What is gluten?
Gluten is composed of two classes of protein: glutenin and gliadin. It is the main storage protein used by some classes of flowering plants to nourish seeds during development and germination(Reference Shewry, Napier and Tatham1).
As a result of its high consumption by humans, seed storage proteins have been studied and characterised for many years. In 1745, wheat gluten was isolated and the structure of the protein ascertained(Reference Beccari2). Further studies have established that gliadin, a prolamin, is responsible for creating the viscoelastic network that gives wheat the ability to produce a doughy texture(Reference Field, Shewry and Miflin3,Reference Shewry, Halford and Belton4) . This quality plays a role in the perceived satiety of gluten-containing products upon consumption and its general acceptance by individuals(Reference Nguyen, Wahlgren and Almli5).
History of gluten
In Eastern Turkey around 10 000 years ago, after the dawn of human civilization, humans required an expansion of food resources. The earliest signs of cultivation have been found in the Fertile Crescent in South West Asia, and this farming expansion lasted until 4000 BC(Reference Harlan and Zohary6). It is believed that Wild Einkorn wheat was often consumed, although this was found to be a labour-intensive grain, requiring extra processing and milling due to its coating(Reference Freeman7). Over time, through hybridisation techniques, higher gluten-containing species that were easier to mill were produced. Subsequently, these newer wheat species spread across Turkey and then throughout the world due to their improved ability to grow in different climates and seasons(Reference Heun, Schäfer-Pregl and Klawan8). As a result, humans no longer relied on Mesolithic hunter-gatherer communities, but became capable of agriculture, especially wheat cultivation(Reference Freeman7).
These ongoing practices to modify wheat have ensured efficient agricultural development leading to the artificial breeding and selection of wheat variants with better adaptation to extreme climate conditions, bread-making qualities and resistance to diseases(Reference van den Broeck, de Jong and Salentijn9). This has contributed to a dramatic change in the genetic variety and possibly immunogenic qualities of wheat over time resulting in two main antibodies used in the diagnostic process of coeliac disease (CD): anti-tissue transglutaminase and anti-endomysial antibody(Reference van den Broeck, de Jong and Salentijn9,Reference Ludvigsson, Bai and Biagi10) .
Currently, about 95 % of the wheat grown worldwide is bread wheat (Triticum aestivum), a hexaploid species that resulted from the spontaneous hybridisations between more ancient tetraploids (Emmer) and diploid species (Einkorn). This selection is likely a result of its superior number and size of seeds(Reference Dubcovsky and Dvorak11). More recently, gluten extraction from plant seeds has become ubiquitous in the processing of many foods as it is utilised to increase elasticity and stability of food products or as a protein supplement in low-protein foods(Reference Kasarda12).
Cereal consumption has gradually increased with time, particularly during the twentieth century, driven in part by the need for rationing and increased agricultural production during the two World Wars(Reference Copping13). The global wheat production today exceeds 700 million tonnes/year(14). It is speculated that this relatively rapid rate of increased gluten exposure has been too great to give our immune systems the time to develop optimal adapted digestive mechanisms, though this ‘evolutionary theory’ has yet to be fully clarified(Reference Catassi15). There are now a number of gluten-related disorders and perhaps as a result of these factors, and there has been a change in their epidemiology. Given the increasing incidence of CD and gluten ingestion, should we revert to older, less modified and more ‘natural’ grains? Should we indeed all be consuming less gluten? In this Horizon article, we discuss the different clinical manifestations of gluten-related disorders, highlighting gaps and areas for future research.
Coeliac disease
Perhaps not surprising, given the history of wheat, the first clinical description of a CD-like illness was documented in East Turkey by the Greek physician Aretaeus the Cappadocian. He described a chronic disorder of the ‘koiliakos’ or ‘abdomen’ in adults with diarrhoea causing wasting due to a ‘lack of internal heat’ and was treated by rest, fasting and prevention of chilling(Reference Adams16). This pattern of dietary alteration, as described previously, followed by the spread of CD has been seen globally and is still being seen today though the reason for this is unclear(Reference Catassi, Gatti and Lionetti17).
CD is now known to be a chronic inflammatory enteropathy caused in part by dietary exposure to gluten, yet the relationship between gluten and CD was only established in the 1940s(Reference Adams16,Reference Mooney, Hadjivassiliou and Sanders18) . More than 80 years later, the pathogenesis of CD is still to be fully elucidated, but the ingestion of gluten in genetically predisposed individuals often carrying the human leukocyte antigen (HLA) DQ2 and/or DQ8 alleles has been shown to result in a T-cell-mediated immune reaction. This is believed to cause small bowel villous atrophy and subsequent clinical manifestations(Reference Marsh19,Reference Sollid, Markussen and Ek20) . However, whilst 25 % of the general population have a HLA compatible genotype, not all acquire CD, highlighting the importance of other contributing factors, requiring further exploration.
The incidence of CD continues to rise globally(Reference Roberts, Morrison-Rees and Thapar21). The reason for this is unclear and needs further study. The frequency of HLA haplotypes remains relatively static therefore other environmental factors must be involved in the pathogenesis of CD. It has been posited that this could be associated with the process of gluten ingestion but also with the microbiome, infant nutrition, season and method of birth, medication use and childhood infections(Reference Catassi, Gatti and Lionetti17,Reference Namatovu, Lindkvist and Olsson22,Reference Ivarsson, Persson and Nyström23,Reference Lionetti, Castellaneta and Francavilla24,Reference Størdal, Haugen and Brantsæter25,Reference Galipeau, McCarville and Huebener26) . Developing a greater understanding of the pathogenesis of CD would help target future methods of prevention and elicit further treatment options.
In those of North Indian descent, where there is a predominantly wheat-based diet, the relative risk of CD is almost seven times greater than in South India, which is a predominantly rice-based diet(Reference Sher, Fraser and Wicks27). Despite this difference, the HLA-DQ2 and/or DQ8 frequencies of North and South Indians are similar (38 % v. 36 %)(Reference Ramakrishna, Makharia and Chetri28). This provides a rare opportunity to study the interplay between the environment and those more susceptible to developing CD. Furthermore, North Indians also have a three times greater risk of CD compared with Europe. Despite this, there still appears to be a lack of awareness and clinical suspicion of CD, as well as adherence to a gluten-free diet (GFD) in CD in areas of high prevalence(Reference Farrukh and Mayberry29). A greater understanding to the barriers to adherence in this group could help treat all patients, but in particular this growing subset of patients.
Clinical presentation
Our understanding of the clinical presentation of CD is entirely different from that in the 1950s as the majority of cases are now diagnosed in adults, and it is recognised that the clinical manifestations are much more heterogeneous. The classical presentation of malabsorption characterised by chronic diarrhoea, weight loss and failure to thrive is relatively rare. Far more commonly, patients present with non-specific GI symptoms similar to irritable bowel syndrome (IBS) can report these symptoms for many years before diagnosis. The second most frequent presentation is iron deficiency anaemia or other haematinic deficiencies. CD is also seen in association with other autoimmune diseases (such as Type 1 diabetes), reduced bone mineral density, sub-fertility, neurological presentations (ataxia or peripheral neuropathy) and dermatitis herpetiformis(Reference Davidson and Fountain30).
Historically, CD was believed to be a rare childhood disease with an incidence of 1 in 8000 reported in the UK in the 1950s(Reference Davidson and Fountain30). However, contemporary epidemiological studies estimate a much greater worldwide prevalence of approximately 1 in 100(Reference Davidson and Fountain30). Despite this recognised greater prevalence, patients in the UK and worldwide still remain undiagnosed, with current estimates that approximately two in three patients with CD are yet to be diagnosed(Reference Davidson and Fountain30). There remains ongoing debate as to the benefits of screening patients for CD.
Diagnosis and treatment
The gold standard for CD diagnosis is a 6-week gluten challenge of 10 g/d which equates to around 4 slices of bread/d(Reference Ludvigsson, Bai and Biagi10). This can be challenging for patients who experience symptoms whilst consuming gluten. It is unclear what the minimum duration and level of gluten exposure are required to diagnose CD. In adults, a small study has shown a 14-d gluten challenge of 3 g of gluten/d may be sufficient for diagnosis(Reference Leffler, Schuppan and Pallav31). In a landmark study, patients were randomised to either 3 g or 10 g of gluten and after 4 h, serum IL-2 levels are increased. The changes in histology can also be seen after 2 weeks in those ingesting 10 g of gluten only. This would support a 2-week gluten challenge being enough to diagnose CD but also identifies a potential role of IL-2 in the early diagnosis of CD. A 4-h test for CD would revolutionise the diagnostic process of CD and merits further research(Reference Leonard, Silvester and Leffler32).
To date, the only therapy for CD is a lifelong GFD(Reference Rubio-Tapia, Hill and Kelly33). This allows gradual small bowel mucosal healing and resolution of symptoms in most individuals. The length of time for mucosal healing can vary and around 10 % of patients can still have incomplete healing at 5 years of GFD adherence(Reference Lee, Lo and Memeo34,Reference Wahab, Meijer and Mulder35) . The Codex standard (used in the UK and Europe), and similarly the Food and Drug Administration in the USA, suggest that foods containing 20 mg/kg or less of gluten or 20 parts per million (ppm) of gluten can be labelled as ‘gluten-free’ and that foods containing between 21 and 100 ppm of gluten can be labelled as ‘very low gluten’(Reference Lanzini, Lanzarotto and Villanacci36). Continued exposure to low levels of gluten from such products may delay healing of the small bowel mucosa.
Over 40 % of patients with CD are dissatisfied with a GFD, highlighting the need for alternative preparations to improve adherence(Reference Aziz, Evans and Papageorgiou37). The use of sourdough may be one way to improve the tolerability of gluten-free foods but needs further exploration. Furthermore, there is preliminary data to suggest that sourdough fermentation may improve the recovery of the small bowel in an ex vivo study(Reference Moroni, Dal Bello and Arendt38,Reference Barratt, Leeds and Sanders39,Reference Calasso, Vincentini and Valitutti40) .
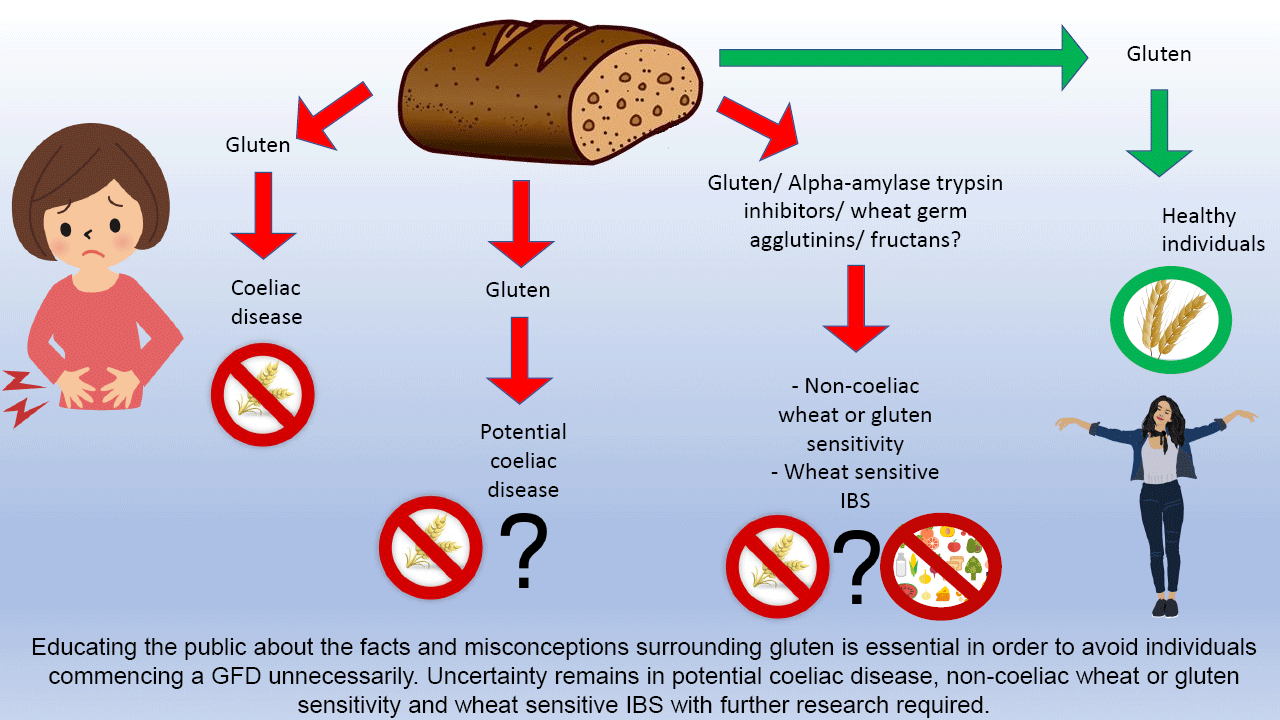
Potential coeliac disease
Around 10 % of patients with CD are diagnosed as potential CD due to having positive coeliac serology (IgA-endomysial antibody or IgA-TTG) but normal duodenal mucosa, on a gluten-containing diet(Reference Biagi, Trotta and Alfano41,Reference Caio, Volta and Sapone42) . The literature is less clear as to the benefits of a GFD in people with potential CD. One proposed strategy is that a GFD could be considered for symptomatic benefit, but in asymptomatic individuals a GFD may not be required as they do not tend to develop villous atrophy(Reference Volta, Caio and Giancola43). Conversely, it has also been suggested that all individuals with positive coeliac serology should be treated with a GFD, regardless of enteropathy(Reference Kurppa, Collin and Viljamaa44). There is a need for long-term follow-up of these patients to assess the long-term consequences of potential CD to better guide clinical management. In the absence of this data, it is important to give patients with potential CD the option of a GFD and help them make an individualised informed decision.
Non-coeliac wheat or gluten sensitivity
The trigger of non-coeliac gluten sensitivity (NCGS) is debated, although wheat appears to be key trigger for symptoms(Reference Carroccio, Mansueto and Iacono45). Patients with NCGS have significantly elevated IgG4 in comparison with patients with CD and healthy individuals. They also have greater levels of IgG2 than healthy individuals. This, in contrast to the greater levels of IgG1 and IgG3 seen in CD, that suggest a difference in the innate immune response between CD and NCGS(Reference Uhde, Caio and De Giorgio46). Patients with NCGS have significantly increased levels of soluble CD14, lipo-polysaccharide-binding protein and antibody reactivity to bacterial lipopolysaccharide and flagellin, suggesting a different consequential systemic immune response compared with patients with CD. However, the underlying mechanism of this remains unclear and needs further investigation(Reference Uhde, Ajamian and Caio47).
The protein content of the wheat grain is around 10–12 %, of which gluten accounts for 80 %(Reference Rubio-Tapia, Ludvigsson and Brantner48). Colonic barrier function has been demonstrated to alter following gluten exposure in these individuals, where there is a lower expression of tight junction proteins, a potentially reversible mechanism(Reference Vazquez-Roque, Camilleri and Smyrk49). In principle, this is supported by several studies which have shown clinical improvement in patients with NCGS following initiation of a GFD. However, when gluten was reintroduced to these individuals in a double blind randomized controlled trial only one third correctly identified gluten based on their response. Therefore, perhaps the causal agent may not be gluten but another component in flour(Reference Biesiekierski, Newnham and Irving50,Reference Di Sabatino, Volta and Salvatore51,Reference Zanini, Baschè and Ferraresi52) .
Other components of wheat have also been shown to alter the gut mucosa, raising the possibility of other non-gluten components of wheat also being associated with NCGS. Alpha-amylase trypsin inhibitors (ATIs) have been shown to induce an innate immune response leading to intestinal inflammation, by pro inflammatory cytokine release(Reference Junker, Zeissig and Kim53), Another component of wheat: wheat germ agglutinins have been demonstrated to alter enterocyte permeability in vitro, although human data is lacking(Reference de Punder and Pruimboom54).
A group of short chain carbohydrates, known as FODMAP (fermentable oligo-, di-, mono- saccharide and polyols) may also be implicated in the aetiology of symptoms. Fructans, a FODMAP found in wheat, has been postulated to lead to symptom generation through large bowel fermentation, with intestinal gas production and distention, in individuals with visceral hypersensitivity(Reference Skodje, Sarna and Minelle55,Reference Spiller56) . However, it is worthwhile noting that extra-intestinal symptoms, commonly seen in NCGS, are not triggered by FODMAP, challenging this hypothesis(Reference Catassi, Alaedini and Bojarski57).
Whilst the literature supports the idea that wheat components are a likely trigger for symptoms in NCGS, further research is required to identify the specific component. In addition, a contributory nocebo response in symptom generation must also be considered. Therefore, given several components in addition to gluten could be potential triggers of symptoms, non-coeliac wheat sensitivity is a commonly used term(Reference Catassi, Alaedini and Bojarski57,Reference De Giorgio, Volta and Gibson58) .
Clinical presentation
The global reported prevalence of non-coeliac gluten sensitivity (NCGS) is variable due to the challenges in diagnosis, but is reported to be between 0·6 %–10·6 %(Reference Rubio-Tapia, Ludvigsson and Brantner48,Reference Golley, Corsini and Topping59,Reference Tanpowpong, Ingham and Lampshire60,Reference DiGiacomo, Tennyson and Green61,Reference Aziz, Lewis and Hadjivassiliou62,Reference Volta, Bardella and Calabrò63,Reference Mardini, Westgate and Grigorian64,Reference van Gils, Nijeboer and IJssennagger65,Reference Carroccio, Giambalvo and Blasca66) . Currently, no accurate biomarkers exist for diagnosis and there is a significant overlap with other conditions, like IBS, with individuals with NCGS meeting the criteria for IBS more than those without, adding to the diagnostic challenge(Reference Aziz, Lewis and Hadjivassiliou62). Furthermore, many individuals self-report gluten sensitivity and struggle to complete strict assessments for formal diagnosis(Reference Catassi, Elli and Bonaz67). Similar to CD, patients with NCGS tend to be middle-aged adults at presentation(Reference Aziz, Lewis and Hadjivassiliou62). Given the poor understanding of the pathogenesis of NCGS, it is difficult to diagnose these patients in a consistent way. In the absence of a gold standard, there is likely significant heterogeneity in the clinical management and diagnosis of these patients.
Presentation can vary, with individuals reporting both intestinal and extra intestinal symptoms related to the ingestion of gluten containing foods. Intestinal symptoms can include bloating, abdominal pain and diarrhoea and commence between a few hours and up to 1 d after gluten ingestion(Reference Volta, Bardella and Calabrò63). As in CD and IBS, extra intestinal symptoms include headaches, myalgia, fatigue, and brain fog(Reference Volta, Bardella and Calabrò63,Reference Dale, Biesiekierski and Lied68) . Interestingly, there appears to be a relationship between NCGS and the development of neurological and psychiatric manifestations such as ataxia, schizophrenia and depression(Reference Catassi, Bai and Bonaz69,Reference Lionetti, Leonardi and Franzonello70) . However, the lack of biomarkers continues to limit diagnosis.
Diagnosis
The first important step prior to the diagnosis of NCGS is to ensure that CD and wheat allergy (WA) are excluded. In individuals with NCGS, IgA-tissue transglutaminase and IgA-endomysial antibody should be negative whilst on a gluten-containing diet. WA is an IgE-mediated reaction, with symptoms occurring rapidly following the ingestion of wheat, within minutes to hours rather than the longer time period seen with NCGS(Reference Aziz, Hadjivassiliou and Sanders71). Also, IgE-mediated WA is seen in 0·1–1 % of children and rarely progresses into adulthood(Reference Sapone, Bai and Ciacci72,Reference Poole, Barriga and Leung73,Reference Czaja-Bulsa and Bulsa74) .
Although non-specific, IgG antigliadin antibodies have been demonstrated to have a higher prevalence in NCGS, reported at 50 %(Reference Catassi, Elli and Bonaz67,Reference Dale, Biesiekierski and Lied68) . Other proposed diagnostic biomarkers such as serum zonulin remain controversial with conflicting results(Reference Talley, Holtmann and Jones75,Reference Barbaro, Cremon and Morselli-Labate76) .
Due to a lack of diagnostic biomarkers, the Salerno experts’ criteria has been suggested for diagnosis. In individuals where CD and WA have been excluded, a double-blind placebo-controlled challenge of gluten (8 g/d) is used to confirm the diagnosis(Reference Catassi, Elli and Bonaz67). However, whilst definitive, in a non-research setting this may be challenging. A practical suggestion for diagnosis in a clinical setting is assessing symptoms on a gluten-containing diet v. GFD for at least 1 week(Reference Leonard, Sapone and Catassi77).
Overlap with other conditions
It has been suggested that NCGS may be a form of mild CD(Reference Khan, Suarez and Murray78). Individuals with NCGS appear to have a higher proportion of the HLA-DQ2/DQ8 haplotype in comparison to the general population(Reference Vazquez-Roque, Camilleri and Smyrk49,Reference Khan, Suarez and Murray78) . In addition, a higher prevalence of gliadin antibodies has been shown in NCGS(Reference Catassi, Elli and Bonaz67). However, it is important to stress that NCGS is diagnosed following the exclusion of CD and that gliadin antibodies and HLA-DQ2/DQ8 haplotypes are non-specific.
IBS is another condition which presents similarly to NCGS. Around 30 % of individuals presenting with IBS have a sensitivity to wheat(Reference Carroccio, Mansueto and Iacono45). This has led to the terminology of ‘wheat-sensitive’ IBS being used(Reference Ferch and Chey79). An important distinguishing feature between these conditions is that individuals with NCGS tend to note gluten as a trigger for their symptoms in comparison to those with IBS(Reference Carroccio, Mansueto and Iacono45,Reference Catassi, Alaedini and Bojarski57) . It is also likely that a subset of individuals with IBS actually have NCGS, with a careful history required to delineate this(Reference Rej and Sanders80). More robust criteria are needed to distinguish between IBS and NCGS which is currently hampered by the lack of diagnostic markers for both, in particular markers for immunoglobuins, CD14 and lipopolysaccharides in NCGS and IBS to see the overlap of immune mediated factors.
Management
Unlike CD where treatment requires a lifelong GFD, the timeframe for management with a GFD for NCGS is unclear and requires further assessment(Reference Dale, Biesiekierski and Lied68). Furthermore, the threshold of gluten restriction in individuals with NCGS is yet to be determined and it is possible that patients with NCGS may require different levels of gluten restriction for symptomatic benefit(Reference Catassi, Elli and Bonaz67,Reference Catassi, Bai and Bonaz69,Reference Sapone, Bai and Ciacci72,Reference Volta, Pinto-Sanchez and Boschetti81) .
Currently, there are no guidelines on whether patients should follow a lifelong GFD, or whether this condition is fluctuating in nature(Reference Dale, Biesiekierski and Lied68,Reference Fasano, Sapone and Zevallos82) . Until guidelines are produced and backed by robust data, it has therefore been suggested that a re-trial of gluten tolerance, after 1–2 years of a GFD can be considered(Reference Khan, Suarez and Murray78). However, as discussed it is unclear whether other triggers are the cause of NCGS and therefore patients may derive benefits from a low FODMAP diet(Reference Khan, Suarez and Murray78).
The implementation of a GFD should be performed by a dietitian, in order to ensure nutritional adequacy, and prevent potential risks of a GFD including micronutrient deficiencies, high fat, sugar and salt intake(Reference Vici, Belli and Biondi83,Reference Skodje, Minelle and Rolfsen84,Reference Potter, Brienesse and Walker85,Reference Wild, Robins and Burley86) .
Gluten-sensitive irritable bowel syndrome
A key trigger for symptom generation in IBS is diet, with up to 80 % of patients with IBS reporting food-related symptoms(Reference Böhn, Störsrud and Törnblom87). Therefore unsurprisingly, patients are keen to explore dietary interventions. Over 60 % of patients want to know what foods to avoid and up to 70 % have tried modifying their diets(Reference Halpert, Dalton and Palsson88).
In terms of dietary interventions for IBS, research has primarily focused on the low FODMAP diet, as well as exploring the role of traditional dietary advice and GFD(Reference Rej, Aziz and Tornblom89). Several randomised controlled trials have been performed assessing these diets, in particular the low FODMAP diet and GFD, with increasing evidence for their use in IBS, particularly at short term follow up(Reference Rej, Aziz and Tornblom89). However, the long term benefits of dietary interventions are less clear, although emerging evidence highlights the efficacy of the low FODMAP diet at long term follow up(Reference Rej, Shaw and Buckle90,Reference Staudacher, Rossi and Kaminski91) . Currently, whilst no diet has been shown to be superior to another, patients should be given a choice based on their individual circumstances, in conjunction with dietetic support.
Wheat sensitivity is common in IBS, reported in 30 % of individuals(Reference Carroccio, Mansueto and Iacono45). In addition, confocal endomicroscopy has shown mucosal changes to wheat in individuals with IBS(Reference Fritscher-Ravens, Schuppan and Ellrichmann92). Whilst this is the case, as highlighted previously, the causal component of wheat which triggers symptoms remains unclear. Fructans, gluten, wheat germ agglutinins and ATIs have all been implicated as potential triggers(Reference Rej, Aziz and Tornblom89). It is likely that there are several dietary triggers for symptoms in different patients with IBS. The first randomised controlled trial to compare FODMAP v. GFD v. Traditional Dietary advice has shown a comparative effect for all three options(Reference Rej, Sanders and Shaw93). Given the lack of biomarkers to accurately assess likelihood of response to a specific dietary therapy, patients are generally offered several dietary options, including a low FODMAP diet, GFD or traditional dietary advice. The final decision should guided by patient choice and dietetic assessment(Reference Rej, Aziz and Tornblom89).
Should healthy individuals be on a gluten-free diet?
The popularity of gluten-free products over the last two decades has dramatically risen and in the UK alone, being now estimated to be worth £835 million/year(94,Reference Aziz, Karajeh and Zilkha95) . Although the increase in gluten-free product availability is in part due to the increasing incidence and awareness of CD, people without CD are also considering or currently undergoing a voluntary GFD(Reference Karajeh, Hurlstone and Patel96). Multiple surveys have shown that there are greater numbers of consumers worldwide following a GFD irrespective of having a diagnosis of CD(Reference Tanpowpong, Ingham and Lampshire60,Reference Aziz, Lewis and Hadjivassiliou62) . Observational studies have reported that up to 13 % of the population may self-report sensitivity to gluten-based products and that up to 5 % of the population may be self-initiating a GFD(Reference Tanpowpong, Ingham and Lampshire60,Reference Aziz, Lewis and Hadjivassiliou62) .
Some people believe a GFD is healthier as opposed to a form of management for a medical condition, whilst others report symptoms following gluten ingestion. Since the 1970s, it has been recognised that people who do not have CD can still present with symptoms following gluten ingestion, however this has become a focus of scientific research only recently(Reference Cooper, Holmes and Fperguson97,Reference Ellis and Linaker98) . This change has been driven by patient demand and a dramatic rise in media popularity of the ‘gluten-free lifestyle’. Despite this increased interest, the only double randomised controlled study of a gluten challenge in healthy volunteers demonstrated that individuals did not develop any significant symptoms(Reference Croall, Aziz and Trott99). Given the current trend, it is reasonable to assume the popularity of the GFD in individuals who would not benefit from this is likely to increase, highlighting the need for education.
Conclusion
Gluten-related disorders are common. In individuals who report symptoms following gluten ingestion, it is crucial to exclude CD. Whilst the prevalence of CD is increasing, the reason for this remains unclear and is worthy of investigation; areas where there are changes towards a more wheat-based diet present an ideal opportunity for this. Given the rise in individuals opting to go gluten free for perceived health benefits despite no clinical indication, it is also essential to educate the public about the facts and misconceptions surrounding gluten to avoid individuals commencing a GFD unnecessarily.
There is emerging evidence to support the role of a GFD for patients with IBS, but due to lack of clarity this should be a collaborative decision-making process between the dietitian and the patient to ensure an individualised approach to management. Finally, there is uncertainty surrounding NCGS, and further research is required to identify the key components responsible for symptom onset in order to ensure the most effective management for patients in the future.
Acknowledgements
S. A. R. and D. S. S. wrote the initial manuscript. A. R. reviewed the manuscript, and D. S. S. approved the final version. The authors contributed equally to this work.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
There are no conflicts of interest.