Vitamin D deficiency has become a worldwide public health problem(Reference Holick and Chen1). There is increasing evidence of an association between a low maternal vitamin D status, evaluated by 25-hydroxyvitamin D (25(OH)D) concentration, and a high risk of pre-eclampsia(Reference Wei, Audibert and Hidiroglou2, Reference Bodnar, Catov and Simhan3), gestational diabetes(Reference Burris, Rifas-Shiman and Kleinman4, Reference Senti, Thiele and Anderson5) and infectious disease(Reference Hensel, Randis and Gelber6, Reference Bodnar, Krohn and Simhan7) as well as an increased risk of reduced fetal bone mineral accrual(Reference Dror, King and Fung8–Reference Mahon, Harvey and Crozier10) and respiratory infections and wheezing(Reference Brehm, Acosta-Perez and Klei11, Reference Camargo, Ingham and Wickens12) in infants.
In addition, non-skeletal health effects have been found, but so far only in observational studies; their causality and significance need to be proven by randomised controlled trials(Reference Chung, Balk and Brendel13). Due to the remarkable high prevalence of vitamin D deficiency, the possible health consequences should not be underestimated as it has been shown that rickets is on the rise in developed as well as developing countries(Reference Ladhani, Srinivasan and Buchanan14, Reference Beck-Nielsen, Brock-Jacobsen and Gram15).
The supply of vitamin D is primarily dependent on sunlight exposure, in particular on UVB irradiation (290–315 nm), of the skin, whereas the intake via food is only of marginal importance(Reference Ladhani, Srinivasan and Buchanan14). The efficiency of vitamin D that is produced in the skin depends on skin melanin pigmentation(Reference Ladhani, Srinivasan and Buchanan14, Reference Bodnar and Simhan16), age(Reference Holick17), sunscreen use(Reference Faurschou, Beyer and Schmedes18) or clothing, including veiling(Reference Prentice19), as well as on latitude and season(Reference Ladhani, Srinivasan and Buchanan14, Reference Holick20). In Europe and in many other parts of the world at a latitude above 35° of the northern or southern hemisphere, it has been shown that UVB irradiation is not sufficient for the synthesis of necessary amounts of vitamin D from November to March(Reference Holick20–Reference Farrar, Kift and Felton22). We hypothesise that pregnant women and their newborns living in Germany at latitudes between 54 and 47°N are at a high risk of vitamin D deficiency at delivery.
During the last couple of years, a number of studies investigating the vitamin D status of pregnant women have shown a high variation in the prevalence of vitamin D deficiency worldwide, ranging between 5 and 96 %(Reference Prentice19, Reference Brannon and Picciano23–Reference Crozier, Harvey and Inskip31). This variation might be due to the divergent cut-off values and analytical methods that are applied in different studies as well as to the geographical and habitual disparities between countries. For example, there are different proportions of individuals who wear a veil as well as variations in the national law regulating food fortification. In Europe, the fortification of food with vitamin D is more common in the Nordic countries, including Norway, Denmark and Sweden, than in Central or Southern European countries(Reference Freisling, Fahey and Moskal32).
Hence, we assume that there is also a need to determine country-specific prevalence data. Especially in Germany, to our knowledge, no data are available concerning the vitamin D status of pregnant women based on the analysis of the 25(OH)D level. The objective of the present study was to estimate the 25(OH)D status and possible risk factors for vitamin D deficiency in a cohort of German pregnant women and in cord blood. Our data should help to improve the clinical practice of healthcare authorities regarding the prevention of vitamin D deficiency during pregnancy and infancy.
Patients and methods
Study design and setting
The present cross-sectional study was conducted between December 2010 and February 2012 at the maternity unit of St Josefs Hospital, a general community care hospital with more than 1000 births per year, located in Giessen, Germany, at a latitude of 50°N. The present trial was registered at the German Clinical Trials Register (DRKS-ID: DRKS00003245).
Study population
On the first day of their admittance to the hospital, a total of 369 women gave written informed consent to participate in the study. Exclusion criteria were a chronic liver or kidney disease, secondary hyperparathyroidism or malabsorption diseases and an intake of medications that are known to affect vitamin D metabolism, including glucocorticoids and anticonvulsants. All women completed a self-administered questionnaire. A total of 261 women (71 %) provided a single blood sample for 25(OH)D analysis and a total of 328 women (89 %) gave their consent for the analysis of a single cord blood sample. Hence, it was possible to match a total of 220 mother–infant pairs. Of the 328 newborns, five were sent to the Neo-Intensive Care Unit, due to low base excess levels in cord blood, dyspnoea (mask respiration required), a pH in cord artery < 7·0, infections or cardiac arrhythmia.
A single maternal blood sample was drawn either at the time of delivery or within 72 h post-partum. A single cord blood sample was drawn at delivery. Blood samples were collected using S-Monovette lithium heparin 7·5 ml tubes (Sarstedt Monovette) and kept at 4°C for a maximum of 4 h until centrifugation. Plasma was removed and stored at − 80°C until analysis. The samples were assayed at the end of a sampling period and defined according to the season of sampling as follows: winter (December to February); spring (March to May); summer (June to August); autumn (September to November). The samples were sent to a central clinical laboratory in Cologne on dry ice. The laboratory participates in national and international quality assurance schemes and is fully accredited according to the DIN EN ISO 17 025 and DIN EN ISO 15 189.
Laboratory analysis
Total 25(OH)D concentration was measured by a direct competitive fully automated chemiluminescent immunoassay (LIAISON 25 OH Vitamin D TOTAL assay; DiaSorin). This standardised assay predicates on the same specificity and analytical sensitivity as the DiaSorin RIA, which was used to establish 25(OH)D reference values for clinical practice(Reference Ersfeld, Rao and Body33, Reference Hollis34). The functional sensitivity of the assay is < 10·0 nmol/l (to convert to ng/ml, divide by 2·5) and the analytical range is 10·0–375·0 nmol/l. The intra- and inter-assay CV were < 3 and < 13 % in a concentration range of 18·0–87·5 nmol/l. The assay has a cross-reactivity of 100 % to 25(OH)D2 and of 104 % to 25(OH)D3. The laboratory reference value for an optimal 25(OH)D level for healthy female adults is ≥ 50 nmol/l. Key parameters of bone metabolism were investigated by the analysis of intact parathyroid hormone (iPTH), alkaline phosphatase and Ca concentrations. iPTH concentration was determined by a chemiluminescent immunoassay (LIAISON N-TACT PTH assay; DiaSorin). The intra- and inter-assay CV were ≤ 8 and < 14 % in a concentration range of 7·80–5·10 pg/ml, respectively. The analytical range is 5·0–400·0 pg/ml. The laboratory reference range for healthy female adults is 7·0–82·0 pg/ml. Total Ca and alkaline phosphatase concentrations were measured by photometric or kinetic colour testing using Olympus automated analysers (Olympus AU640 and AU600; Lismeehan Company).
Definition of vitamin D deficiency cut-offs
Vitamin D status can be evaluated by measuring the concentration of the major circulating vitamin D metabolite, 25(OH)D, reflecting dietary intake and endogenous production(Reference Hollis34, Reference Ross, Manson and Abrams35).
For Ca and bone metabolism, it has been well described that at 25(OH)D levels < 50 nmol/l, the PTH concentration starts to increase rapidly, whereas it begins to plateau at 25(OH)D levels >75 nmol/l(Reference Holick36, Reference Thomas, Lloyd-Jones and Thadhani37). Consequently, the risk of bone resorption and secondary hyperparathyroidism is increased(Reference Bischoff-Ferrari, Giovannucci and Willett38, Reference Bischoff-Ferrari, Dietrich and Orav39). With regard to the non-classical effects of vitamin D, there are observations indicating that 25(OH)D levels of 50 nmol/l or less are associated with major clinical diseases(Reference de Boer, Levin and Robinson-Cohen40). Therefore, we used 50 nmol/l as the vitamin D deficiency cut-off, which is currently being proposed by a number of authorities, for example the International Osteoporosis Foundation, the Canadian Osteoporosis Society and the Endocrine Society(Reference Dawson-Hughes, Mithal and Bonjour41–Reference Holick, Binkley and Bischoff-Ferrari43).
Assessment of secondary outcome measures
Women had to complete a questionnaire to record factors that have been shown to be associated with the 25(OH)D level, including baseline demographics and habitual and health-related variables(Reference Campagna, Settgast and Walker44). Data of the medical history of each participant were determined by the external quality assurance, a standardised form routinely used in St Josefs Hospital to assess anthropometric, medical and socio-economic data for general statistical reasons.
The recorded demographical and habitual variables were statistically considered as independent variables (independent risk factors) and dichotomised as follows: age by median ( ≤ 30 or 31–40 years); pre-pregnancy BMI calculated from height and pre-pregnancy weight that was recorded from the external quality assurance and classified using the WHO cut-off for overweight ( ≤ 25 or >25 kg/m2)(45); parity, i.e. number of previous pregnancies (nulliparous or ≥ 1 para); country of origin (European and non-European or other countries of origin); type of skin (light or medium–dark, modified by Fitzpatrick(Reference Fitzpatrick46)); wearing a veil (yes or no); years spent at school (no degree, < 8 years or ≥ 10 years); physical activity ( < 1 or ≥ 1 h/week), i.e. referring to an average physical activity load during pregnancy; time spent outdoors ( < 1 or ≥ 1 h/d). The intake and frequency of vitamin D-containing supplements were assessed by asking whether the women had taken a vitamin D-containing supplement or other supplements during pregnancy or not and, if yes, what the brand name was. Questions to assess health-related variables included those on malabsorption disease, including ulcerative colitis, Crohn's disease, allergies, diabetes mellitus, asthma and the use of medications that are known to affect vitamin D metabolism, including glucocorticoids and anticonvulsants. Data of the medical history and baseline characteristics of women and their newborns were recorded from the external quality assurance.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local ethics committee of the medical department of the Justus Liebig University Giessen (AZ 189/10).
Sample size calculation and statistical analysis
Using a paired t test for independent samples, we estimated that with 300 subjects (seventy-five pregnant women and newborns per season), we would have 80 % statistical power at a two-sided significance level of α = 0·05 to detect a minimal difference of 7 nmol/l between the true value of the median 25(OH)D concentration and the sample median in our cohort considering a standard deviation of 10 nmol/l, according to the literature(Reference Thomas, Fudge and Whiting47–Reference Nicolaidou, Hatzistamatiou and Papadopoulou49).
To take into account the absence of a Gaussian distribution, an additional 10 % was considered in the sample size calculation. Normality tests were carried out using the Shapiro–Wilk test. Non-normally distributed data are shown as medians with interquartile ranges. Comparisons of medians for non-normally distributed data were carried out using non-parametric tests including either the Kruskal–Wallis test in conjunction with the Mann–Whitney U test as a post hoc test and a Bonferroni corrected P value < 0·016 or the Wilcoxon signed rank test. The Mann–Whitney U test was used to test for significant differences between two independent samples. Yates' correction was used where appropriate. Correlations between continuous variables were assessed using Spearman's rank coefficient of correlation (r s).
Univariate, bivariate and multivariate logistic regression models were used to estimate the crude and adjusted OR with 95 % CI for vitamin D deficiency, using the 25(OH)D level as a dichotomous categorical variable and the proposed risk factors as independent variables. A two-sided P value < 0·05 was considered as statistically significant and only variables with P< 0·05 in the univariate logistic regression analysis were included in the final multivariate model. All analyses were conducted using the IBM SPSS statistics version 20.
Results
In the present study, 77% of the maternal blood samples and 69 % of the cord blood samples had 25(OH)D levels < 50 nmol/l. The overall median maternal and cord blood 25(OH)D levels were 25·0 (interquartile range 12·6–45·5) and 34·1 (interquartile range 17·7–58·6) nmol/l, respectively (Table 1).
Table 1 Maternal 25-hydroxyvitamin D (25(OH)D) levels for the total cohort (n 261), intact parathyroid hormone (iPTH), alkaline phosphatase (ALP) and calcium levels for the number of available samples, and cord blood 25(OH)D levels for the total cohort (n 328) (Medians and interquartile ranges (IQR); number of subjects and percentages)

* P value for the Mann–Whitney U test comparing medians between 25(OH)D categories.
Women who were from a non-European country, who were physically active < 1 h/week, who spent < 1 h/d outdoors or had ≥ 1 previous pregnancy had significantly lower median 25(OH)D levels than those from a European country of origin with a physical activity level ≥ 1 h/week and those who spent ≥ 1 h/d outdoors (Table 2).
Table 2 25-Hydroxyvitamin D (25(OH)D) levels (nmol/l) of pregnant women (n 261) according to the demographical characteristics (Medians and interquartile ranges (IQR); number of subjects and percentages)

Median values were significantly different between categories by the Mann–Whitney U test: *P< 0·05, ***P< 0·001.
† P value for the χ2 test or Fisher's exact test for categorical variables.
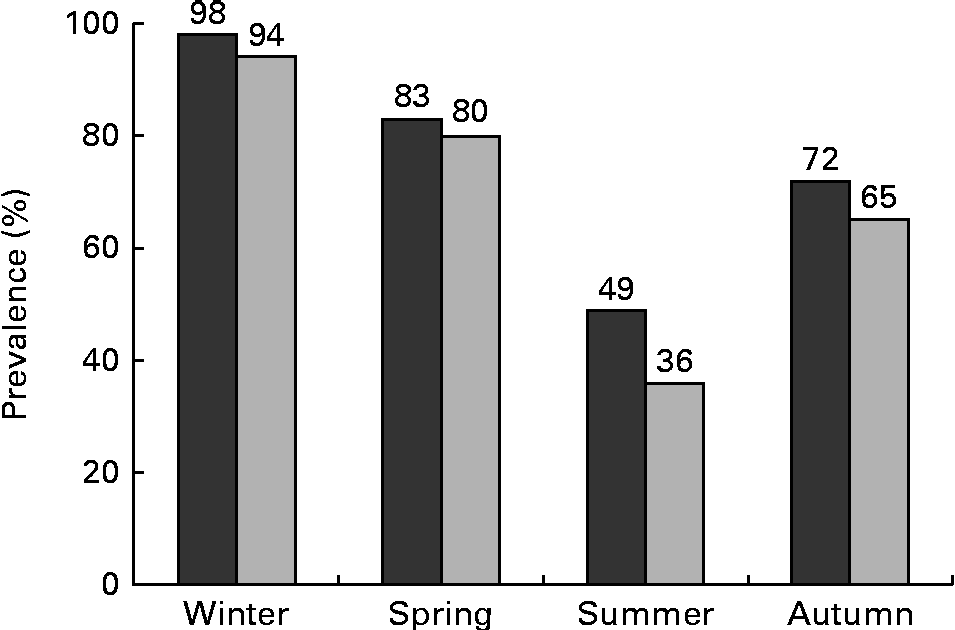
Considering 50 nmol/l as the cut-off, 98 % of the maternal blood samples and 94 % of the cord blood samples had 25(OH)D concentrations below this level in winter (Fig. 1). Even during the summer months, 49 % of the maternal blood samples and 35 % of the cord blood samples had 25(OH)D concentrations under this threshold.

Fig. 1 Prevalence of vitamin D deficiency, 25-hydroxyvitamin D < 50 nmol/l, in pregnant women (n 261, ![]() ) and in cord blood (n 328,
) and in cord blood (n 328, ![]() ), stratified by season.
), stratified by season.
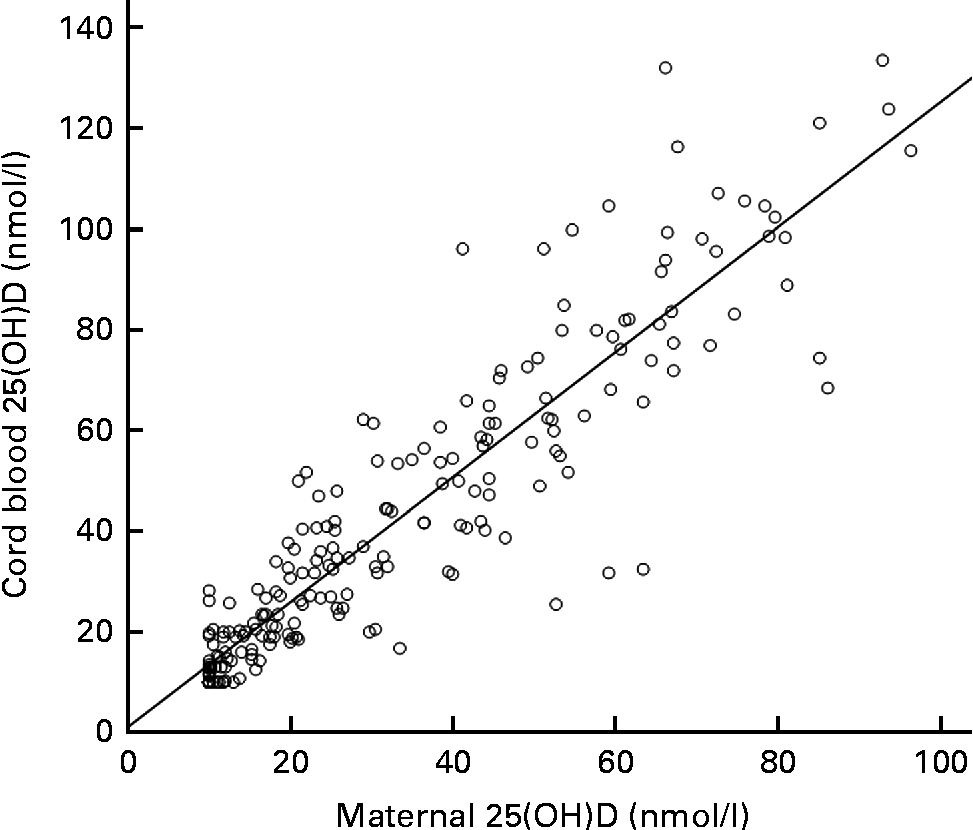
Overall, a strong positive correlation between the maternal and cord blood 25(OH)D levels could be observed (r s 0·93, P< 0·001; Fig. 2). Median maternal and cord blood 25(OH)D levels were significantly different between the seasons, with the highest median maternal levels being observed in summer (50·5 (interquartile range 30·4–66·2) nmol/l) and the lowest in winter (11·8 (interquartile range 10·0–17·8) nmol/l). The same seasonal variation was observed for the cord blood 25(OH)D levels. Median 25(OH)D levels were 61·4 (41·5–87·5) nmol/l in summer and 16·3 (10·0–26·8) nmol/l in winter (Bonferroni corrected P< 0·001). Data are given in the Supplementary material (available online).

Fig. 2 Correlation of maternal and cord blood 25-hydroxyvitamin D (25(OH)D, nmol/l). r s 0·94, P< 0·001.
In the following, we discuss only maternal biochemical parameters (Table 1). We found a significantly inverse correlation of maternal 25(OH)D and iPTH levels (r s − 0·6, P< 0·001). Vitamin D-deficient women had significantly higher median iPTH levels than those with 25(OH)D levels ≥ 50 nmol/l (45·60 (interquartile range 27·43–65·50) pg/ml and 16·71 (interquartile range 9·98–24·73) pg/ml, respectively; P< 0·001). Ca and alkaline phosphatase levels showed a significantly positive correlation with 25(OH)D levels (r s 0·33, P< 0·001, and r s 0·19, P< 0·05, respectively). The Ca level was significantly inversely correlated with the iPTH level (r s − 0·39, P< 0·001).
When using a univariate logistic regression analysis to calculate crude OR and 95 % CI, a significant association between maternal vitamin D deficiency and season, a non-European country of origin, physical inactivity < 1 h/week, pre-pregnancy BMI >25 kg/m2, no vitamin D supplement intake, parity ( ≥ 1 previous pregnancy) and the iPTH level could be observed (Table 3). Season itself had the strongest effect on maternal 25(OH)D levels (OR 47·07, 95 % CI 10·76, 205·75, P< 0·001; Table 3). The risk of 25(OH)D levels < 50 nmol/l during winter was forty-seven times as high as that during summer. The OR for spring was 5·2 (95 % CI 2·27, 11·79, P< 0·001) and for autumn, it was 2·7 (95 % CI 1·2, 6·2, P= 0·02).
Table 3 Factors associated with maternal vitamin D deficiency (25-hydroxyvitamin D (25(OH)D) <50 nmol/l) (Crude and adjusted odds ratios with 95 % confidence intervals)

OR, crude OR; aOR, adjusted OR; iPTH, intact parathyroid hormone.
* Reference category for season is summer.
† P value for the univariate logistic regression analysis.
‡ P value for the bivariate logistic regression analysis controlling for season (categorical).
In the multivariate logistic regression analysis controlling for season and significant independent risk factors (e.g. vitamin D supplement intake, pre-pregnancy BMI, parity and time spent outdoors as categorical variables as well as the iPTH level as a continuous variable), physically inactive women were about 2·7 times more likely to have vitamin D deficiency than those reporting to be physically active ≥ 1 h/week (adjusted OR 2·67, 95 % CI 1·06, 6·69, P= 0·032). Women from a non-European country of origin had 3·2 times the odds of being vitamin D deficient than those from a European country of origin (adjusted OR 3·21, 95 % CI 1·0, 10·28, P= 0·047; data not shown).
No correlation with vitamin D status was found for the time spent outdoors, age, type of skin and years spent at school analysed as continuous and categorical variables.
Discussion
To our knowledge, the present results are the first 25(OH)D data to be presented for pregnant women living in Germany. From previous studies, we know that, especially during winter, there is a high risk of vitamin D deficiency for the general population(Reference Prentice19, Reference Hintzpeter, Mensink and Thierfelder50). However, the very high prevalence in our cohort had not been expected, and the results support our hypothesis that pregnant women are at a high risk of vitamin D deficiency at the time of delivery. Our data are based on a 25(OH)D cut-off of 50 nmol/l as recommended by various authorities(Reference Dawson-Hughes, Mithal and Bonjour41–Reference Holick, Binkley and Bischoff-Ferrari43). In 2011, the North American Institute of Medicine released a report on the dietary reference intakes for Ca and vitamin D, considering a 25(OH)D level < 30 nmol/l as vitamin D deficiency(51). On applying their recommendation to our data, the prevalence of vitamin D deficiency did not change much. For example, during the winter months, 92 % of the maternal blood 25(OH)D levels and 79 % of the cord blood 25(OH)D levels were < 30 nmol/l.
Like in many other countries, the German Nutrition Society has recently changed the dietary reference intake for vitamin D for different population groups(52). For pregnant women, the recommendations were set to 20 μg vitamin D/d compared with the former 5 μg/d. This seems to be a major step towards improving the vitamin D status. However, these dietary reference intakes include the following statement of limitation: 20 μg vitamin D/d are only recommended if there is no cutaneous synthesis of vitamin D(52). From a practical point of view, we are convinced that this is difficult to comply with: how should an individual know how much vitamin D he or she should take? The endogenous vitamin D synthesis after exposure to sunlight can hardly be estimated without measuring the 25(OH)D level. Consequently, we suspect that there will be no improvement of the current vitamin D supply, with respect to not only pregnant women, but also the vast majority of the population.
After adjusting for independent risk factors, we identified physical inactivity ( < 1 h/week) and a non-European country of origin as risk factors for maternal vitamin D deficiency, 25(OH)D < 50 nmol/l. Within the scope of the questionnaire, the actual sunlight exposure (time spent outdoors) as well as the exact load of physical activity was not determined. Furthermore, at the end of pregnancy, the extent of physical activity is very likely to decrease. Hence, only a very conservative conclusion can be drawn from these results, as the use of a questionnaire is always highly susceptible to recall bias by the participants.
To determine risk factors for vitamin D deficiency, we adjusted our data for the season of blood sampling. As expected, season itself had the strongest effect on maternal 25(OH)D levels (OR 47·07, 95 % CI 10·76, 205·75, P< 0·001; Table 3).
We also found that at normal Ca levels, vitamin D-deficient women had significantly higher median iPTH levels than those with a 25(OH)D level >50 nmol/l, indicating an increased risk of bone demineralisation. These findings could be seen as a metabolic compensation due to the low 25(OH)D level. PTH is strongly involved in Ca and vitamin D metabolism. Vitamin D deficiency or slightly decreased Ca levels stimulate PTH secretion and can cause secondary hyperparathyroidism. Consequently, Ca is mobilised from the bones, which might have various health impacts including an increased risk of osteoporosis as well as impaired bone mineralisation(Reference Bowyer, Catling-Paull and Diamond48, Reference O'Brien, Donangelo and Ritchie53, Reference Haddow, Neveux and Palomaki54). In addition, the developing fetus is strongly dependent on maternal placental Ca and 25(OH)D transfer(Reference Haddow, Neveux and Palomaki54). It is assumed that the circulating 25(OH)D crosses the placenta readily, whereas the active hormone, 1,25-dihydroxyvitamin D3, does not. Consequently, the formation of 1,25-dihydroxyvitamin D3 in the fetal kidney is exclusively dependent on the maternal substrate that crosses the placenta and hence the maternal 25(OH)D level(Reference Kaludjerovic and Vieth55–Reference Evans, Bulmer and Kilby58).
We observed significantly higher median cord blood 25(OH)D levels compared with the maternal levels, which differ from most of the published data (e.g. Dror et al. (Reference Dror, King and Fung8)). However, results from other studies are consistent with our observations. In their review from 2010, Dror & Allen(Reference Dror and Allen59) stated that the 25(OH)D level in cord blood at delivery may range from 68 to 108 % of maternal levels. Significantly lower median maternal 25(OH)D levels compared with cord blood levels have also been reported by others(Reference Thomas, Fudge and Whiting47–Reference Nicolaidou, Hatzistamatiou and Papadopoulou49); the reasons for the discrepancies are not known yet. There might be a link with the C-3 epimer concentration of the 25(OH)D molecule that has been shown to be present especially in cord blood. However, the chemiluminescent immunoassay LIAISON method that we used is not affected by the C-3 epimer(Reference Singh60, Reference Lensmeyer, Poquette and Wiebe61).
In the present study, vitamin D supplement intake was not related to vitamin D status after controlling for season. This might be explained by the low number of pregnant women reporting the intake of supplements. Only 20 % of the women stated that they took a vitamin D-containing supplement. Despite a daily supplement intake in the range of 0·14–25 μg, the majority of these women still showed vitamin D deficiency. The same was true for veiling as a possible risk factor for vitamin D deficiency. In our cohort, only 3 % of the women were wearing a veil, which included eight women in total. Due to the low sample size, this risk factor was excluded from the present analysis. The type of skin did not significantly influence maternal 25(OH)D levels.
We would like to mention that the present study had some limitations. It was only possible to determine the vitamin D status of women at the end of pregnancy. 25(OH)D concentrations at the beginning of pregnancy and during the whole gestation should be investigated in future studies. Furthermore, due to a lack of blood volume and due to the short half-life of iPTH, it was not possible to measure iPTH, alkaline phosphatase and Ca concentrations in all the 261 women. Data on the modifiable factors, including the identified risk factors physical activity and country of origin, were determined by a self-administered questionnaire, which is prone to recall bias. Furthermore, questions concerning vitamin D intake from food, physical outdoor activity, visit to a solarium, holidays per year and use of sunscreen (sun cream per d) had to be excluded from the analysis due to incomplete or missing answers. In a prospective approach, a more controlled setting is desirable to obtain more definite answers.
In conclusion, to our knowledge, these results are the first data to be presented for Germany, showing that an extremely high number of pregnant women and their newborns are prone to severe vitamin D deficiency, almost regardless of season. The present results clearly indicate that there is a great need for urgent action especially by public healthcare authorities that are in charge of preventing vitamin D deficiency during sensitive stages of life. The daily intake of vitamin D should be increased considerably; however, its extent needs to be clarified in future studies. Only recently, the European Food Safety Authority(62) has increased the tolerable upper intake level of vitamin D for adults including pregnant and lactating women from 50 to 100 μg/d. This amount is far beyond the current dietary reference intake in Germany and many countries worldwide.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513001438
Acknowledgements
We thank the team of P. G. of the maternity unit of St Josefs Hospital in Giessen, Germany, for sample collection and their dedicated support. Also, we wish to acknowledge all participating mothers. The present research received no grant from any funding agency in the public, commercial or not-for-profit sector. The contributions of the authors are as follows: C. K. initiated and designed the study; P. G. was responsible for data collection and supervised the recruitment of the subjects; W. B. was responsible for laboratory analysis of the samples; C. W. and C. K. evaluated the data and wrote the manuscript. All authors read and approved the final manuscript. None of the authors reports a conflict of interest.