Impact statement
Fibre fragments are among the most common microplastics found in the environment. Standard test methods were introduced in 2021 and 2023 to measure fibre fragmentation propensity during laundry. However, the recommended methods for analysing the test results are slow and/or require access to rare and expensive equipment, potentially limiting its adoption. This article introduces a high-throughput, low-cost, accessible method for quantifying fragmented fibres that has lower variability than gravimetric methods at critical fragmentation levels.
Introduction
Microplastic fibres are ubiquitous pollutants in both natural (Barrows et al., Reference Barrows, Cathey and Petersen2018; Lambert and Wagner, Reference Lambert, Wagner, Wagner and Lambert2018; Xu et al., Reference Xu, Zhang, Gu, Shen, Yin, Aamir and Li2020; Acharya et al., Reference Acharya, Rumi, Hu and Abidi2021) and urban environments (Dris et al., Reference Dris, Gasperi, Mirande, Mandin, Guerrouache, Langlois and Tassin2017, Reference Dris, Gasperi, Tassin, Wagner and Lambert2018; Zhang et al., Reference Zhang, Zhao, Du, Cai, Wang and Shi2020). Fibres cause a myriad of health issues in animals when ingested (Kwak et al., Reference Kwak, Liu, Wang, Lee, Lee and An2022), and concerns are emerging over their impact on human health (Gasperi et al., Reference Gasperi, Wright, Dris, Collard, Mandin, Guerrouache, Langlois, Kelly and Tassin2018; Prata, Reference Prata2018; Abbasi et al., Reference Abbasi, Keshavarzi, Moore, Turner, Kelly, Dominguez and Jaafarzadeh2019; Amato-Lourenço et al., Reference Amato-Lourenço, dos Santos Galvão, de Weger, Hiemstra, Vijver and Mauad2020). The predominant path of fibre pollution to waterways and oceans may be from washing textiles, with the effluent being discharged through wastewater treatment plants (Gatidou et al., Reference Gatidou, Arvaniti and Stasinakis2019), although atmospheric distribution may also contribute (Dris et al., Reference Dris, Gasperi, Saad, Mirande and Tassin2016).
Commercial fibre capture devices and washing machine filters are marketed for capturing fragmented fibres (FF) in washing machines, but they are not completely effective (Napper et al., Reference Napper, Barrett and Thompson2020) and are therefore optional. Therefore, the quantity of FF released from textiles must be reduced by design. Routine testing is required to inform the design of less polluting textile materials and processes and to set boundaries for maximum allowable fragmentation. This test must be reliable and cheap if everyone is to use it.
Initial causal studies of FF employed various methods, including using domestic washing machines and cannister-style accelerated laundering machines (Tiffin et al., Reference Tiffin, Hazlehurst, Sumner and Taylor2022). All studies filtered effluent through filter membranes to collect FF for analysis, then a relatively equal split of quantification methods can be found in the literature, with gravimetric quantification using analytical balances providing the mass of fibres (Hartline et al., Reference Hartline, Bruce, Karba, Ruff, Sonar and Holden2016; Napper and Thompson, Reference Napper and Thompson2016; Pirc et al., Reference Pirc, Vidmar, Mozer and Kržan2016; Sillanpää and Sainio, Reference Sillanpää and Sainio2017; Kelly et al., Reference Kelly, Lant, Kurr and Burgess2019; McIlwraith et al., Reference McIlwraith, Lin, Erdle, Mallos, Diamond and Rochman2019; Zambrano et al., Reference Zambrano, Pawlak, Daystar, Ankeny, Cheng and Venditti2019; De Falco et al., Reference De Falco, Di Pace, Cocca and Avella2019b, Reference De Falco, Di Pace, Cocca, Avella, Scholz, Fox, Mayershofer, Cocca, Di Pace, Errico, Gentile, Montarsolo, Mossotti and Avella2020; Cesa et al., Reference Cesa, Turra, Checon, Leonardi and Baruque-Ramos2020; Cotton et al., Reference Cotton, Hayward, Lant and Blackburn2020; Lant et al., Reference Lant, Hayward, Peththawadu, Sheridan and Dean2020; Napper et al., Reference Napper, Barrett and Thompson2020; Tiffin et al., Reference Tiffin, Hazlehurst, Sumner and Taylor2022) and visual counting providing the number of fibres (Browne et al., Reference Browne, Crump, Niven, Teuten, Tonkin, Galloway and Thompson2011; Hernandez et al., Reference Hernandez, Nowack and Mitrano2017; Sillanpää and Sainio, Reference Sillanpää and Sainio2017; Carney Almroth et al., Reference Carney Almroth, Åström, Roslund, Petersson, Johansson and Persson2018; De Falco et al., Reference De Falco, Gentile, Avolio, Errico, Di Pace, Ambrogi, Avella and Cocca2018, Reference De Falco, Cocca, Guarino, Gentile, Ambrogi, Ambrosio and Avella2019a; Belzagui et al., Reference Belzagui, Crespi, Álvarez, Gutiérrez-Bouzán and Vilaseca2019; Haap et al., Reference Haap, Classen, Beringer, Mecheels and Gutmann2019; McIlwraith et al., Reference McIlwraith, Lin, Erdle, Mallos, Diamond and Rochman2019; Yang et al., Reference Yang, Qiao, Lei, Li, Kang, Cui and An2019; Athey et al., Reference Athey, Adams, Erdle, Jantunen, Helm, Finkelstein and Diamond2020; Cai et al., Reference Cai, Yang, Mitrano, Heuberger, Hufenus and Nowack2020; Kärkkäinen and Sillanpää, Reference Kärkkäinen and Sillanpää2021; Özkan and Gündoğdu, Reference Özkan and Gündoğdu2021). Gravimetric methods, although also time-consuming, are quicker than manual counting and have been adopted as standard in the American Association of Textile Chemists and Colorists (AATCC) and International Standards Organisation (ISO) methods.
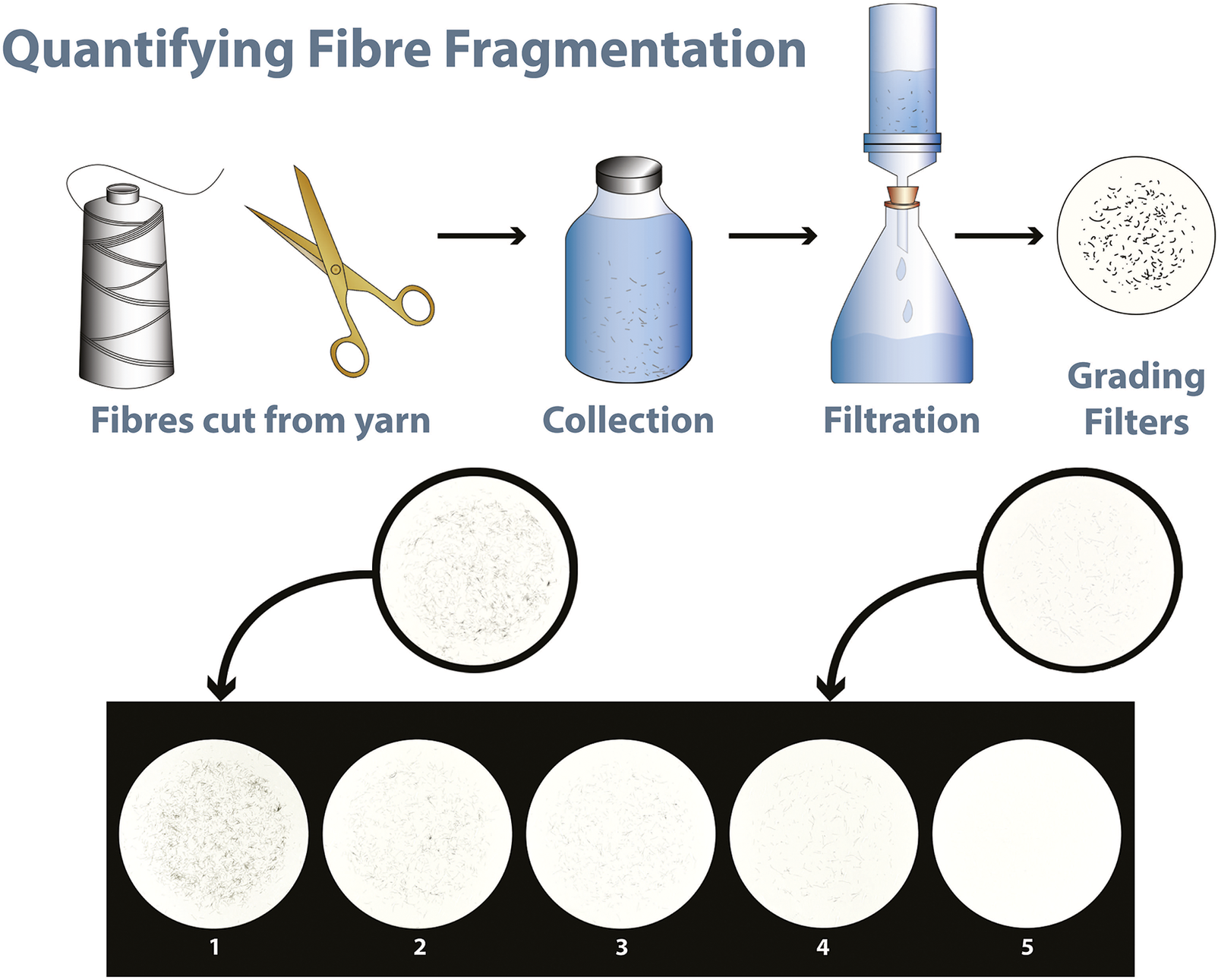
Standard methods for quantifying FF were first published in 2021 with AATCC TM212-2021, followed by ISO 4484-1:2023 (ISO 4484 also has parts 2 and 3 not discussed here), and DIN SPEC 4872:2023-02 in 2023. All methods require four textile test specimens, double-rolled hems and a finished size of 100 ± 10 mm × 240 ± 10 mm. Specimens were weighed on analytical balances with a resolution of at least 0.1 mg before washing. ISO recommends oven-drying test specimens to a constant mass, AATCC recommends oven-drying or conditioning test specimens for 4 h before weighing, and DIN recommends removing contaminant fibres with a lint roller but no drying or conditioning, which would increase measurement variability. All methods produce FF from textiles using laboratory-scale accelerated laundry machines with rotating 1200 ml stainless steel cannisters, 360 ml water, 50 stainless steel balls and one test specimen per cannister. ISO and AATCC methods recommend filtration to collect FF from the wastewater, onto pre-weighed filter membranes, and gravimetric methods to quantify fibre fragmentation. Before testing, filter membranes are rinsed, oven-dried or conditioned for a minimum of 4 h, and then weighed ready for filtration. After filtration, the filters with FF are oven-dried or conditioned, weighed, and the total FF mass is calculated and reported. FF is also calculated as a percentage of the test specimens’ mass. The time required to oven-dry or condition test specimens and filters before and after wash testing means a single test takes more than 9 h, leading to high costs and slow throughput. The DIN method uses dynamic image analysis to automatically analyse cannister effluent with image analysis sensors to detect, count and characterise: the total FF quantity per gram of textile material; mean FF length (μm); and FF length distribution. This method quickly provides detailed information about the fibres released, but the specialist additional equipment required may limit its accessibility. Note that most textile laboratories already have accelerated laundry devices for the popular colour fastness to wash test.
Visual scales are commonly used in international standard methods to grade visual changes in textile specimens caused by test conditions. The grade dictates whether the textile ‘passes’ (suitable for purpose) or ‘fails’, requiring either improvements or rejection. For example, the extent of pilling, fuzzing, and matting on textile specimens after abrasion is exclusively subjectively judged against a 5-point scale aided by photographed images of progressively worse surface deterioration (ISO 12945-4:2020). In colour fastness tests, a ‘grey scale’ is widely used and trusted, despite the option of (expensive) instrumental methods, to assess both colour change in the original test specimen (ISO 105-AO2:1995) and staining of adjacent fabrics (ISO 105-AO3:2019). In these, and all other methods using a visual scale, a grade 5 indicates ‘no change’ between the test specimen and control (untested) specimen, a grade 4 indicates slight change and is the most common ‘pass’ grade used by industry. Scales continue to grade 1, showing the greatest (worst) change in appearance. Subjective grading of test specimens is widely used and trusted in the textile industry to inform cost-effective buying decisions.
The objectives of this study were:
-
1. To develop and evaluate a high-throughput, low-cost, visual fibre fragmentation scale (FFS).
-
2. Establish the relative accuracy and repeatability of subjective grades assigned using a FFS in comparison to the objective gravimetric methods recommended in the AATCC and ISO standards.
Methods
Ten FFSs were developed, with the parameters shown in Table 1. This article presents the results of evaluations of the tenth scale, J, using seeded test specimens for validation purposes and some discussion of learning from earlier FFS versions. Scale J was reported as it used the filter size recommended in the newly released standard ISO and AATCC test methods and had enough fragments on it to compare to a wide range of materials.
Table 1. Total length of 95 filament yarn (in mm) cut into fibre fragments (FFs) to create fibre fragmentation scales (FFSs) A–J. Scales with ‘2-mm’ fragments were measured and those showing ≈2/≈1 were free cut to the approximate maximum length shown

Materials
Fibre fragments
FFs were prepared from the same yarn for all scales and seeded samples: black WonderFil 100% polyester, 300 denier, textured 95 filament yarn (meaning that a 1 mm length of yarn would generate 95 fibre fragments [FFs]).
Filters
70 mm Whatman glass-fibre GF/B filters (1 μm pore diameter) were used in scales A-G.
47 mm Whatman Millipore APFA glass-fibre filters without binder (1.6 μm pore diameter) were used in scales H-J.
Methods for making FFSs and seeded specimens
Cleaning
All equipment was triple rinsed with soft tap water without particulate matter before use and between specimens. Surfaces were wiped clean with damp, lint-free paper towels. Nitrile gloves and white laboratory coats were worn to prevent contamination.
Fibre fragments
Yarn was measured and cut to the required total length, as shown in Table 1. Yarn sections cut for scale J were weighed on an ultra-microbalance to facilitate comparison to scale grades. Fibres were cut using two methods: FF for scales A, B, D and G were cut using a rotary cutter and an engraved steel rule to measure 2 mm fragments as consistently as possible. FF for scales C, E, F, H, I and J were ‘free cut’ using sharp tailor snips and metal tweezers to create a range of fragment sizes with the maximum lengths shown in Table 1. Fragments were immediately suspended in 100 ml of tap water in a clean glass jar ready for filtration. Tools were rinsed and a magnifying glass was used to ensure no FF remained on tools after cutting. Fragments for ‘seeded specimens’ were free cut, prepared in the same way and matched the quantities used in the scales to enable calculation of grade accuracy. Four replicate specimens were prepared for each grade on the scale, and an additional four replicate specimens were prepared using 2 mm yarn for test specimens whose ‘correct grade’ would be 4.5. Scale images were selected from the replicate specimens with the appropriate number of fibre fragments shown in Table 1.
Filtration
Each FF specimen suspended in water was filtered using vacuum filtration onto filter membranes to create scale grades 1–4. Grade 5s (blank controls) were prepared in the same way, except the water filtered contained no fibres. Following filtration of the seeded ‘effluent’, glass jars were rinsed with 100 ml of tap water filtered through the same filter, and a wash bottle was used to rinse FF from the edge of the filtration funnel. Care was taken to ensure drops of water did not disturb the FF distribution pattern. In the production of scales E-J if the FF distribution was disturbed, an additional 40 ml of water was used to re-suspend the FF, and the fragments were allowed to re-settle. Filters were placed in clean Petri dishes and covered before drying.
Weighing the filters
Scales H–J used 47 mm glass-fibre filters without binder and could therefore be weighed accurately. Four replicate filters for each scale grade, and those with 2 mm yarn (190FF), were pre-rinsed three times with 20 ml of soft tap water, placed into clean pre-labelled glass Petri dishes, covered, and oven-dried for 4 h at 50 °C. The filters were immediately weighed on an ultra-microbalance (Satorius SC2) readable to 0.0001 mg and a microbalance (Sartorius A210P) readable to 0.0001 g/0.1 mg to obtain the pre-filtration oven-dry mass. Filters were conditioned at 20 ± 2 °C and 65 ± 5%RH according to ISO 139:2005 for >16 h and then re-weighed (pre-filtration conditioned mass). Each weighed filter paper was then used to filter a specimen of fibre fragments. Filters were placed back into their Petri dish, covered, oven-dried for 4 h and immediately weighed (post-filtration oven-dry mass), then conditioned for >16 h and re-weighed (post-filtration conditioned mass). The mass difference was calculated and used as the mass of FF in each specimen.
Creating FFSs
Dry filters were photographed with a Canon EOS 77D camera, zoom 18–55 mm lens (ISO:200, F:9, 1/80 shutter speed, auto white balance, 2 s timer. File saved as large jpeg) under standardised lighting conditions (two soft box lamps) in a photographic studio. Early scales A–D were used ‘as produced’, without pre-selection for even fibre distribution, which made them hard to use. Scales E–J were visually assessed to ensure an even fibre distribution pattern on each filter. Filters with excessive fibre clumping, or areas without fibres due to water droplets were harder to grade and rendered the specimen unsuitable for a scale image. Images were edited in Photoshop to correct the white balance and ensure accurate size for printing. Images for scales C–J were given black backgrounds. Two versions of each image were prepared: one for the ranking tests, given an ambiguous code name for researcher-only identification, the second was placed onto 5-point scale templates and labelled with its grade number. Images were printed to scale on smooth, pure white, 90 gm−2 paper at 4800 × 1200DPI with 70 mm diameter filter images for scales A–G and 47 mm for scales H–J.
Ranking test method
The first step in evaluating the effectiveness of each FFS or scale was to ask volunteers (‘observers’) to independently rank scale images in order from highest to lowest fibre presence. The images were rearranged until observers were satisfied with their ranking order. The time taken to rank scale images, any comments made during the ranking process and the ranking order were noted. Observers were asked to perform grading tests if their ranking order was perfect.
Most observers were Heriot-Watt University textiles/fashion staff, PhD, MSc or honours year students, but members of the public and industry representatives also participated. All were recruited using convenience sampling methods (email invites). Our final evaluations were undertaken with three members of the public, six staff/students and three people working in the textile industry (at Lochcarron of Scotland). Some observers had experience conducting fibre fragmentation wash tests and/or using visual scales to evaluate test results, while many had no laboratory experience.
Images were viewed under artificial ‘daylight’ (D65) or in bright daylight. Staff/student and industry observations were made under D65 light in a VeriVide light box, three observers ranked specimens under natural daylight to establish whether the light source had a significant impact on test results.
Method for grading with the FFS
Observers were shown a FFS consisting of five numbered filter photographs. Grade 5 had no FF and grade 1 had most FF, as shown in Table 1 and Figure 1. The observer was given individual images of seeded, or real, FF test specimens and asked to match the test specimen to its closest matching grade on the scale. The assigned grade could be on (1, 2, 3, 4 or 5) or between grades (e.g., AX shown in Figure 1 was 4.5). If there were more FF than shown on grade 1, observers could assign grade 0. Most observations were made under D65 light in a VeriVide light box, but one set of observations (three individual observers) were conducted in bright natural daylight to determine whether the use of a D65 light box was strictly necessary for consistent gradings.

Figure 1. Fibre fragmentation scale J and example filter specimen AX, seeded with 190 FF, that would be correctly assigned a grade 4.5 and AO that would be correctly assigned a grade 2.
In our evaluation of the final scale J, tests were repeated with the real filters contained in glass Petri dishes to ensure that similar results were obtained when viewing actual filter papers compared to photographic images of filter papers (FF could be disturbed when real filters were moved, while photographs of the filters ensured all observers graded identical images). Genuine wash test specimens were also graded using FF scales D–G (not reported here but results were comparable).
Results were recorded by hand in observer-specific tables, and subsequently copied into Microsoft Excel. The mean grades assigned by three observers for all replicate specimens was calculated and compared to the ‘correct grade’ (correct grades were seeded with the same total length of free-cut FF as replicate specimens).
In this article, we report the results of measurements made using our latest FFS, scale J, and pertinent observations based on evaluations of earlier scales.
The correlation between ‘correct grades’ and ‘observed grades’ of both individuals and the group means was calculated and tested for significance. The variance (standard deviation, standard error and coefficient of variation) and confidence intervals were calculated for each set of data. The data were analysed in Microsoft Excel.
Results and discussion
Gravimetric measurement of fibre fragmentation
Table 2 shows the mean mass of filtered FF weighed using a microbalance readable to 0.0001 g (0.1 mg, as recommended in TM212-2021 and ISO 4484-1:2023) and on a higher precision ultra-microbalance readable to 0.0001 mg (ISO recommends “resolution of at least 0.1 mg”). Mean values quoted for the filtered fragments were calculated as the mean difference between the pre-filtration mass of the blank filter (after rinsing and drying) and post-filtration and drying as specified in the AATCC and ISO test methods. The seeded FF mass is the mean oven-dry mass of yarn before it was cut into fragments and suspended in water ready for filtration.
Table 2. Mass of fibre fragments (in mg) weighed using AATCC/ISO standard microbalance (Sartorius A210P) readable to 0.0001 g (0.1 mg) and higher than standard ultra-microbalance (Satorius SC2) readable to 0.0001 mg

Table 2 shows that the mean mass of filtered FF was lower than the initial mass of seeded FF in all cases. Table 2 also shows that filters lost mass in the rinsing process when no fibre fragments were present (0 mm/0 mg fibre seeded). Therefore, for filters with some seeded FF, some of the mass lost was due to filters losing mass with each rinse cycle (in a separate experiment we tested five rinse cycles and the blank filter mass reduced each time, with peak mass loss on the third rinse) and some may have been lost due to FF getting stuck (microscopically) on scissor blades, tweezers, or in and around the jar and filter equipment used to contain them. These reductions in fragment mass were statistically significant for all but one of the ultra-microbalance measurements (2 mm oven-dried was not statistically different at 95% confidence level). However, this reduction in fragment mass was only statistically significant for two-thirds of oven-dry filter measurements and one-third of conditioned filter measurements weighed on the microbalance recommended by the AATCC (and minimum recommended by ISO). This lack of statistical significance in the lower precision measurements is believed to be due to the low relative accuracy and high relative variability of measurements in relation to the masses measured on the microbalance. The standard microbalance was recording these tiny masses at the limits of its precision, recording only one significant digit, for example, 0.2 mg measurement read as 0.0002 g on the microbalance. Despite the high variability in the mass measured, the filtered FF mass correlated to the mass of seeded FF mass at 99% significance regardless of the balance or drying method used.
Table 2 also shows that the microbalance recommended in the AATCC/ISO standards recorded a mean of 0 mg FF when 2 mm of yarn had been cut into ≈190 fragments. Thus, readings of ‘zero’ fibre fragments using this gravimetric method are misleading. Further, the variability in mass recorded was very high, particularly for the measurement of lower, but significant, fibre fragmentation masses. The standard deviation was frequently 50–100% of the mean mass when measured on the standard microbalance. The measurements taken on the higher precision ultra-microbalance had relatively high, but significantly lower variability than the standard microbalance demonstrating that balance resolution was to blame for more of the mass measurement variability than the test method or specimens. We conclude from this that gravimetric measurement of fibre fragmentation is only advisable when using balances with higher resolution than 0.1 mg. However, sourcing a balance capable of measuring to resolutions of 0.0001 mg with a measuring pan more than 47 mm is very challenging (our measuring pan was 20-mm diameter and therefore not recommended for these measurements, but as the results show the variability was comparatively low).
The AATCC method also suggests reporting the fragment mass as a percentage of the specimen mass to the same number of significant digits as the mass itself. We did this on a nominal mid-weight fabric of 225 g−2 and for the microbalance measurements, this gave our specimens mean fragmentation rates of 0.001% for 4 and 8 mm total fragmented yarn length, 0.003% for 16-mm yarn and 0.005 or 0.006% for oven-dried or conditioned specimens of 32-mm yarn, respectively. As already discussed, 2 mm (≈190 FF) was unmeasurable on 0.1 mg resolution balances and filters without any FF weighed less after filtration with pure water than pre-filtration.
Thus, the problems with gravimetric quantification of FF extended beyond long drying times, and associated energy use. Negative mass values for blank filters should be accounted for if the total FF mass reported is to be accurate. High inherent measurement variability at low FF quantities, could be problematic during fabric development and selection, and false zero masses could be misleading to both brands and consumers.
Grading fibre fragmentation using photographs of filters against a photographic scale
All observers who ranked our FFS J grade images were able to rank them perfectly and quickly in order from most to least fibre presence. Therefore, all qualified to do the grading test using scale J.
Table 3 shows that the mean grade assigned to images of replicate test specimens by four sets of three independent observers were all within half a grade of the correct grade. The correlation between the mean grades assigned by each set of observers and the correct grade, total length of fibre fragments and mean mass of fibre fragments present on the filters was statistically significant at 99% confidence in every case.
Table 3. Mean FFS version J grades assigned to printed photographs of four replicate specimen filters (by four sets of three independent observers), compared to ‘correct grade’ and total length of fragmented yarn

Table 3 shows that the variability in the individual grades contributing to the mean result was significantly lower for all four sets of observer gradings than the variability in fragment mass measurements at lower fibre fragment prevalence. Every observer graded all replicate filters with no fibre presence as grade 5 (no fragments present) and every observer graded any filter with 2 mm/≈190 seeded fragments as either 4 or 4.5 demonstrating that they could distinguish between filters with no FF and those with very small numbers of fragments. When we compare this to the mean fragment mass method recommended in AATCC TM212-2021 (and ISO 4484-1:2023), Table 3 also shows that the microbalance measuring to 0.1 mg resolution was not capable of consistently registering the presence of small quantities of fibre fragments and this is problematic because it could validate brands claiming ‘zero fibre fragmentation’ when there could be enough FF to be visible to the naked eye, as shown in Figure 1. Conversely, our human observers appeared to find it easier to accurately grade smaller quantities of FF associated with grades 4 and 4.5 than higher levels associated with grades 1 or 2. Further, our human observers were significantly more consistent than the 0.1 mg precision microbalance up to and including grade 3, weighing approximately 0.2 mg.
There was no statistically significant difference in the mean grades assigned by four different sets of observers to test specimens graded 1, 2, 3, 4 or 5. However, all public observers using natural daylight, and industry observers, correctly graded all specimens seeded with 2 mm/0.0702 mg yarn as grade 4.5. The lack of variance in their results meant that their grades were statistically significantly higher than those assigned mean grade 4.3 by the two sets of observers using D65 light. Other than this, the background of the observers had no significant impact on the results. However, some individual observers appear to have a particular talent for accurate matching while others find it more difficult.
A minority of observers, evaluating earlier FFS versions (that are not reported here), were unable to complete the ranking test accurately and these observers also gave some grades that were at odds with most other observers. A minority of people (approximately 10%) appear to see the filter images/fibre fragments quite differently to most observers. However, there was no statistically significant correlation between prior experience of visual grading and accuracy in ranking or grading our samples. Therefore, we recommend training observers using the ranking test and a series of seeded specimen grading tests, and only using observers who demonstrate accuracy in the training exercise for actual specimen gradings to ensure consistency and accurate gradings.
The use of a D65 light source/light box compared to bright natural daylight had no significant impact on the mean results obtained in this or earlier tests (not reported here). Using natural daylight could reduce the costs associated with this test method for laboratories without a light box. However, using the light box gave us the flexibility to undertake tests at any time and regardless of light/weather conditions.
Grading real filters against the FFS
Table 4 shows that the mean fibre fragmentation grade assigned to real filters (held in glass Petri dishes) and photographs of the same filters, by the same six observers (assigned to set 1 or 2), were all within half a grade of the correct grade. There was no statistically significant difference between the mean grades given to real filters, compared to photographs of real filters, with one exception. One observer in set 1 graded the grade 5 specimens as 4.5 and while they did so they commented that the blank filters did not look identical to the grade 5 scale images because the (real) filters had ridges that were “possibly just the filter and not actually fibres”. Since observers had been instructed that grade 5 should only be given if there were absolutely no fibres present, and the texture difference between the filters and grade 5 image could conceivably be fibres, this observer chose grades of 4.5 for the ‘blank’ filters. Other observers also commented on the difference in texture but selected grade 5 for all ‘blank’ filters.
Table 4. Mean FFS version J grades assigned to real test filters, by two sets of independent observers on four replicate test specimens, compared to the ‘correct grade’ and fragment mass, also grades assigned to printed photographs of the same filters by the same observers

This issue could be resolved in future work by printing the scale onto a material with the same texture as the filters or potentially printing directly onto filter membranes. Evaluations of earlier versions of our scale also demonstrated the importance of the printing techniques, papers used, background colour and fibre fragment distribution in facilitating easy, accurate and reproducible grading. Black backgrounds, free-cut FF and regular distribution of variable but broadly similar sized fragments all contributed to easy and reliable assignment of FF grades.
Genuine wash test filters have also been graded using earlier versions of our FFSs D–G with high levels of observer agreement (low variability) indicating that they are similarly effective to the results reported in this article.
Conclusions
Observers ranked the final FFS images in the correct order very quickly and easily. Ranking the scale images was an effective and efficient method of training new observers, and we recommend ranking a set of scale images and grading a set of seeded specimens before grading real test specimens. Any observer who cannot rank the grade images correctly and easily should not be responsible for grading any test specimens.
The FFS concept works effectively as a method of analysing the results of wash tests. Visual grading of test specimens has several advantages over the gravimetric methods recommended in AATCC method TM212-2021 and ISO 4484-1:2023:
-
1. High-throughput method: Grading test specimens takes less than 1 min per specimen for each observer on average, which is considerably quicker than gravimetric methods (minimum 490 min due to required drying times). Grading the recommended four replicate test specimens using three independent observers should take 10–12 min in total.
-
2. Cheap method: Grading test specimens requires no additional equipment or energy (e.g., for drying) beyond the wash testing and filtration equipment required for all fibre fragmentation wash tests. Use of a light box and D65 light source meant that grading could be done at any time of day but delivered similar results compared to grading undertaken in bright daylight. Purchasing a set of ranking and grading images need not be expensive, although the quality of image/print/paper was very important so we would recommend using a standardised FFS.
-
3. Easy access method: This method could be widely accessible to all researchers and industrial users due to its low cost, intuitive design, quick training of observers and ease of use.
-
4. Highly reproducible method: the mean grade, quickly and subjectively assigned, was more reproducible and less variable than the objective measurement of mass using a microbalance with 0.1 mg resolution, as recommended in AATCC TM212-2021 and minimum recommended in ISO 4484-1:2023, at low fibre presence (less than 0.3 mg).
-
5. FFS method is more accurate at detecting low fibre presence than minimum standard gravimetric methods: our observers consistently and reliably graded filters at grade 4.5 that would have (incorrectly) shown ‘zero’ fragmentation using AATCC and ISO Standard methods recommending the use of a microbalance with 0.1 mg, or at least 0.1 mg, resolution, respectively.
-
6. Promote effective communication of fibre fragmentation propensity to consumers: As our scale shows actual fibre fragments, it could be used to communicate the fragmentation propensity, or lack thereof, to potential consumers. Grade numbers could also be communicated to consumers if sufficient explanation were given. If this were desirable then it may be more logical for the grade numbers to be reversed so that increasing grade numbers indicated increasing fragmentation, or letters could be used (mimicking energy efficiency ratings for electrical goods) with an AA grade fabric exhibiting ‘acceptable/minimal’ fragmentation, for example, what we have shown here as grade 4.5.
Future work
Traditionally in the textile industry, a grade of 4, 4.5 or 5 on a visual scale would be considered ‘acceptable’ for a good quality product, while products achieving a grade of 3.5 or lower would be rejected or require material development to improve its performance. We have tested a range of different fragment numbers at each of our grades. Our results show that observers are very sensitive to small numbers of fibre fragments and could reliably make distinctions between ‘pass’ and ‘fail’ results. However, consensus should be reached between all stakeholders on what the ‘pass’ mark, or grade 4, should be. This should be followed by further development work to make that consensus visual scale on paper with similar texture to the filters.
Textiles are extremely variable with myriad variables introduced at each stage of production (fibre, yarn, fabric, colouration, finishing) and many of these will influence fibre fragmentation propensity. Consideration should be given to whether multiple scales, representing different standards should be available for different textile categories. For example, we would anticipate that faux fur and fleece fabrics would shed more fibres than a high-sett plain weave. Wide consultation would be necessary to establish whether there should be a scale for such high-shedding fabrics, or whether such fabrics should be phased out in the coming years based on the amount of fibre pollution they generate.
This article introduces the concept of using a 5-point scale to visually grade white filters with black fibre fragments. A high contrast between fibre and filter paper colours is likely to give more accurate results than lower contrasts. Future work should evaluate the method’s effectiveness for different coloured fibre fragments against white and black filter papers.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/plc.2024.30.
Data availability statement
The data summarised in this article are available on request from the authors.
Acknowledgements
Sincere thanks to all our observers, including participating employees of Lochcarron of Scotland, CGG and Helly Hansen.
Author contribution
S.M. and L.M. planned the work and manuscript. S.M. drafted the introduction, prepared all test specimens and FFS, collected and collated most of the data. L.M. collected data from the industry observers reported here, drafted the method, results, discussion and conclusions. Both authors edited the manuscript and approved the final version for publication.
Financial support
This work was supported by a Heriot-Watt University James Watt Scholarship (2020-3).
Competing interest
The authors declare none.








Comments
Dear Steve, Cressida and Ben
Many thanks again for organising such an inspiring conference. As discussed, please find attached our paper introducing and evaluating a new method of quantifying fibre fragmentation from textiles: Low cost, high throughput quantification of microplastics released from textile wash tests: Introducing the Fibre Fragmentation Scale
This paper describes the results of evaluating known quantities of fibre fragments on filter papers. Fibre fragments were seeded into ‘effluent’ and filtered following standard methods. Our results show that this method is more accurate at the critical pass/fail decision point when compared to standard gravimetric methods and it is much quicker, uses less energy and requires no additional specialist equipment. This paper is a development from the work that we presented at your conference and responds to recent publication of standard test methods. We have consulted with 3 companies, all of whom see potential in this method. Issam Yousef and his R&D team at Helly Hansen in Norway and Olawale Ibrahim at CGG in Wales used our method to evaluate the scale before the one we have reported here (this was done remotely). We undertook all tests reported here in person to ensure identical processes and so have only reported results of ‘Industry observations’ for local company, Lochcarron of Scotland.
Both authors made significant contributions to all aspects of the paper. The concept was originally mine and I was allocated a PhD Scholarship from Heriot-Watt University to pursue this research. Sophia was awarded the scholarship and has undertaken almost all the practical work. We work closely together in a genuine collaboration. We are delighted to be able to submit this paper to you and hope that you like it.
This work has not previously been published, AI was not used in any capacity in this research or paper preparation, and we have no conflicts of interest to declare. There is no funding beyond the scholarship to fund this work.
Yours sincerely
Lisa Macintyre PhD
Associate professor of textiles and School Director of Doctoral studies
I am the corresponding author for the paper: [email protected]