Introduction
Landscape alteration and degradation is an unfortunate consequence of a rapidly expanding human population. This pressure on the environment can take two forms: direct harvesting of plants and animals for food or harvesting of key resources on which wildlife may depend (Du Plessis, Reference Du Plessis1995). In southern African savannahs trees are routinely harvested for firewood and building material, which is a significant threat to cavity-dwelling vertebrates such as birds and small mammals (Du Plessis, Reference Du Plessis1995). Certain arboreal lizards such as tree agamas Acanthocercus atricollis atricollis and skinks Trachylepis spp. are not only dependent on trees for refuge (Cooper & Whiting, Reference Cooper and Whiting2000; Reaney & Whiting, Reference Reaney and Whiting2002) but also use them as foraging vantage points (Vitt et al., Reference Vitt, van Loben Sels and Ohmart1981). Furthermore, removal of trees influences the thermal environment available to lizards (Vitt et al., Reference Vitt, Avila-Pires, Caldwell and Oliveira1998), which may have cascading effects in which certain species increase in abundance at the expense of others (Vitt et al., Reference Vitt, Avila-Pires, Caldwell and Oliveira1998). Consequently, when trees are harvested selectively the landscape is transformed (Germaine & Wakeling, Reference Germaine and Wakeling2001), and these species may be negatively affected. Therefore, not only are humans competing with wildlife for space but also for key resources.
Smart et al. (Reference Smart, Whiting and Twine2005) recorded higher densities of tree agamas in degraded communal lands compared to a nearby conserved area, noted the presence of tree agamas in rural villages but did not quantify their abundance, and found that local residents had a negative perception of tree agamas and may be persecuting them. The aim of our study was therefore to establish whether tree agamas and humans can coexist in a high-density peri-urban environment and whether human perceptions of tree agamas result in any form of persecution that could affect their viability. A second motivation for focusing on tree agamas is that they are arboreal and therefore dependent on trees, a natural resource used by humans. Consequently, tree agamas may face a dual threat: habitat destruction and persecution by humans.
We surveyed tree agamas in local villages and adjacent disturbed rangelands in the same general area as Smart et al. (Reference Smart, Whiting and Twine2005) for comparison with their measures of tree agama density in a nearby protected area. Because Smart et al. (Reference Smart, Whiting and Twine2005) had established that tree agamas are more densely populated in disturbed areas compared to protected areas we restricted our surveys to a complex of adjoining villages and nearby communal, disturbed rangelands. We addressed anthropomorphic threats to A. a. atricollis by integrating field surveys of tree agamas with questionnaire surveys of local village residents. Our study asked the following questions: (1) Do A. a. atricollis occur in reduced numbers in villages compared to nearby rangelands that are less disturbed? We specifically tested the prediction that tree agamas would occur at lower densities in villages. (2) Are any differences in abundance explained by habitat features? (3) What are the traditional beliefs concerning tree agamas and could human attitudes have negative impacts on local populations of A. a. atricollis?
Study area and species
Fieldwork was conducted in seven adjacent villages in Bushbuckridge Local Municipality, Mpumalanga Province (Fig. 1) and adjoining communal rangeland, in an area encompassing 13.3 km2 during September of both 2003 and 2007. Each village was distinct from the next and separated by at least 2–3 km. Bushbuckridge is characterized by high population density, poverty and land-use practices typical of African savannah rangelands such as cattle overstocking and intensive harvesting of firewood (Shackleton, Reference Shackleton2000). As such, villages are largely devoid of vegetation except for scattered trees (mainly marula Sclerocarya birrea caffra), hedges and small-scale vegetable gardens. In the late 1990s the mean population density was 176 people km-2 (Pollard et al., Reference Pollard, Perez de Meniguren, Joubert, Shackleton, Walker, Poulter and White1999) and the cattle stocking rate was 0.88 livestock units ha-1 (four times the recommended stocking rate; Parsons et al., Reference Parsons, Shackleton and Scholes1997). A detailed description of the vegetation, climate and socio-economic conditions of Bushbuckridge is given in Shackleton (Reference Shackleton2000).

Fig. 1 Location of the study area in the Mpumalanga Province of South Africa.
A. a. atricollis occurs from eastern South Africa northwards into Zimbabwe and Mozambique (Branch, Reference Branch1998). While there has been no recent conservation assessment of this species (IUCN, 2008), it is a wide-ranging (Branch, Reference Branch1998) and relatively common species (authors, pers. obs.). Tree agamas are large (largest snout-vent length: 167 mm), diurnal, arboreal, sexually dichromatic (females are olive coloured with black marbling, and mature males have a bright blue head and throat and a vertebral stripe) and insectivorous (Reaney & Whiting, Reference Reaney and Whiting2002). A. a. atricollis is a classic ambush forager that spends most of its time waiting for potential prey on tree trunks or lateral branches (Reaney & Whiting, Reference Reaney and Whiting2003).
Methods
To estimate whether tree agamas are more abundant in villages than in surrounding rangelands we systematically surveyed trees that were sufficiently large to harbour tree agamas (minimum diameter 0.09 m) and recorded the number of lizards in each tree. We sampled 300 trees: 150 in the villages and 150 in the adjacent rangelands. Because temporal differences in lizard activity patterns may affect abundance estimates we blocked our sampling procedure so that, within each day, we alternated our sampling site between different habitat types every 10–20 tree surveys. We also balanced each day’s starting site (village vs rangeland) so that equal numbers of trees were sampled in both habitat types on the same day, and each habitat type was sampled an equal amount of times within a given time frame across different days (i.e. early morning, mid morning, late morning, midday, early afternoon, late afternoon). Surveys for tree agamas were conducted by two people approaching a tree from opposite sides. We first scanned a tree with binoculars from > 10 m before slowly approaching. If we recorded a tree agama, we did not survey any trees within a 10 m radius in case their presence influenced whether a conspecific would occupy a neighbouring tree. We also estimated perch height (cm) and tested for a significant difference between villages and surrounding areas.
We measured the diameter of each tree at breast height (c. 160 cm) to the nearest centimetre and estimated tree height to the nearest 1 m. We did not identify tree species but did score whether a tree was a marula because they are maintained for fruit production and dominate the landscape.
We gauged human attitudes and behaviour towards tree agamas through household surveys. Members of a household were asked a series of questions about tree agamas (Table 1). Two pictures of the tree agamas (one of each sex) were shown to the interviewee to ensure correct identification. Three primary themes were covered in the survey: (1) familiarity with A. a. atricollis, (2) beliefs and perceptions about tree agamas, and (3) whether A. a. atricollis and village residents are able to coexist. As previous research in this community established that a negative perception exists towards tree agamas (Smart et al., Reference Smart, Whiting and Twine2005) we asked the question ‘Do tree agamas bring good luck?’. A high proportion of negative responses would suggest that other responses were answered truthfully. The survey was always carried out by KC with the help of a translator fluent in Tsonga. Households were randomly sampled in Sigagula village (Fig. 1), which is relatively central in the study area. We stopped interviewing after 49 households because of the consistent nature of responses.
Table 1 Survey questionnaire presented to 49 households to gauge their attitudes and beliefs towards tree agamas Acanthocercus atricollis atricollis. Respondents were first presented with pictures of male and female agamas to confirm that they were familiar with the lizard. Question number 3 is a control question (see text for further details).

Because relatively high numbers of trees did not contain A. a. atricollis we used non-parametric Mann–Whitney tests to determine if there was any significant difference in the number of lizards per tree in the villages compared to surrounding rangelands. To examine what factors best predicted lizard abundance (physical characteristics of trees or location) we used general linear modelling. For this we used PROC GENMOD in SAS v 9.1 (SAS Institute, Cary, USA) for a Poisson regression model that allows categorical and continuous data. We used stepwise subset selection, in which the criterion for remaining in the model was P = 0.1. We then performed a Type 3 analysis to examine the interactive effect of explanatory variables on the main effect. The response variable was number of lizards and the explanatory variables were location and tree height and diameter. All statistical tests are two-tailed.
Results
We found a significantly (![]() = 48.95, P < 0.00001) higher number of tree agamas in villages (n = 97) compared to surrounding rangelands (n = 21; Fig. 2). The mean number of tree agamas per tree was also significantly higher in villages (0.65 ± SE 0.06) than in surrounding rangelands (0.14 ± SE 0.04; Mann–Whitney Z = 7.4, P < 0.00001). Mean perch height was not significantly different (t 91 = 1.77, P =0.08) between villages (220.4 ± SE 16.3 cm) and surrounding rangelands (287.1 ± SE 39.22 cm).
= 48.95, P < 0.00001) higher number of tree agamas in villages (n = 97) compared to surrounding rangelands (n = 21; Fig. 2). The mean number of tree agamas per tree was also significantly higher in villages (0.65 ± SE 0.06) than in surrounding rangelands (0.14 ± SE 0.04; Mann–Whitney Z = 7.4, P < 0.00001). Mean perch height was not significantly different (t 91 = 1.77, P =0.08) between villages (220.4 ± SE 16.3 cm) and surrounding rangelands (287.1 ± SE 39.22 cm).
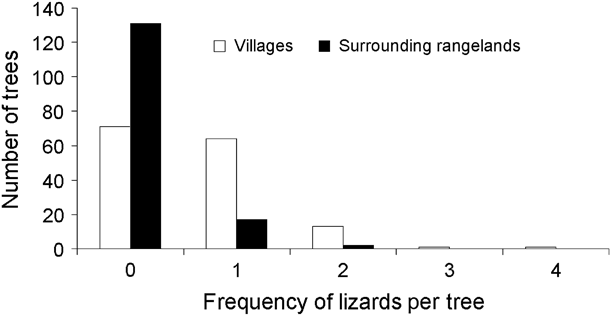
Fig. 2 Frequency of tree agamas encountered on trees in villages and surrounding rangelands.
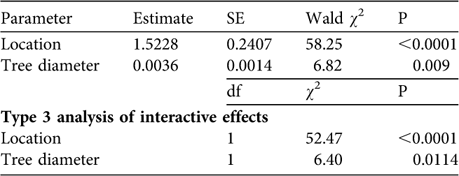
We found no significant differences in the characteristics of trees containing tree agamas between villages and surrounding rangelands: mean diameter (villages: 242.82 ± SE 7.06 cm, n = 79; rangelands: 258.68 ± SE 24.77 cm, n = 19; Mann–Whitney Z = 0.08, P = 0.94) and mean height (villages: 10.27 ± SE 0.25 m, n = 79; rangelands: 9.16 ± SE 0.55 m, n = 19; Mann–Whitney Z = 1.74, P = 0.08). There was a good fit (Pearson ![]() = 270.5, P = 0.91) for the Poisson regression model describing lizard abundance in relation to location and physical characteristics of trees and the data were not overdispersed, thereby meeting the assumptions of the model. Our final model retained lizard location and tree diameter as explanatory variables for tree agama abundance (Table 2). When interactive effects were examined (Type 3 analysis), location (village vs rangeland) was a more significant predictor of lizard abundance than tree diameter (Table 2).
= 270.5, P = 0.91) for the Poisson regression model describing lizard abundance in relation to location and physical characteristics of trees and the data were not overdispersed, thereby meeting the assumptions of the model. Our final model retained lizard location and tree diameter as explanatory variables for tree agama abundance (Table 2). When interactive effects were examined (Type 3 analysis), location (village vs rangeland) was a more significant predictor of lizard abundance than tree diameter (Table 2).
Table 2 General linear modelling (Poisson regression) of predictors of tree agama abundance. Tree diameter has a marginal effect on tree agama abundance but location is highly significant. Significantly more tree agamas were sighted in villages compared to surrounding rangelands (see text for further details).
Seventy-four percent (n = 191) of trees sampled (n = 259) were marula; 80 (31%) trees contained tree agamas, of which 53 (66%) were marula. Tree agamas used marula trees significantly more (![]() = 18.2, P < 0.0001) than all other tree species combined, and this did not depend on location (villages: n = 31 of 51 trees, 66%; surrounding areas: n = 11 of 23 trees, 48%; Yates’ corrected
= 18.2, P < 0.0001) than all other tree species combined, and this did not depend on location (villages: n = 31 of 51 trees, 66%; surrounding areas: n = 11 of 23 trees, 48%; Yates’ corrected ![]() = 0.62, P = 0.43).
= 0.62, P = 0.43).
Of the 49 households interviewed 98% of respondents recognized the tree agama and all reported seeing them often. The question ‘Is it true that tree agamas bring good luck?’ was answered positively by only 4% of interviewees, allowing us to accept the remaining responses with reasonable confidence. Smaller subsets of the population held certain other negative beliefs: 20% of respondents believed that tree agamas are capable of killing livestock, 18% that if A. a. atricollis bites a person, the agama will hold on to its victim and will have to be burnt off with hot maize meal, 6% that the tree agama's brain is poisonous, and 12% that tree agamas have medicinal properties. As a result of these perceptions of tree agamas a large proportion of respondents (n = 41, 84%) actively excluded tree agamas from trees on their property, either killing them with a stone (n = 7, 14%) or by wrapping plastic around the tree trunk (n = 34, 69%). Where residents had wrapped plastic around a tree we did not observe any agamas. Almost half (n = 23, 47%) of all respondents reported they had killed tree agamas.
Discussion
We found the converse of what we had predicted: tree agama density was higher in villages, where human density is high, than in surrounding communal rangelands. We expected that the more degraded landscape of villages, coupled with a negative human perception of tree agamas, would have an additive effect that would reduce lizard density. This paradox may be resolved by several explanations that need not be mutually exclusive. The primary factors likely to promote tree agama population growth are food availability, the suppression of competition and the removal of natural predators. A less likely explanation is that fire may have a greater effect on agamas in rangelands than in villages, either directly or indirectly through reduction of food availability. However, rangelands that surround villages are typically overgrazed and have relatively low fuel loads (Smart et al., Reference Smart, Whiting and Twine2005), suggesting that fire is unlikely to significantly affect agama populations.
Tree agamas eat large numbers of ants and beetles, although grasshoppers comprise a high proportion of their diet by volume (Reaney & Whiting, Reference Reaney and Whiting2002). Grass and vegetation in general are limited in villages, which comprise mostly bare ground. Therefore, although we did not measure insect prey availability, it is unlikely that grasshoppers and ants would be more abundant in villages, given that both groups are likely to experience low food availability. Alternatively, other factors may have promoted insect availability, or agamas may have switched to eating insects that are more readily available in villages.
The removal of natural predators can cause an increase in the abundance of prey species (Case et al., Reference Case, Bolger, Richman, Fiedler and Kareiva1998). Snakes, a known predator of A. a. atricollis (authors, pers. obs.), are generally killed on sight in villages (Smart et al., Reference Smart, Whiting and Twine2005). Other potential predators include feral domestic cats and raptors, both of which are less common in villages (Lewis, Reference Lewis1997). Therefore, tree agamas are probably experiencing lower natural predation pressure in villages than in the surrounding rangelands.
Based on the interviews we conclude that village tree agamas face the additional threat of human persecution. However, we did not quantify the frequency with which residents kill tree agamas, so it is possible that killing by humans is relatively insignificant and therefore insufficient to offset natural predation levels.
Many factors drive the community ecology of savannah ecosystems but chief among those that affect the plant community are the actions of herbivores (Owen-Smith, Reference Owen-Smith1988). In undisturbed African savannahs with typical browsing pressure marula trees probably occurred at low densities (Cunningham & Shackleton, Reference Cunningham, Shackleton, Lawes, Eeley, Shackleton and Geach2004). However, given that marula fruit has been a key resource for at least the past 2,000 years of agriculture in southern African savannahs it is likely that human action has increased their abundance (Cunningham & Shackleton, Reference Cunningham, Shackleton, Lawes, Eeley, Shackleton and Geach2004). In particular, disturbed areas not only have fewer browsers because of anthropogenic disturbance but many trees are protected from browsing by fencing.
Tree agamas have been reported to select trees of a particular species and physical complexity (Reaney & Whiting, Reference Reaney and Whiting2003). At our study site the landscape has been radically altered by years of selective tree harvesting, resulting in a tree community with low diversity. In particular, marula trees are among the more common large trees. Marula are large enough and sufficiently complex structurally to be readily used by tree agamas, so the availability of marula trees may be a key factor contributing to their resilience in rural settlements. If levels of tree harvesting for fuelwood in communal areas increase, tree agamas may in future become more restricted to human settlements, where they could use trees that are grown specifically for economic purposes. The future of tree agamas in rural communities therefore appears to be linked to the economic benefits afforded from growing marula trees in homesteads. Paradoxically, tree agamas represent an example of a native species that has benefited from historical human action and is currently relatively abundant despite the best efforts of humans to exclude them from their domain. Although tree agamas appear to be secure and occur in relatively high abundance in a modified landscape, we need a better understanding of how degraded ecosystems function and whether altering the abundance of top level predators may increase the potential for detrimental cascading effects on other, less conspicuous groups, such as invertebrates.
Acknowledgements
This study was funded by grants to MJW from the National Research Foundation of South Africa and the University of the Witwatersrand. WT was funded by the National Lottery Distribution Trust Fund. For field assistance we thank Skusi Nardini, Solly B. Zitha and Themba H. Ngobeni. We also greatly appreciate the support and cooperation of the Timbavati community.
Biographical sketches
Martin Whiting’s primary research interests span behavioural and evolutionary ecology, including conservation biology. He works primarily with reptiles, and lizards in particular. The majority of his field work has been in southern Africa, although he has also worked on species of conservation concern in North America and Australia. Kinesh Chetty is involved in the implementation of Clean Development Mechanism projects in sub-Saharan Africa aimed at promoting the development of clean energy technologies in developing countries. Wayne Twine manages a research programme focusing on human-environment interactions in rural common-property resource systems. Pau Carazo’s principal area of research is behavioural ecology. He has conducted research on social communication in lacertid lizards and on sperm competition and sexual conflict in beetles.






