The associations of diet quality with the risk of non-communicable chronic diseases are well documented. It is estimated that about 11 million deaths in 2017 were attributable to dietary factors(Reference Afshin, Sur and Fay1). Accordingly, high Na intake and low intake of whole grains and fruits are the main worldwide dietary risk factors(Reference Afshin, Sur and Fay1). Several meta-analyses of prospective cohort studies have documented that higher and/or lower intakes of healthy/unhealthy dietary components are associated with the risk of CVD(Reference Bechthold, Boeing and Schwedhelm2,Reference Schwingshackl, Schwedhelm and Hoffmann3) , type 2 diabetes (T2D)(Reference Schwingshackl, Hoffmann and Lampousi4), total and site-specific cancers(5) and mortality(Reference Schwingshackl, Schwedhelm and Hoffmann6).
Besides studying the association of single dietary components with the risk of chronic diseases, dietary pattern analysis has been introduced to reflect the overall quality of the diet and investigate the association of individual’s dietary habits and preferences with disease risk(Reference Schwerin, Stanton and Riley7–Reference Wirfält and Jeffery10). Dietary pattern analysis takes interactive and synergistic effects of dietary components into account(Reference Hu11) and accounts for cumulative effects of risk-increasing and/or risk-decreasing dietary components(Reference Sacks, Obarzanek and Windhauser12). In this method, empirical dietary patterns are explored by pattern-based analytic methods (e.g. factor analysis, unsupervised cluster analysis, etc.), and investigating their association with non-communicable chronic diseases risks may serve as a complementary approach in parallel with investigating the association of single foods or nutrients with disease risks.
The associations of empirical dietary patterns with the risk of non-communicable chronic diseases have been widely investigated. There is evidence that healthy or unhealthy dietary patterns are associated with the risk of the metabolic syndrome(Reference Shab-Bidar, Golzarand and Hajimohammadi13) T2D(Reference Maghsoudi, Ghiasvand and Salehi-Abargouei14), CVD(Reference Zhang, Shu and Si15) and site-specific cancers(Reference Alizadeh, Djafarian and Alizadeh16,Reference Alizadeh, Shab-Bidar and Mohtavinejad17) . However, the interpretation of the results may have been limited by inclusion of cross-sectional, case–control and retrospective cohort studies. Retrospective and cross-sectional studies are subject to recall and selection biases and as a result do not present reliable evidence. In addition, the strength of the evidence obtained by published meta-analyses has not been adequately assessed. Besides studying the association of dietary pattern with disease risk, it seems necessary to evaluate the quality of the evidence and determine whether the results of the published meta-analyses are unbiased.
Umbrella review studies have emerged as an attempt to present a wide picture of published meta-analyses on a specific topic(Reference Aromataris, Fernandez and Godfrey18). Umbrella reviews search and present the results of the published meta-analyses, assess the methodological quality and evaluate the quality of the evidence and the accuracy of the estimates obtained by published meta-analyses. Thus, to present a wide and accurate picture of the association of overall diet quality with disease risk, we aimed to perform an umbrella review of published meta-analyses of prospective observational studies on the association of empirical dietary patterns with the risk of non-communicable chronic diseases including CVD, T2D, site-specific cancers and neurological disorders as well as all-cause and cause-specific mortality. There is no available meta-analysis of interventional studies on empirical dietary patterns and disease risk, and therefore, we did not include interventional studies in this review.
Methods
Systematic search
Two authors (S. S. and A. J.) performed an independent systematic literature search according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement(Reference Moher, Liberati and Tetzlaff19). We searched PubMed and Scopus databases up to September 2019 to find meta-analyses of prospective cohort studies evaluating the association of empirical dietary patterns with the risk of non-communicable chronic diseases. The systematic search was completed by screening of the reference lists of all relevant reviews and meta-analyses. The set of keywords used for the systematic search is presented in online Supplementary Table S1.
Selection of meta-analyses
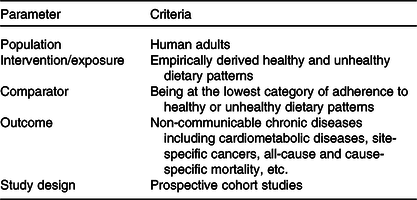
Inclusion and exclusion criteria were defined according to the population, intervention/exposure, comparator, outcome and study design framework (Table 1). For the purpose of the present umbrella review, we selected published meta-analyses with the following criteria: (1) meta-analyses of observational studies that were conducted in the general population aged 18 years or older; (2) assessed dietary intakes by established dietary assessment tools (e.g. FFQ, diet history, 24 h dietary recalls and dietary records); (3) reported empirically derived dietary patterns identified by pattern-based analytic methods such as factor analysis, principal component analysis, cluster analysis or reduced rank regression as exposure; (4) considered the incidence of non-communicable chronic diseases including T2D, CVD, neurological disorders, total and site-specific cancers and all-cause and cause-specific mortality as outcome and (5) reported multivariable adjusted summary risk estimates and their corresponding 95 % CI. Primary studies, studies with no summary risk estimate (e.g. narrative reviews and systematic reviews without meta-analysis) and meta-analyses that only had one primary prospective cohort study were excluded. If more than one published meta-analysis on the same association was identified, we selected the one with the largest number of primary prospective cohort studies(Reference Jayedi and Shab-Bidar20,Reference Neuenschwander, Ballon and Weber21) . In general, the meta-analysis with the largest number of primary prospective cohort studies included the same primary studies as meta-analyses including fewer studies, with one or more additional recent primary cohort studies. Therefore, we selected the one with the largest number of primary prospective cohort studies to include more evidence in this review.
Table 1. Population, intervention/exposure, comparator, outcome, and study design criteria for inclusion and exclusion of studies
Data extraction
Two authors (A. J. and S. S.) independently extracted the following data from eligible meta-analyses: first author’s name, publication year, exposure, number of primary studies and number of participants/cases. We also extracted the following data from primary studies included in each meta-analysis: first author’s name, number of participants/cases, statistical model used for identifying healthy and unhealthy dietary patterns, dietary factors contributed to identified healthy and unhealthy dietary patterns, multivariable relative risks that controlled for the maximum number of confounders and their 95 % CI, and confounding variables that were included in that model. Disagreement was resolved by consensus.
Assessment of methodological quality and quality of evidence
Two independent authors (A. J. and S. S.) performed quality assessments. Disagreements were resolved by consensus between the two authors. To evaluate the methodological quality of published meta-analyses, we used the validated AMSTAR tool(Reference Shea, Grimshaw and Wells22,Reference Shea, Hamel and Wells23) . This tool is a useful tool to evaluate the quality of conduct of each published meta-analysis. The score ranges from 0 to 11, and accordingly, meta-analyses with 8–11, 4–7 and ≤3 points were considered high, moderate and low quality, respectively(Reference Sharif, Janjua-Sharif and Ali24). The quality of the evidence obtained by each published meta-analysis was assessed by the NutriGrade score(Reference Schwingshackl, Knüppel and Schwedhelm25). For the purpose of the present review, we used a modified version of this scale(Reference Neuenschwander, Ballon and Weber21). This score considers the following components to evaluate the quality of the evidence obtained by published meta-analyses: (1) risk of bias, study quality or study limitations; (2) precision of the estimate; (3) heterogeneity; (4) directness; (5) publication bias; (6) funding bias; (7) effect size and (8) dose–response association. The score ranges from 0 to 10. Accordingly, the quality of the evidence was categorised as follows:
-
Very low (0–3·99): There is very low confidence in the effect estimate; meta-evidence is very limited and uncertain.
-
Low (4–5·99): There is low confidence in the effect estimate; further research will provide important evidence on the confidence and likely change the effect estimate.
-
Moderate (6–7·99): There is moderate confidence in the effect estimate; further research could add evidence on the confidence and may change the effect estimate.
-
High (8–10): There is high confidence in the effect estimate, and further research probably will not change the confidence in the effect estimate.
Statistical analysis
For each outcome, we extracted multivariable relative risks that controlled for the maximum number of confounders and their 95 % CI from each primary prospective cohort study that was included in selected meta-analyses. Then, we recalculated summary relative risk (SRR) and its corresponding 95 % CI by using the DerSimonian and Laird random-effects model(Reference DerSimonian and Laird26). Some of the published meta-analyses used a fixed-effects model to combine primary relative risks. Thus, this method helps to present comparable SRR across different outcomes(Reference Neuenschwander, Ballon and Weber21). In addition, this method provided sufficient information for quality assessment (including τ 2, I 2 and publication bias).
Some of the primary studies included in selected meta-analyses reported sex-specific effect sizes only, and in such cases, some of the included meta-analyses considered these studies as two separate studies. In such cases, we combined sex-specific estimates using a fixed-effects model and included the combined effect size for our analyses.
To recalculate SRR for each meta-analysis, primary studies with the following criteria were excluded (1) cross-sectional, case–control and retrospective cohort studies; (2) baseline cross-sectional evaluations within prospective cohort studies; (3) studies with unadjusted risk estimates; (4) studies that were not conducted in the general population such as studies that were conducted in patients with CVD, cancer or other diseases, studies that assessed dietary patterns during pregnancy (e.g. studies that evaluated postpartum depression) and studies that were conducted among heavy athletes (e.g. studies that assessed fracture risk in cross-country runners); (5) studies that used index-based dietary patterns as exposure (e.g. considered healthy eating index as healthy diet) and (6) studies that assessed the association of specific dietary components with the risk of chronic disease (e.g. fruits and vegetables or whole grains as exposure). We excluded primary studies listed under points 1–6 from each meta-analysis and then recalculated risk estimates with the use of a random-effects model. This approach helped to ensure that all primary studies were conducted in the general population, used posteriori-defined dietary patterns as exposure and had prospective observational design. With the use of this approach, we were able to present comparable SRR across different outcomes. For each meta-analysis, we evaluated between-study heterogeneity by using the I 2 statistic and its 95 % CI(Reference Higgins, Thompson and Deeks27). Because I 2 is dependent on the study size, we also calculated τ 2, which is independent of study size(Reference Riley, Higgins and Deeks28). Potential publication bias was assessed with the use of Egger’s test(Reference Egger, Smith and Schneider29). All analyses were conducted with STATA software, version 13 (Stata Corp).
Results
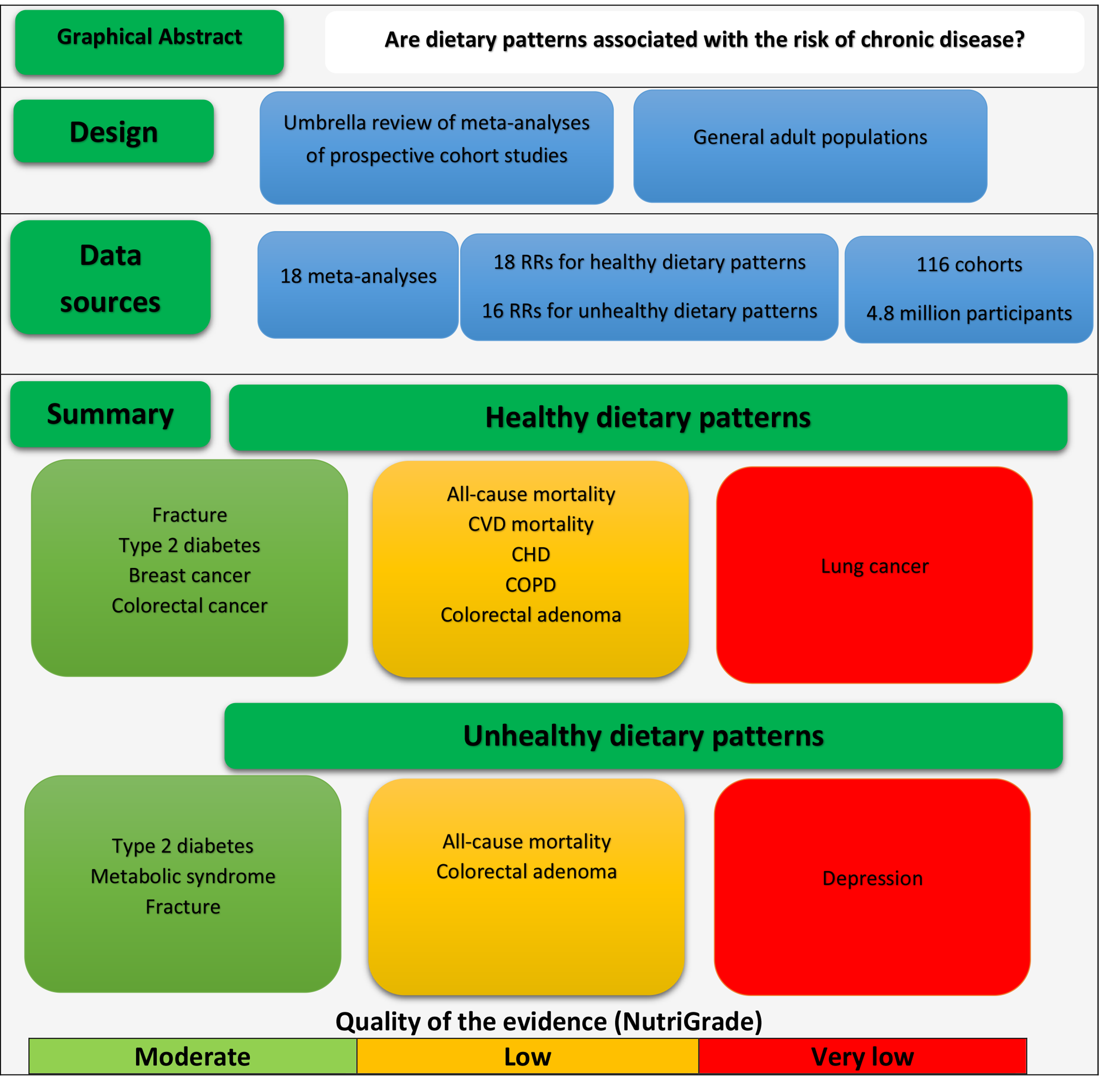
We identified 916 records through database searching. We reviewed titles and abstracts of all retrieved articles, and eventually, seventy articles were fully reviewed for eligibility. Of those, sixteen meta-analyses, reporting eighteen SRR for healthy dietary patterns and sixteen SRR for unhealthy dietary patterns obtained from 116 primary prospective cohort studies with 4 801 734 participants, were considered eligible for the analyses(Reference Alhazmi, Stojanovski and McEvoy30–Reference Zheng, Shu and Si45). Reasons for excluding studies are presented in Fig. 1, and a list of studies excluded by full-text assessing is provided in online Supplementary Table S2.

Fig. 1. Literature search and study selection process. COPD, chronic obstructive pulmonary disease.
Characteristics of the studies included in the umbrella review
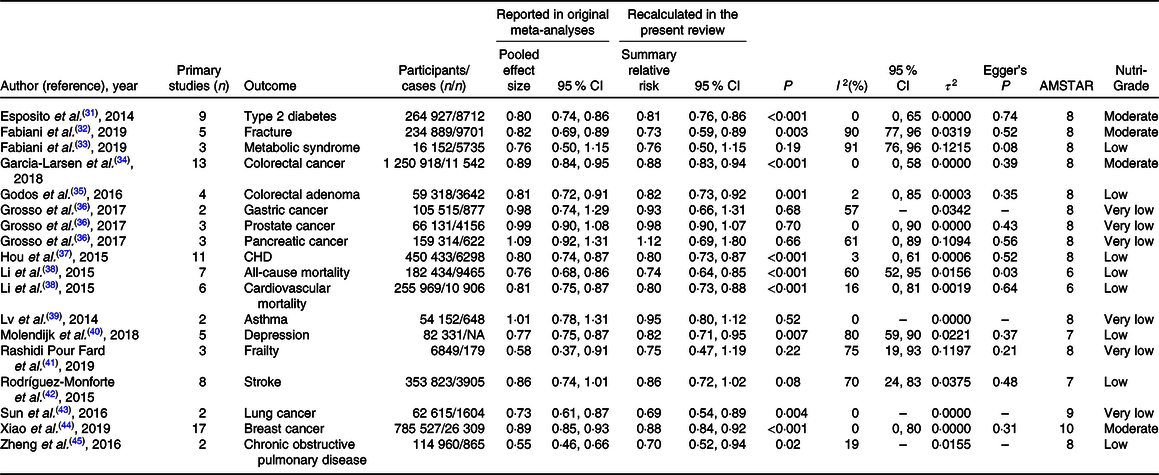
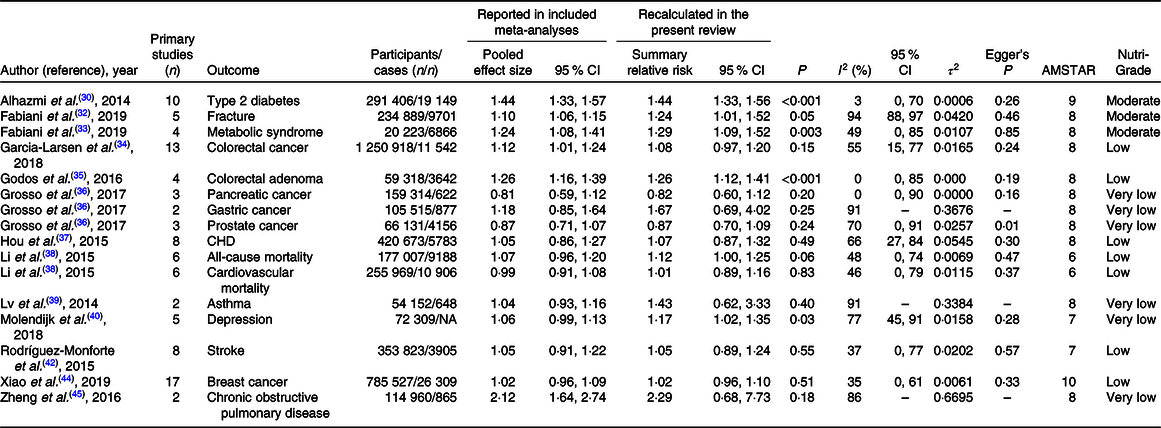
The systematic search identified six published meta-analyses for T2D, four meta-analyses for CHD, colorectal cancer (CRC), depression and pancreatic cancer, three for stroke and gastric cancer and two meta-analyses for fracture, metabolic syndrome and prostate cancer. For other outcomes, only one published meta-analysis was available. If more than one published meta-analysis on the same association was identified, we included the one with the largest number of primary prospective cohort studies. Included meta-analyses assessed the following outcomes in their analyses: all-cause and CVD mortality(Reference Li, Hou and Chen38), CHD(Reference Hou, Li and Wang37), stroke(Reference Rodriguez-Monforte, Flores-Mateo and Sanchez42), T2D (healthy pattern)(Reference Esposito, Chiodini and Maiorino31), T2D (unhealthy pattern)(Reference Alhazmi, Stojanovski and McEvoy30), fracture(Reference Fabiani, Naldini and Chiavarini32), depression(Reference Molendijk, Molero and Ortuno Sanchez-Pedreno40), chronic obstructive pulmonary disease (COPD)(Reference Zheng, Shu and Si45), asthma(Reference Lv, Xiao and Ma39), frailty(Reference Lv, Xiao and Ma39), metabolic syndrome(Reference Fabiani, Naldini and Chiavarini33), colorectal adenoma(Reference Godos, Bella and Torrisi35), lung cancer(Reference Sun, Li and Li43), breast cancer(Reference Xiao, Xia and Li44), CRC(Reference Garcia-Larsen, Morton and Norat34) and prostate, pancreatic and gastric cancers(Reference Grosso, Bella and Godos36). For lung cancer(Reference Sun, Li and Li43) and frailty(Reference Rashidi Pour Fard, Amirabdollahian and Haghighatdoost41), there was only one available meta-analysis that reported SRR for healthy patterns. We did not find any meta-analysis on the association of unhealthy patterns and the risk of frailty and lung cancer. The general characteristics of included meta-analyses for the relation of healthy and unhealthy dietary patterns and the risks of chronic diseases are presented in Tables 2 and 3, respectively.
Table 2. General characteristics of the published meta-analyses of healthy dietary patterns, methodological assessment (AMSTAR) and assessment of quality of the evidence (NutriGrade)

NA, not available.
Table 3. General characteristics of the published meta-analyses of unhealthy dietary patterns, methodological assessment (AMSTAR) and assessment of quality of the evidence (NutriGrade)

NA, not available.
The magnitude and direction of the associations were similar among meta-analyses with the same outcomes. The exception was the metabolic syndrome, for which a published meta-analysis found no significant association in the subgroup of cohort studies(Reference Shab-Bidar, Golzarand and Hajimohammadi13), but a more recent meta-analysis with three new studies found a significant association(Reference Fabiani, Naldini and Chiavarini33). A list of meta-analyses excluded by full-text assessing is provided in online Supplementary Table S2.
Most of the published meta-analyses combined prospective, cross-sectional and retrospective studies in their analyses. We identified one published meta-analysis for obesity, hypertension and endometrial and ovarian cancers (online Supplementary Table S2) that included only one prospective cohort study in their analyses. Thus, these meta-analyses were not included in our review. There were ≥10 primary studies available for the analyses of T2D, CHD, CRC and breast cancer, 5–9 studies for fracture, depression, stroke and all-cause and CVD mortality and <5 studies for the analyses of COPD, colorectal adenoma, asthma, frailty, metabolic syndrome, and pancreatic, prostate, gastric and lung cancers.
All primary studies included in the published meta-analyses reported multivariable relative risks. Of the 116 primary prospective cohort studies included in the eligible meta-analyses, 95 % (n 110) controlled for age, 76 % (n 88) controlled for sex, 93 % (n 108) for smoking status, 90 % (n 104) for BMI and energy intake, 78 % (n 91) for physical activity, 64 % (n 74) for educational status and 39 % (n 45) for alcohol intake in their multivariable analyses. Only 49 % (n 57) accounted for family history of the disease assessed as outcome in that study.
Characteristics of dietary patterns
We reviewed dietary components that contributed to healthy and unhealthy dietary patterns that were identified in each primary prospective cohort study. Of the 116 primary prospective cohort studies included in this review, 109 studies used principal component analysis or factor analysis, four studies used reduced rank regression and three studies used cluster analysis to explore dietary patterns. The most frequent terms used for healthy patterns were healthy (54 %), prudent (28 %) and fruit and vegetables (12 %). The most frequent terms used for unhealthy patterns were unhealthy (45 %), Western (22 %), high fat (8 %), traditional (6 %), animal pattern (5 %) and sweets and fats (2 %). The most frequent foods that contributed to healthy dietary patterns in the 116 primary prospective cohort studies were as follows: vegetables (83 %, n 96), fruits (73 %, n 85), fish and seafoods (53 %, n 62), whole grains (40 %, n 46), low-fat dairy products (22 %, n 26), poultry (22 %, n 26), soya (20 %, n 23), legumes (18 %, n 21), olive oil (16 %, n 19), nuts and seeds (15 %, n 17) and beans (9 %, n 10). The most frequent foods that contributed to unhealthy dietary patterns were red and processed meat (86 %, n 100); refined grains (49 %, n 57); French fries (45 %, n 52); high-fat dairy products (33 %, n 38); sweets (30 %, n 35); desserts (24 %, n 28); egg (21 %, n 24); sugar-sweetened beverages (16 %, n 19); butter (16 %, n 19); pizza, poultry and snacks (11 %, n 13); animal fat (10 %, n 12) and cakes and biscuits (8 %, n 10).
Methodological quality
The overall AMSTAR scores for each meta-analysis are presented in Tables 2 and 3, and detailed scores are presented in online Supplementary Table S3. All included meta-analyses had a score of ≥6. Of the sixteen included meta-analyses, thirteen meta-analyses were conducted with a high-quality approach (≥8 points) and other three ones were performed with a moderate quality method (6 and 7 points). The main reasons for lower quality scores were due to the fact that included meta-analyses did not provide a list of excluded studies and did not consider the scientific quality of evidence in preparing their conclusions and recommendations (online Supplementary Table S3).
Quality of evidence
Of the thirty-four SRR reported in this review, there was statistically significant association for 47 % of the associations (n 16). The quality of the evidence was rated moderate for 21 % of the associations (n 7), low for 44 % of the associations (n 15) and very low for 35 % of the associations (n 12). All SRR for which the quality of the evidence was rated moderate had statistically significant associations. For SSR for which the quality of the evidence was rated low (n 15), there was significant association for eight SSR. For SRR with very low quality of evidence (n 12), there was only three significant associations. The overall NutriGrade scores for each meta-analysis are provided in Tables 2 and 3.
Healthy dietary patterns and chronic disease
For healthy dietary patterns, there was moderate-quality evidence that healthy dietary pattern was associated with a lower risk of T2D (SRR 0·81, 95 % CI 0·76, 0·86). This was also the case for fracture, breast cancer and CRC (Table 4). We also found significant inverse associations for CHD, COPD, colorectal adenoma, depression and all-cause and CVD mortality, but the quality of the evidence was rated low. Healthy dietary pattern was not associated with the risk of stroke. For other outcomes, there were no significant associations, and the quality of the evidence was rated very low (Table 4).
Table 4. Quality of the evidence for association between healthy dietary patterns and the risk of non-communicable chronic diseases (Summary relative risks and 95 % confidence intervals)
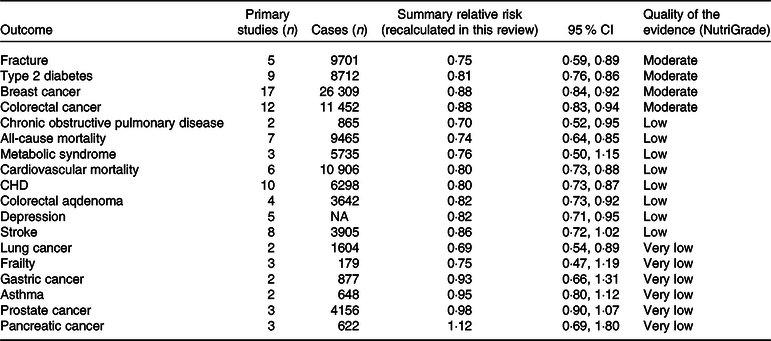
NA, not available.
Unhealthy dietary patterns and chronic disease
The results for unhealthy dietary patterns are presented in Table 5. We found moderate-quality evidence that unhealthy dietary patterns significantly increased the risk of T2D, fracture and the metabolic syndrome. There were also significant associations between unhealthy patterns and the risk of colorectal adenoma, all-cause mortality and depression, but the quality of the evidence was rated low, low and very low, respectively. For other outcomes, there were no significant associations. Detailed NutriGrade scores for each outcome are provided in online Supplementary Table S4.
Table 5. Quality of the evidence for association between unhealthy dietary patterns and the risk of non-communicable chronic diseases (Summary relative risks and 95 % confidence intervals)
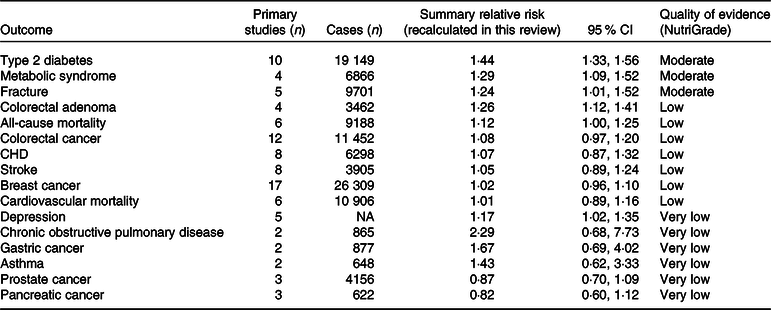
NA, not available.
Heterogeneity and publication bias
The results for publication bias are presented in Tables 2 and 3. There was evidence of publication bias in the analyses of healthy dietary pattern and the risk of all-cause mortality and the metabolic syndrome. This was also the case for unhealthy pattern and prostate cancer. In the analyses of COPD, asthma and gastric and lung cancers, there were only two available studies, and thus, we were unable to test the potential for publication bias. For other outcomes, there was no evidence of publication bias. There was low evidence of between-study heterogeneity in the analyses of healthy diet and the risk of T2D, CRC, CHD, CVD mortality, asthma and prostate and lung cancers. For unhealthy patterns, there was low evidence of heterogeneity for T2D, colorectal adenoma and pancreatic cancer.
Discussion
This umbrella review evaluated the evidence obtained by published meta-analyses of prospective cohort studies on the association between empirically derived healthy and unhealthy dietary patterns and the risk of non-communicable chronic diseases. We found moderate-quality evidence for the inverse association between healthy diets and the risk of T2D, fracture and colorectal and breast cancers. This was also the case for the positive association of unhealthy dietary patterns with the risk of T2D, fracture and the metabolic syndrome. There was low-quality evidence for the inverse association between a healthy diet and the risk of COPD, CHD, colorectal adenoma, depression and all-cause and CVD mortality, as well as for a positive association between unhealthy dietary pattern and the risk of colorectal adenoma, and depression. For other outcomes, there were no significant associations, and the quality of the evidence was rated low or very low.
Dietary pattern analysis was introduced as a complementary approach for investigating diet–disease associations(Reference Slattery46). There is convincing evidence that higher or lower intake of several food groups are associated with the risk of non-communicable chronic diseases including T2D(Reference Schwingshackl, Hoffmann and Lampousi4), CVD(Reference Bechthold, Boeing and Schwedhelm2) and mortality(Reference Schwingshackl, Schwedhelm and Hoffmann6). This method accounts for risk-decreasing and risk-increasing dietary components existed in individual’s diet and therefore presents a wide overview of the potential effects of individual’s dietary habits on disease risks.
The results demonstrated that vegetables, fruits, fish, whole grains, low-fat dairy products, poultry, soya, legumes, olive oil, nuts, beans and seeds are the most frequent constituents of healthy dietary patterns across the world, respectively. In contrast, red and processed meat, French fries, refined grains, high-fat dairy products, sweets, deserts, egg, sugar-sweetened beverages, butter, snacks, cakes, biscuits and pizza are the most frequent constituents of unhealthy dietary patterns, respectively. According to the current guidelines, healthy eating pattern has been defined as a diet rich in vegetables, fruits, fish, whole grains and low-fat dairy products and low in red and processed meat, refined grains and sugar-sweetened beverages(47,48) . Our results also confirmed current evidence that higher intake of ultra-processed foods such as sugar-sweetened beverages, sweets, snacks, processed meat and desserts are associated with a higher risk of CVD and cancer(Reference Fiolet, Srour and Sellem49,Reference Srour, Fezeu and Kesse-Guyot50) .
However, some important issues should be noted. We included cohort studies that used empirically derived dietary patterns as exposure. Dietary pattern analysis takes interactive and synergistic effects of dietary components into account. Therefore, the totality of food groups/nutrients, and not a single a food or nutrient, is the basis of the pattern. The definition of healthy and unhealthy dietary patterns, contribution and the factor loadings of food groups in a given dietary pattern may differ across primary studies(Reference Salari-Moghaddam, Larijani and Esmaillzadeh51). Healthy dietary patterns had different components with different factor loadings across primary prospective cohort studies included in each meta-analysis. In addition, the classification of a particular food or food group as healthy or unhealthy is not always clear-cut given that the scientific evidence in the primary studies may disagree or the evaluation of the findings by different researchers may differ. For example, dairy products may be considered beneficial for certain populations or in the context of particular diseases but not when evaluated in a different setting.
We evaluated the methodological quality of included meta-analyses by using a validated AMSTAR tool(Reference Shea, Grimshaw and Wells22,Reference Shea, Hamel and Wells23) . The results indicated that of the sixteen published meta-analyses included in the present review, thirteen meta-analyses were performed with a high-quality method (AMSTAR score ≥8) and other three ones were conducted with moderate quality approaches (AMSTAR scores 6 and 7). As mentioned earlier, the main reasons for lower quality scores were due to the fact that included meta-analyses did not provide a list of excluded studies and did not consider the scientific quality of the evidence in preparing their conclusions and recommendations. All meta-analyses assessed the quality of primary studies included in their analyses, but only two meta-analyses reported the quality of the evidence in the abstract or conclusions sections, or considered the quality of primary studies in the subgroup analyses(Reference Alhazmi, Stojanovski and McEvoy30,Reference Garcia-Larsen, Morton and Norat34) .
All meta-analyses included in this review were performed with high and moderate quality approaches, as assessed by AMSTAR score. However, the AMSTAR evaluates the quality of conduct of each published meta-analysis and do not assess the quality of the evidence. Being at high quality by AMSTAR score does not reflect the quality of the evidence. Meta-analyses may be high quality by AMSTAR and may present moderate-, low- or very low-quality evidence.
Of the sixteen meta-analyses included in this review, eleven meta-analyses(Reference Esposito, Chiodini and Maiorino31–Reference Fabiani, Naldini and Chiavarini33,Reference Godos, Bella and Torrisi35,Reference Hou, Li and Wang37,Reference Li, Hou and Chen38,Reference Rashidi Pour Fard, Amirabdollahian and Haghighatdoost41–Reference Zheng, Shu and Si45) used the Newcastle–Ottawa Scale(Reference Stang52) to assess the quality of primary studies, one meta-analysis used the American Dietetic Association Quality Criteria Checklist(53), one meta-analysis used the National Institute for Clinical Excellence methodological checklist for cohort and case–control studies(Reference Garcia-Larsen, Morton and Norat34), one published meta-analysis used its own checklist(Reference Molendijk, Molero and Ortuno Sanchez-Pedreno40) and one used the standardised critical appraisal instrument from the JBI Meta-Analysis of Statistics Assessment and Review Instrument(Reference Alhazmi, Stojanovski and McEvoy30). Only one published meta-analysis assessed the overall quality or strength of the evidence by a validated tool(Reference Godos, Bella and Torrisi35). Grosso et al.(Reference Grosso, Bella and Godos36) performed a comprehensive systematic review and meta-analysis of observational studies on the association of posteriori-defined dietary patterns with total and site-specific cancer risk. They used the modified Joint World Health Organization–Food and Agriculture Expert Consultation criteria for evidence in nutrition(54) to rate the strength of the evidence. In the present umbrella review, we used the NutriGrade score to rate the quality of the evidence(Reference Schwingshackl, Knüppel and Schwedhelm25). This score is a useful tool to judge the meta-evidence of randomised controlled trials and cohort studies in nutrition research(Reference Schwingshackl, Schwedhelm and Hoffmann3,Reference Schwingshackl, Hoffmann and Lampousi4,Reference Schwingshackl, Schwedhelm and Hoffmann6,Reference Neuenschwander, Ballon and Weber21,Reference Galbete and Schwingshackl55,Reference Schwingshackl, Hoffmann and Missbach56) and considers nutrition-specific aspects such as dietary assessment methods and diet-associated biomarkers(Reference Schwingshackl, Knüppel and Schwedhelm57).
In this review, there were seventeen and twelve available primary prospective cohort studies for breast cancer and CRC, respectively. However, the associations of healthy and unhealthy dietary patterns with the risk of cancers of other sites have not been well investigated. There were <5 primary prospective cohort studies available for prostate, lung, gastric and pancreatic cancers. In addition, one published meta-analysis assessed the association of diet patterns with the risk of renal cancer(Reference Alizadeh, Shab-Bidar and Mohtavinejad17), but they did not include prospective cohort study in their analysis. Four published meta-analyses of diet patterns and obesity(Reference Mu, Xu and Hu58), endometrial cancer(Reference Si, Shu and Zheng59), hypertension(Reference Wang, Shen and Liu60) and ovarian cancer(Reference Wang, Yao and Sun61) included only one prospective cohort study. There was also no available prospective cohort study for oral/pharyngeal cancers(Reference Grosso, Bella and Godos36). This was also the case for COPD and asthma, for which only two primary prospective cohort studies were available. Thus, further prospective cohort studies are needed to fully investigate the association of healthy and unhealthy dietary patterns with site-specific cancer risks.
We found moderate quality of evidence that healthy dietary patterns decreased the risk of T2D, CRC, fracture and breast cancer, with relatively sufficient number of studies available for the analyses. There was also low-quality evidence for the inverse association of healthy diets with the risk of CHD and all-cause and CVD mortality. There are several reasonable explanations which create a link between diet patterns and chronic diseases risk. There is convincing evidence that dietary habits can affect cardio-metabolic risk factors including blood pressure(Reference Hojhabrimanesh, Akhlaghi and Rahmani62,Reference Ndanuko, Tapsell and Charlton63) , lipid profile(Reference Shridhar, Satija and Dhillon64), insulin resistance(Reference Esmaillzadeh, Kimiagar and Mehrabi65), endothelial function(Reference Sijtsma, Meyer and Steffen66), oxidative stress(Reference Mirmiran, Hadavi and Mottaghi67) and systemic inflammation(Reference Silveira, Oliveira and Andrade68). In addition, a recent meta-analysis of cross-sectional studies suggested that higher adherence to healthy dietary patterns may decrease the risk of central fatness(Reference Rezagholizadeh, Djafarian and Khosravi69).
Our results indicated that both healthy and unhealthy dietary patterns were associated with the risk of depression. It is proposed that dietary habits can affect immune function and oxidative stress in the brain and thereby are related to depressive symptoms(Reference Schwingshackl, Hoffmann and Missbach56). Chronic systemic inflammation and mitochondrial dysfunction are the two additional diet-related biological mechanisms that are implicated in the development of depression and mental illness(Reference Galbete and Schwingshackl55). However, the interpretation of the results is limited by the low number of primary studies included in the analyses, high evidence of between-study heterogeneity and very low to low quality of the evidence. Thus, on the basis of the present results, there is no convincing evidence to relate dietary patterns to depression.
There exists limited evidence regarding the beneficial effect of dietary interventions, without any additional intervention, on depressive symptoms(Reference Francis, Stevenson and Chambers70–Reference McMillan, Owen and Kras72). Although meta-analyses of interventional studies suggested that improving diet quality may improve depressive symptoms in adults(Reference Firth, Marx and Dash73,Reference Opie, O’Neil and Itsiopoulos74) , in most of original trials, depressive symptoms were assessed as a secondary outcome, and many studies have compared the effect of two differing diets, or involved lifestyle change such as diet, exercise and sleep combined.
In this umbrella review, we found that healthy diets were associated with a lower risk of COPD, CRC, CHD, breast cancer and CVD mortality, but unhealthy diets were not associated with higher risks. It is proposed that greater adherence to unhealthy diets may also be accompanied by higher consumption of some healthy foods such as fish and olive oil(Reference Zazpe, Sanchez-Tainta and Toledo75), and this, at least in part, can attenuate harmful effects of unhealthy diets. Another possible explanation is that cardioprotective effects of healthy diets mediated primarily through plant-based foods are reduced because of greater intakes of meat products instead of more beneficial plant foods in diet(Reference Trichopoulos and Lagiou76). It is also possible that detrimental effects of unhealthy diets may be mediated partly by increasing the risk of adiposity. A recent meta-analysis of cross-sectional studies has suggested that higher adherence to healthy dietary patterns may decrease the risk of central fatness(Reference Rezagholizadeh, Djafarian and Khosravi69). Another meta-analysis of observational studies suggested that higher consumption of red and processed meat, the most frequent food contributed to unhealthy/Western dietary patterns, may be correlated with higher BMI and waist circumference(Reference Schwingshackl, Knüppel and Schwedhelm57). Of the 116 primary prospective cohort studies included in this umbrella review, 104 studies (90 %) controlled for BMI in their multivariable analyses. Thus, adjustment for obesity measures may attenuate harmful effects of unhealthy diets(Reference Xiao, Xia and Li44).
We presented original pooled effect sizes that reported in included meta-analyses to present a comparison between recalculated SRR in this review and original pooled effect sizes. The pooled effect sizes became stronger in the analyses of healthy and unhealthy dietary patterns and fracture, became non-significant in the analyses of unhealthy dietary pattern and CRC and COPD and healthy pattern and frailty and did not change materially for other associations. The observed differences were due to the fact that included meta-analyses combined empirically derived and index-based dietary patterns or combined prospective cohort studies with case–control studies or with baseline cross-sectional evaluations within prospective cohort studies and did not perform subgroup analyses on the basis of study design.
This umbrella review has several strengths. This is the first try, to our knowledge, that gathered existing evidence obtained by published meta-analyses of prospective cohort studies on the association of empirical dietary patterns with the risk of chronic disease. We excluded case–control, cross-sectional and retrospective cohort studies, studies conducted in patients, studies with unadjusted risk estimates and studies that did not consider empirical dietary pattern as exposure. We recalculated SRR with the use of a random-effects model to present comparable results across different outcomes. We evaluated the methodological quality of published meta-analyses by the use of a validated AMSTAR tool. We also rated the quality of the evidence obtained by published meta-analyses to help readers make better and more realistic judgement about potential beneficial or harmful effects of healthy and unhealthy dietary patterns. In addition, we described characteristics of healthy and unhealthy dietary patterns identified in primary prospective cohort studies which indicated that higher intake of plant-based foods, low-fat dairy products and fish and lower intake of red and processed meat, refined grains, sweets, snacks and ultra-processed foods may be good dietary suggestions for health promotion. Finally, more than 90 % of the primary prospective cohort studies included in the present umbrella review considered age, BMI, smoking status and energy intake in their multivariable analyses and 78 % controlled for physical activity. Thus, we were able to show the associations of the overall diet quality with disease risks, independent of the effects of traditional confounding variables.
We also were faced with some important limitations which should be noted, especially for future investigations. First, of the 116 primary prospective cohort studies, only fifty-seven studies (49 %) controlled for family history of the disease assessed in that study. In addition, residual confounding by unknown variables should be acknowledged. Second, of the thirty-four SRR reported in this review, <5 primary prospective cohort studies were available for the analyses of COPD, colorectal adenoma, asthma, frailty, metabolic syndrome and pancreatic, prostate, gastric and lung cancers. Only one prospective cohort study was found for hypertension, obesity and ovarian and endometrial cancers. In addition, there was no prospective cohort study available for renal and oral/pharyngeal cancers. Much of the evidence for these outcomes was obtained from case–control and cross-sectional studies. Thus, further prospective cohort studies are needed to investigate the potential effects of healthy and unhealthy dietary patterns on the risk of these outcomes. Third, although dietary patterns analysis presents a total picture of the diet and our results demonstrated that components of healthy and unhealthy dietary patterns were relatively similar across the world; there are ethnically specific dietary patterns with their own specific and diverse constituents(Reference Nettleton, Steffen and Mayer-Davis77,Reference Tucker78) . Thus, health outcomes of these ethnically specific dietary patterns must be more investigated. Fourth, almost all primary studies included in eligible meta-analyses assessed baseline dietary intakes and did not perform repeated dietary assessment during follow-up period. Fifth, for published meta-analyses with the same outcomes, we selected and included those with the largest number of primary prospective studies. However, almost all included meta-analyses mentioned that they searched the reference lists of all relevant meta-analyses, and therefore, it is unlikely that some primary studies have been missed due to inclusion of meta-analyses with the largest number of primary studies. Nevertheless, systematic reviews without meta-analyses were not included in this review, and some primary studies may have been published after publication of each meta-analysis. Therefore, some primary prospective cohort studies may have been missed in this review, and as a result, some of the results could have been influenced by missing studies.
Conclusion
This umbrella review evaluated the evidence obtained by published meta-analyses of prospective cohort studies on the association of empirical dietary patterns with the risk of non-communicable chronic diseases and indicated that healthy dietary patterns may be associated with a lower risk of T2D, COPD, CHD, fracture, depression and all-cause and CVD mortality. The associations for site-specific cancer risks have been less investigated. Exceptions were colorectal and breast cancers, for which inverse associations were found. More research is needed for outcomes for which the quality of the evidence was rated low, such as respiratory disease, mental illness and site-specific cancers.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
A. J. and S. S.-B. conceived and designed the study; A. J. and S. S. conducted systematic search, screened articles and selected eligible articles; A. J. and S. S. extracted the information from eligible studies and performed quality assessments; A. J., and S. S.-B. performed analyses and interpreted the results; A. J., S. S., and A. A. wrote the first draft of the manuscript; S. S.-B. critically revised the manuscript. S. S.-B. is the guarantor. All authors have read and approved the final manuscript. All authors had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520002330