authors declare none.





Comments
No Comments have been published for this article.