Introduction
Human visceral leishmaniasis (HVL) is a neglected tropical disease with chronic and infectious presentation that can reach up to 95% of lethality without treatment [1]. It is an increasingly prevalent disease in Latin America and Brazil, the latter of which accounts for 95.1% of the region's cases [2]. Despite the high morbidity and lethality, most infections by Leishmania infantum in humans are asymptomatic. Individuals with low socioeconomic conditions and immune deficiency usually are more susceptible to the development of clinical signs [Reference Pagliano3]. Thus, one of the most important risk factors for visceral leishmaniasis (VL) is the co-infection with human immunodeficiency virus (HIV), especially in situations where anti-viral treatment is not effective [Reference Pagliano3–Reference Cota8].
In Brazil, VL and HIV are serious public health problems. The national average incidence rate (IR) of HIV infection over the last five years was 19.1 cases/100 000 inhabitants. In the southeastern region of Brazil, the IR in 2015 was 18 cases/100 000 inhabitants, whereas in Belo Horizonte, the capital of the state of Minas Gerais (MG), the IR in 2015 was 26.3 cases/100 000 inhabitants [9], which indicate a more serious epidemiological situation in the municipality, compared to regional and national averages. According to the Belo Horizonte Municipal Department of Health (MDH-BH), 1711 cases of VL in humans (HVL) were reported between 1994 and 2015, with a lethality of 27.6% in 2015 [10], rates that are well above the national and international averages [2]. However, it is important to emphasise that areas with high prevalence of HIV and Leishmania largely overlap [Reference Pagliano3]. In Brazil, the country that has the largest number of cases of co-infection in Latin America, 2% of VL patients from 2001 to 2005 were co-infected with HIV, while in 2012, 8.5% of VL cases were co-infected [Reference Alvar11, Reference Lindoso12].
Therefore, considering the high mortality from VL in Brazil, especially in Belo Horizonte, it is relevant to invest in the development of new control measures. In order to improve this situation, it is important to consider the local characteristics of the disease, such as its social determinants, in order to better understand the eco-epidemiology of the disease and thus provide more accurate information to reduce morbidity and mortality.
Indeed, despite the high number of reported cases of VL–HIV co-infection, aspects of its epidemiology, clinical features and management remain unknown [Reference Cota13]. Some studies have already evaluated risk factors related to deaths due to leishmaniasis in Brazil [Reference Cota13–Reference Madalosso16], but few have evaluated the social determinants associated with death and VL–HIV co-infection in this country. Therefore, the aim of this study was to evaluate the distribution of cases and the social determinants associated with death from HVL and VL–HIV co-infection in Belo Horizonte, MG, between 2006 and 2013, and so draw attention to Brazilian and international public health issues about the growing public health problem that VL and VL–HIV confection poses to the country, despite the control measures adopted against these diseases in Brazil.
Methods
An observational retrospective study was conducted by analysing data related to VL in Belo Horizonte, MG, Brazil. Information regarding the incidence and lethality in human cases in this city between 2006 and 2013, and its association with risk factors, were analysed.
Data collection
Belo Horizonte is the capital of the State of Minas Gerais, the sixth largest city in Brazil. It consists of a population of 2 479 165 inhabitants distributed over an area of 331.4 km2, with a population density of 7167 inhabitants/km2 estimated in 2013. The majority of the population (91%) in the city is literate; the gross national product per capita, according to the 2011 statistical data was about US$ 7,136.29, and the basic sanitation coverage was about 96.1% [17]. According to the Brazilian Institute of Geography and Statistics (IBGE) [18], the literacy rate (%) is the proportion of literate people of an age group in relation to the total number of people of the same age group. A person is considered as literate if he/she knows how to read and write simple text in Portuguese. Furthermore, according to the IBGE [18], the coverage of basic sanitation (%) is the proportion of the population with access to safe sewage disposal and treated water.
Information on cases and deaths from HVL were obtained from the databases of the Management of Epidemiology and Information (GEEPI) and Zoonoses Control (GECOZ), both databases being managed by the MDH/BH. For confirmed cases of HVL in Belo Horizonte, data relating to the death, the date of occurrence of the disease, age, sex, education, skin colour and co-infection with HIV were collected. In addition, the start date of the symptoms and probable infection sites were obtained from the Brazilian Notifiable Diseases Information System (SINAN).
The SINAN is a public health information system that is compiled mainly by reporting and investigating cases of diseases and injuries that are listed in the national list of compulsory notifiable diseases [19]. Thus, the databases used in the present study are those related to the occurrence of HVL provided by the MDB/BH, based on data generated by SINAN and comprise the passive and active surveillance actions performed by the MDH/BH within the scope of the National Program of Control of Visceral Leishmaniasis in the municipality.
Statistical analysis
Descriptive analyses of all variables selected from the databases were conducted. In order to characterise the morbidity, mortality and lethality of HVL in Belo Horizonte, the following proportion-type indicators were used: (i) incidence rate (IR), (ii) mortality rate (MR) and (iii) lethality rate (LR).
(i) IR = ([cases of HVL relapses]/population) × 100 000
(ii) MR = (HVL deaths/population) × 100 000
(iii) LR* = (deaths HVL/[cases of HVL + relapses]) × 100
*The calculation of LR per year included prevalent cases (those whose onset of symptoms occurred in the years preceding the year of death), in addition to relapses, in the denominator.
Bivariate descriptive statistical analysis of the above indicators and social determinants such as education level, sex, age, skin colour and co-infection with HIV were evaluated. The chi-square (χ 2) or Fisher's exact tests were used in these analyses, the latter when there were fewer than five observations in at least one cell in the test contingency table. The variables involved in these analyses were those available in the information system from which the data analysed in this study originated.
For the variables associated with a minimum confidence level of 95% (P < 0.05) by Chi-square or Fisher's exact tests, the risk was calculated using relative risk (RR) (when the dependent variable was death from HVL) or by chance using odds ratio (OR) (when the dependent variable was the co-infection with HIV). Confidence intervals were calculated at 95% (95% CI) for both measures of association.
All statistical analyses were performed using SPSS 20.0 software.
The study was approved by the Ethics Committee on Human Research of the Federal University of Lavras (UFLA), under protocol 832274/2014.
Results
Between 2006 and 2013, there were 866 cases of HVL in Belo Horizonte, MG, with 111 deaths (average annual IR = 4.18 cases/100 000 inhabitants; average annual MR = 0.56 deaths/100 000; annual average LR = 11.17%). In 2008, a spike in the IR was observed, followed by a decline that accelerated from 2011. In 2013, lower values of the evaluated series were observed. Although the LR remained high over the years evaluated, it showed a peak in 2009 (19.61%). In that same year, the highest MR value was observed (1.20 cases/100 000 population) (Fig. 1).
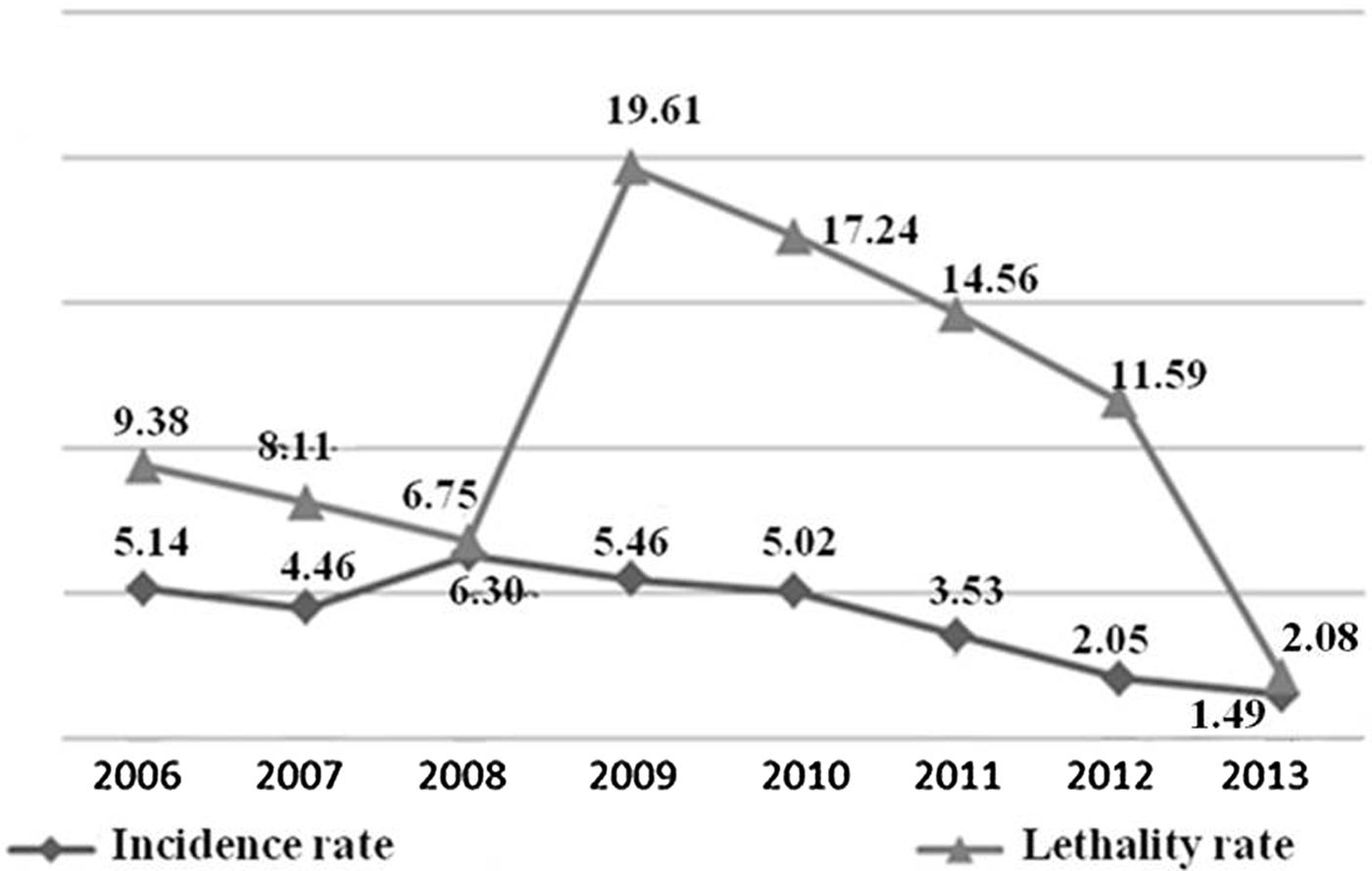
Fig. 1. Epidemiological indicators1 incidence rate (/100 000 inhabitants), mortality rate (/100 000 inhabitants) and lethality rate (%) of human visceral leishmaniasis in Belo Horizonte, 2006–20132. 1Incidence rate = reported cases/population (×100 000); lethality rate (%) = deaths/incident cases + prevalent (relapses and cases whose first symptoms appeared in the years prior to death) 2until July 2013; estimated population for 2014 (01/07) = 2.491.1097.
With respect to age, after categorisation of age into intervals of 5 and 10 years (Fig. 2), following the same standardisation method of the IBGE census of 2010 [17], it was observed that most of the higher IR occurred among young individuals, and a higher LR of the disease among elderly people. After the application of the χ 2 test, this finding was confirmed, that is, the risk of dying from leishmaniasis increased with advancing age (Table 1).
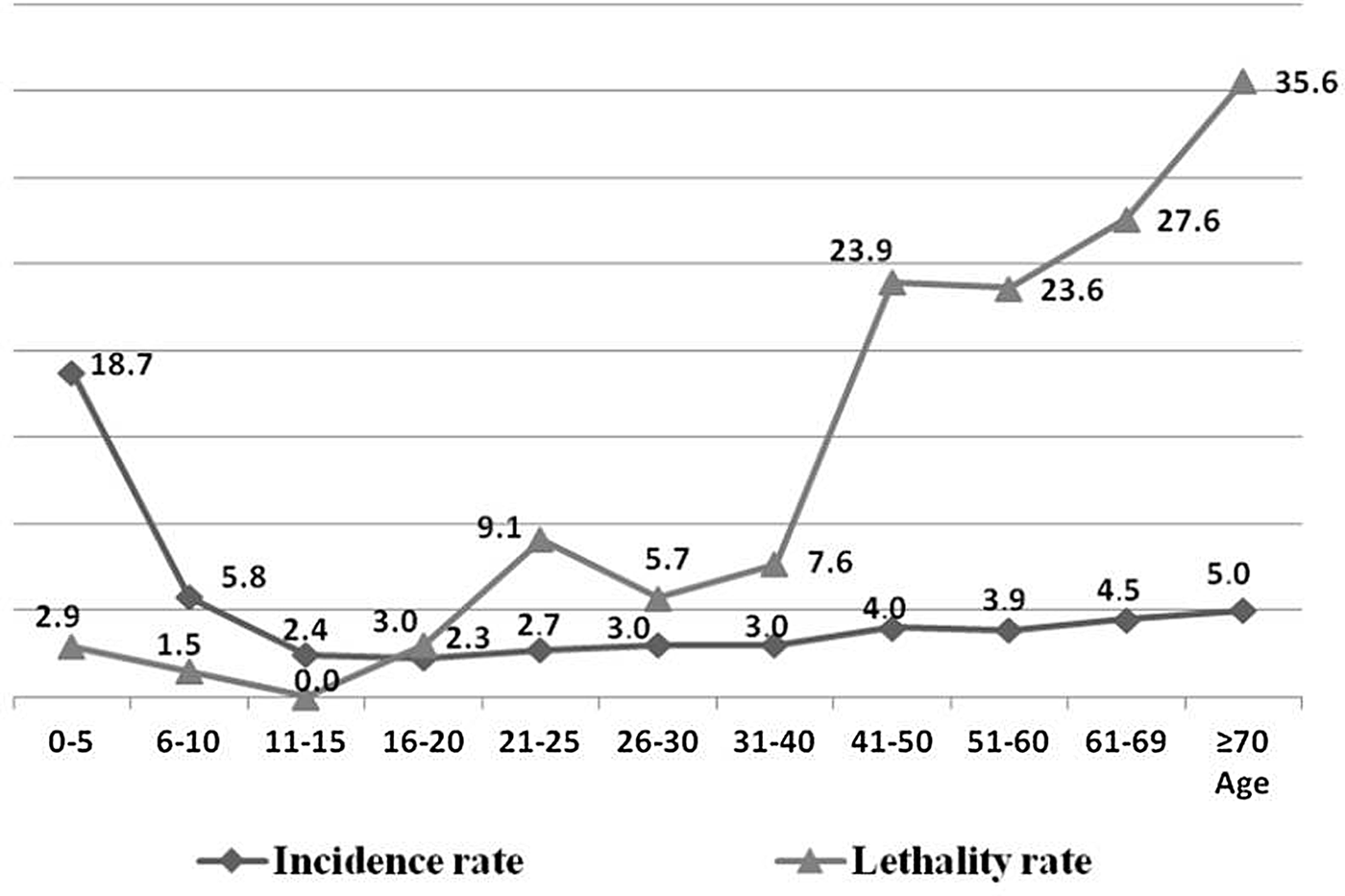
Fig. 2. Average annual epidemiological rates1 of human visceral leishmaniasis in Belo Horizonte, according to age groups, 2006–20132. 1Average values of IR and MR over the 8 years evaluated; IR = [incidence rate = reported cases/population (× 100 000)]/8; LR (%) = lethality rate = deaths/incidents + prevalent cases (relapses). 2Population variation adjusted per year according to demographic census (2010) [17].
Table 1. Association between age and death from human visceral leishmaniasis (HVL) in Belo Horizonte, Minas Gerais, Brazil, 2006–2013, using the χ 2 test

Regarding skin colour, people characterised as black or mulatto had higher morbidity (black = 144 cases; brown = 215 cases; 41.5%), followed by white (151 cases, 17.4%). It is important to note that in most cases (33.8%), skin colour was ignored in the development of the information system. Among deaths where skin colour was reported, there was no statistically significant difference (P = 0.408) in the occurrence of deaths due to skin colour in Belo Horizonte (n = 103; 99.0%), despite the higher LR that occurred between individuals with white skin (14.6%), followed by mulatto (12.1%) and black (10.5%).
With respect to schooling, among the 866 cases of HVL described in the system, in 338 (39%) schooling was characterised as ignored (not known), and information was missing for 43.2% of the cases. Among the cases whose education was characterised (n = 135; 15.6%), the distribution of VL cases was similar between categories of years of study, but the LR was more pronounced among individuals with lower education. It was found that 36 (26.7%) subjects had up to 3 years of education, 50 (37%) from 4 to 7 years, and 49 (36,3%) had over 8 years of education. However, regarding LR, it was observed that of the 15 deaths where education was characterised (13.9% of total), the largest LR was observed in subjects with up to 3 years of education (16.7%), followed by those with 3 to 7 years (10.0%) and those with more than 8 years of education (8.2%). Statistically, the relationship between years of study and deaths by VL could not be confirmed (P = 0.215), possibly because of lack of the relevant data (86.1%) regarding the observed deaths for this variable in the information system, which can display bias, especially for those with lower education.
When evaluating the epidemiological morbidity and mortality rates of HVL among different age groups and between both the sexes (Table 2), it was found that there were more cases of VL in males (61.9%) in Belo Horizonte than in females. After applying the χ 2 test of association, it was confirmed that the highest LR was among male individuals, who had approximately twice the risk of dying from HVL (P = 0.005, RR = 1.89, 95% CI = 1.21–2.97). Furthermore, it was observed that the IR was highest in the age group of children from 0 to 5 years, followed by children from 6 to 10 years old, for both sexes. In addition, there was an increase in the LR with age, between females and males, with the latter reaching an LR of 50% of individuals over 70 years old.
Table 2. Average annual epidemiological rates of human visceral leishmaniasis in Belo Horizonte, Minas Gerais, according to age groups and sex, 2006–2013
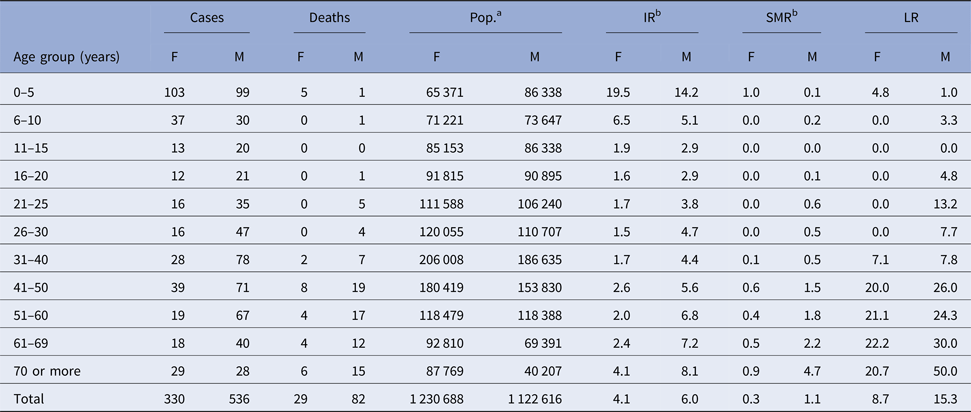
a Population according to the census (2010) [17].
b Average values of IR and SMR over the eight years evaluated; IR = [incidence rate = reported cases/population ( × 100 000)]/8; SMR = specific mortality rate = [deaths in the group/population in that group (×100 000)]/8; LR (%) = lethality rate = deaths/incident and prevalent cases (relapses).
F, female; M, male.
It is also noteworthy that in Belo Horizonte there was no statistical association between HVL–HIV co-infection and education and skin colour of the individual. In addition, it is important to note that among 488 cases of VL that reported knowing their HIV status, 70 (14.3%) had the co-infection. Among all cases, 335 (38.6%) subjects reported being unaware of their HIV status. The LR of co-infected cases (27.3%) was significantly higher than the rates of those not co-infected (12.1%). Statistically, this was confirmed by the finding that co-infected individuals were 2.26 times more likely to die from VL than those not co-infected (P = 0.012; RR = 2.26; 95%CI = 1.18–4.32). Furthermore, male individuals had a 2.4 greater chance of having the co-infection than females (P < 0.001; OR = 2.69; 95%CI = 1.57–4.61).
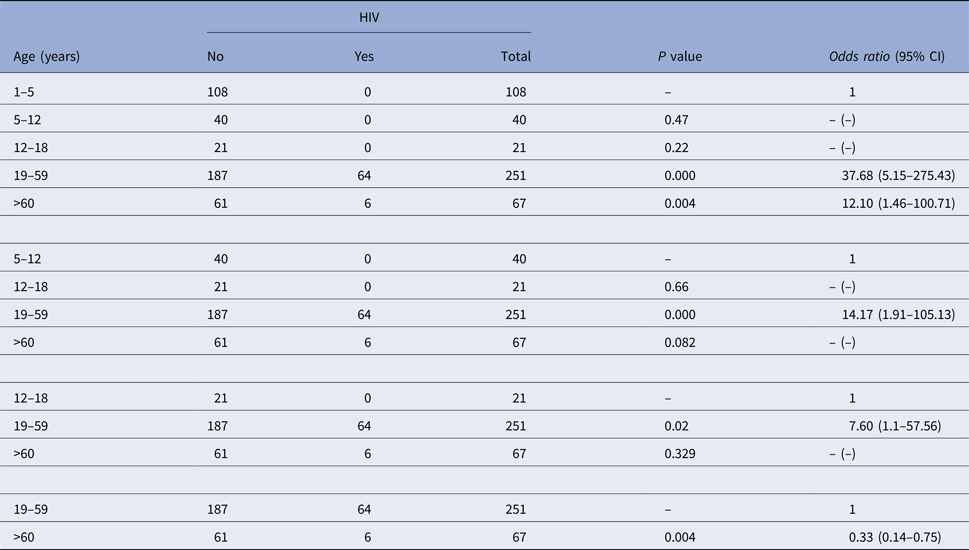
In this study, it was found that individuals with higher age were more likely to have HIV co-infection (P < 0.05) (Table 3). The median age for the cases of VL who had co-infection with HIV was 41 years old (interquartile range = 17 years) and the median in those who did not have it was 26 years old (interquartile range = 43.25 years).
Table 3. Association between age and co-infection with Leishmania infantum and HIV in Belo Horizonte, Minas Gerais, Brazil, 2006–2013 using the χ 2 test
Discussion
In Belo Horizonte, HVL is still a serious public health problem. The disease in the city, despite the historical endemicity and the existence of a control programme developed by public health systems, presented an average lethality of above 11% and an IR of 4,18/100 000 population between 2006 and 2013, especially among individuals in a situation of vulnerability, such as young people, the elderly and those co-infected with HIV.
In recent decades, a growing number of VL cases have been reported in major cities, including Belo Horizonte, MG. Araújo et al. [Reference Araújo20] noted in 1994, when the first cases of HVL were reported in the Belo Horizonte, an IR of 1.4 cases/100 000 inhabitants and an LR of 20.7%. Between 1994 and 2007, Lopes et al. [Reference Lopes21] observed an average IR of 3.6 cases/100 000 inhabitants and an average LR of 11.7%. Furthermore, between 2007 and 2009, Araújo et al. [Reference Araújo20] found an average IR of 6.2 cases/100 000 inhabitants, with an LR of 14.7%. These results, together with those of the present study (IR = 4.18 cases/100 000 inhabitants and LR = 11.16%), demonstrate that the persistence of the disease as a public health problem is on the rise in the country, despite the control measures instituted and regulated by the Brazilian government.
In 2008, in Belo Horizonte, the highest IR of the analysed period was reported and one of the highest ever reported in the history of the city [Reference Lopes21]. Regarding the LR, it is important to highlight the increase observed in this indicator in 2009, followed by a steady decline until 2013. This increase in 2009 may be associated with the active search for information related to deaths by HVL, initiated in 2008 and performed by the MDH/BH, in other information systems besides SINAN, such as the SIM, on the occurrence of HVL deaths that were not previously present in the SINAN. Thus, there was a rise in LR in 2009, partly explained by the improvement in the sensitivity of the health information system with regard to the investigation of deaths from HVL.
Elderly and young ages are commonly characterised as risk factors for morbidity and mortality from HVL [Reference Karimi6, Reference Madalosso16, Reference Maia-Elkhoury22, Reference Aissi23]. In the present study, higher disease morbidity with young age and severity with higher age have been verified. It was observed that the risk of dying from being an HVL case is the same among individuals from 1 to 18 years (P > 0.05) (Table 1). However, after that age, the risk increases significantly (P < 0.05), and those aged over 60 years are at an increased risk of dying from the disease compared to all other age groups (P < 0.05). In this regard, there was a high LR of the disease among people over 40, especially those older than 70 years old, reaching up to 35.6% (Fig. 2), reinforcing that the elderly constitute a risk group in this disease and therefore, preventative measures, especially for the elderly, should be implemented by health services. According to Oliveira et al. [Reference Oliveira24], the higher susceptibility of children can be explained by immunological immaturity, which is aggravated by malnutrition, a condition that is common in endemic areas. The distribution of cases of VL by age and sex in Belo Horizonte is similar to that observed in other cities of Brazil, with greater susceptibility of children up to 10 years old and among male adults [19, Reference Barata25, Reference Mestre and Fontes26].
In the present study, it was observed that social factors in Belo Horizonte are still related to the likelihood of becoming ill or dying from VL. Thus, regarding skin colour, it was verified that people characterised as black or mulatto had higher morbidity, followed by white. Regarding schooling, LR was more pronounced among individuals with lower education. It is worth noting that the effect of these determinants on the occurrence of VL can be explained by the greater poverty among those with a lower level of education and black or mulatto skin colour [27], which predisposes individuals to social vulnerability, and thus to greater susceptibility to diseases such as VL. In fact, according to a case-control study conducted by Argaw et al. [Reference Argaw5] on the risk factors for HVL between residents and migrants from different regions of Ethiopia, and a meta-analysis of studies conducted by Belo et al. [Reference Belo28] evaluating the risk factors associated with HVL in the Americas, the risk factors have a social character, essentially linked to poverty and low educational level. This leads to increased vulnerability of the population, not only for HVL, which can occur as a secondary disease to other diseases, such as AIDS, but for other diseases as well.
Indeed, VL has emerged as a major complication in patients infected with HIV [29]. It is known that the individual development of VL and AIDS may accelerate the onset or progression of the other [Reference Karimi6, 29], considering that drug interaction, treatment failure, relapse and increased mortality are known consequences of VL–HIV co-infection [Reference Karimi6, Reference Cota8, Reference Druzian14]. In addition, considering that AIDS in Brazil in 2015 showed a tendency of an increase among individuals aged between 15 and 25 years and over 50 years, HIV infection may further increase the LR in VL patients in Brazil, especially in these age groups [9, Reference Mahy30].
Recently, an increase has been reported in the number of geographical areas with HIV and VL co-infection [9, Reference Monge-Maillo31], and in Belo Horizonte this same pattern has been observed. In the city, the co-infection of L. infantum and HIV has been a major health issue, considering that of the majority of cases of VL that reported knowing their HIV status had the co-infection. Moreover, among all cases, 335 (40.7%) subjects reported being unaware of their HIV infection status. This indicates that the prevalence of co-infection may be even higher since HIV infection is a risk factor for the development of clinical signs of VL, which is a determinant for seeking medical attention. This is subsequently reported as a notifiable condition.
In this study, the association between infection with HIV and death from VL, observed through the descriptive analysis of the data, was confirmed statistically. The results presented in this study related to the epidemiology of L. infantum and its relationship with HIV suggest that one of the risk factors that have contributed to the high rates of morbidity, mortality and lethality of VL in Belo Horizonte is the co-infection with HIV. HIV was present in many of the subjects analysed, culminating in LRs higher than in the average population, especially among individuals with ages ranging from 19 to 59. This suggests that co-infection depends mainly on the exposure to the risk of contracting HIV, which is more likely to occur among adults than among younger individuals. It is therefore interesting to note that the risk of co-infection among individuals over 60 years old tended to decrease, with no statistically significant difference (P > 0.05) in the chance of co-infection among people over 60 years of age and those aged between 12 and 18 years.
The risk of HIV co-infection and death has also been reported in other studies conducted in Brazil [Reference Argaw5–Reference Madalosso16], which suggest that measures against leishmaniasis are also related to education for the prevention of infection by HIV, as well as campaigns to encourage the search for diagnosis, and the application of the recommended treatment. Even in this context, educational campaigns are necessary and must be continued in order to increase public awareness of the importance of control of VL in order to promote community participation in reducing the conditions that facilitate the spread of the disease [Reference Lobo32].
In conclusion, this study demonstrated that VL is a serious disease with high lethality and morbidity rates in Belo Horizonte, especially in the presence of HIV/AIDS co-infection. Elderly, young age and the male sex are risk factors for VL in Belo Horizonte. Among the social determinants studied, the co-infection by HIV is also an important factor related to deaths from HVL in the city.
Acknowledgements
The authors acknowledge the Municipal Health Department of Belo Horizonte for their support on the research data and the Graduate Program in Veterinary Sciences (PPGCV/UFLA), where the study was developed.
Financial support
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Research scholarship to C.M.B.M.R.
Declaration of interest
None.







