Non-alcoholic fatty liver disease (NAFLD) is defined as the presence of more than 5 % hepatic fat accumulation without other causes of chronic liver disease such as excessive alcohol consumption, hepatitis C or medications(Reference Chalasani, Younossi and Lavine1). It has now become the most common liver disease, affecting at least 25–30 % of adults in the general population and up to 70–90 % of persons with obesity or type 2 diabetes(Reference Younossi, Koenig and Abdelatif2). NAFLD may further progress to fibrosis, cirrhosis and even hepatocellular carcinoma(Reference Vernon, Baranova and Younossi3). In addition, NAFLD is associated with an increased risk of incident CVD and type 2 diabetes(Reference Morrison, Zaccardi and Khunti4). However, there is still no approved drug for the treatment of NAFLD. Therefore, lifestyle modifications consisting of diet, exercise and weight loss remain the main focuses of management in NAFLD(Reference Chalasani, Younossi and Lavine1).
There has been a growing interest in the benefit of specific foods against NAFLD progression. Edible mushrooms are popular worldwide because of their taste and nutritional value. Mushrooms provide carbohydrate including fibre, protein and a range of vitamins (e.g. niacin), minerals and polyphenols(Reference Zhang, Li and Zhou5,Reference Wang, Zhang and Wu6) . Abundant evidence has suggested that mushrooms can counter inflammation(Reference Muszynska, Grzywacz-Kisielewska and Kala7) and oxidative stress(Reference Kozarski, Klaus and Jakovljevic8), which are major risk factors involved in the pathogenesis of NAFLD(Reference Al Rifai, Silverman and Nasir9,Reference Masarone, Rosato and Dallio10) . Moreover, several animal studies have shown that mushroom intake can ameliorate NAFLD(Reference Iniguez, Perez-Matute and Villanueva-Millan11–Reference Shimizu, Mori and Ouchi13). However, to date, the potential effects of habitual mushroom intake on NAFLD have not been evaluated in population-based studies.
Given that mushrooms are widely consumed in China and no epidemiological data are available on an association between mushroom consumption and NAFLD, the present study aimed to investigate an association between mushroom intake and NAFLD in the general population. We hypothesised that greater consumption of edible mushrooms is associated with a lower prevalence of NAFLD.
Methods
Study design and participants
The Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) Cohort Study is an ongoing prospective cohort study. Detailed descriptions of the TCLSIH Cohort Study have been published previously(Reference Gu, Li and Bao14,Reference Wang, Gu and Zheng15) . Briefly, the study was initiated in 2007 and all participants underwent a real-time ultrasonographic examination as part of health examinations since January 2010. Moreover, these participants were administered a detailed lifestyle questionnaire survey (including their dietary intake, physical activity (PA), household income, occupation, educational level, etc.) since May 2013. The study protocol was approved by the Institutional Review Board of Tianjin Medical University. All participants provided written informed consent.
For the present analysis, we used baseline data from the TCLSIH Cohort Study from 2013 to 2016 (n 32 165). We excluded subjects with a history of CVD (n 1552), cancer (n 248), alcoholic fatty liver disease (n 1099) or other liver diseases (n 124). Furthermore, we excluded subjects who were diagnosed with NAFLD from January 2010 to May 2013 or those with a self-reported history of the disease (n 4364), as they could have changed their eating habits after being diagnosed. Those who had missing data on dietary intake were also excluded (n 542). After these exclusions, this cross-sectional study consisted of 24 236 participants (Fig. 1).
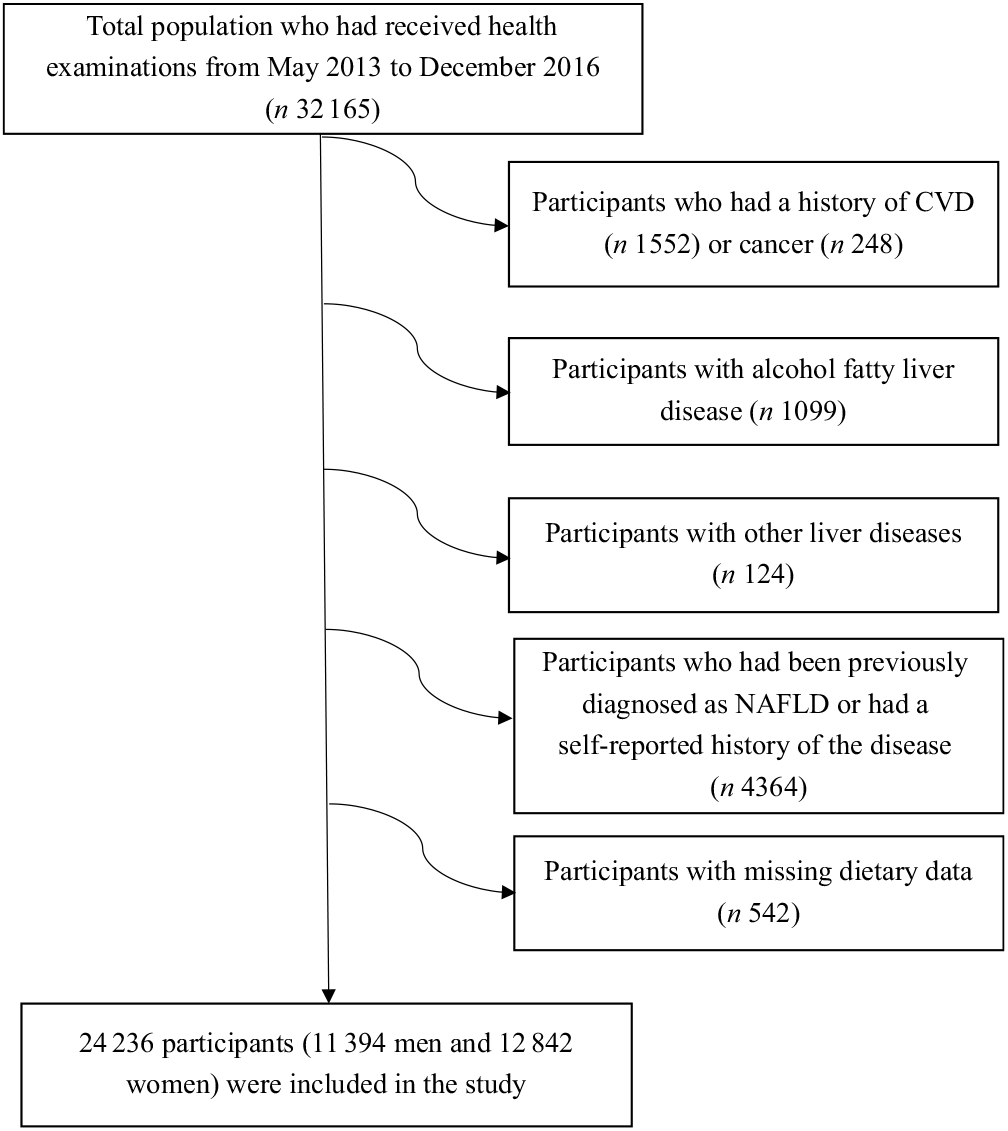
Fig. 1. Selection of subjects for the study. NAFLD, non-alcoholic fatty liver disease.
Diagnosis of non-alcoholic fatty liver disease
Fatty liver disease was diagnosed using abdominal ultrasonography, all unaware of the study aims. Abdominal ultrasonography was performed by experienced sonographers on a high-resolution ultrasound machine (TOSHIBA SSA-660A; Toshiba) with a 2–5 MHz curved array probe. Diagnosis of fatty liver disease required the presence of at least two of the following three abnormal findings(Reference Farrell, Chitturi and Lau16): diffusely increased liver near-field ultrasound echo (‘bright liver’) and increased liver echotexture when compared with the kidneys, vascular blurring and deep attenuation of ultrasound signal. Participants with fatty liver disease and without significant alcohol consumption (<140 g/week for men and <70 g/week for women) were defined as having NAFLD(Reference Gao and Fan17). Furthermore, as a confirmatory analysis, participants who had NAFLD and elevated serum alanine aminotransferase (ALT) levels (ALT >30 U/l for men and >19 U/l for women) were defined as having NAFLD with elevated ALT(Reference Prati, Taioli and Zanella18,Reference Ruhl and Everhart19) .
Assessment of dietary intake
Diet was assessed by a self-administered FFQ that included 100 food items with specified serving sizes. The FFQ inquired about the mean consumption frequency of selected food items during the previous month using seven frequency categories ranging from ‘almost never eat’ to ‘twice or more per day’ for foods and eight frequency categories ranging from ‘almost never drink’ to ‘four or more times per day’ for beverages. The reproducibility and validity of the FFQ were assessed in a subsample of 150 participants from the TCLSIH study by comparing the data from two FFQ collected approximately 3 months apart and 4-d weighed dietary records including one weekend day. The Spearman’s rank correlation coefficients comparing the FFQ to weighed dietary records were 0·49 for total energy, 0·35–0·54 for nutrients (n-3 fatty acid, fat and carbohydrate) and 0·69 for mushrooms. The Spearman correlation coefficients between two FFQ were 0·68 for total energy, 0·62–0·79 for food items (fruits, vegetables, sweet foods and beverages) and 0·75 for mushrooms. Energy and nutrient intakes were calculated by using an ad hoc computer programme developed to analyse the FFQ. Nutrient-composition data for foods were based on the Chinese Food Composition Table(Reference Yang20). Intakes of foods and nutrients were expressed as intakes per 2000 kcal (8368 kJ) of energy intake, calculated as: (food or nutrient intake/total energy intake) × 2000 kcal (8368 kJ).
Mushroom intake was assessed through one specific question. The question considered the consumption of edible mushrooms, including Lentinula edodes, Auricularia auricula-judae, Pleurotus ostreatus, Flammulina velutipes, Agaricus bisporus and other. Participants answered the question using seven possible responses as follows: almost never, <1 time/week, 1 time/week, 2–3 times/week, 4–6 times/week, 1 time/d and ≥2 times/d. In the present study, a typical serving size of mushrooms was 50 and 30 g for men and women, respectively. Mushroom consumption was categorised into three groups: ≤1 time/week, 2–3 times/week and ≥4 times/week.
Assessment of covariates
Height and weight were measured by using standard protocols with participants wearing light clothing and no shoes. BMI was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured to the nearest 0·1 cm using a non-stretchable measuring tape at the umbilical level with subjects standing and breathing normally. Information on age (birthdate), sex, educational level, occupation, household income, smoking status, drinking status and medical disease was collected using a structured questionnaire.
Fasting blood samples were drawn 12 ml from the cubital vein. Serum was used to measure fasting blood glucose (FBG) and lipids. FBG was measured using the glucose oxidase method, total cholesterol and TAG were measured using enzymatic methods, LDL was measured using the polyvinyl sulphuric acid precipitation method and HDL was measured using the chemical precipitation method. Serum ALT was measured using the International Federation of Clinical Chemistry method. These measurements were performed by using appropriate kits on a Cobas 8000 analyser (Roche).
After 5 min of rest, blood pressure (BP) was measured twice at the upper right arm using the TM-2655 oscillometric device (A&D), while the participants were in a seated position. The mean of two readings was used for analysis. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg or having a history of hypertension(Reference Chobanian, Bakris and Black21). The metabolic syndrome was defined according to the criteria of the American Heart Association scientific statements of 2009(Reference Alberti, Eckel and Grundy22). PA in the most recent week was evaluated by using the short form of the International Physical Activity Questionnaire(Reference Craig, Marshall and Sjostrom23). Total PA was calculated as metabolic equivalent hours per week (metabolic equivalent × h/week).
Statistical analysis
To normalise the data, we used the natural logarithm transformation for all continuous variables. Baseline characteristics of participants were expressed as geometric means and 95 % CI for continuous variables and percentages for categorical variables. Participant characteristics were compared using ANCOVA for continuous variables and logistic regression analysis for categorical variables, with adjustments for age. Moreover, in Table 1, to evaluate whether the baseline variables increased or decreased with increasing mushroom consumption, we presented the P values for linear trend.
Table 1. Age-adjusted characteristics of the participants according to categories of mushroom consumption (n 24 236)*
(Least square geometric mean values and 95 % confidence intervals; percentages)
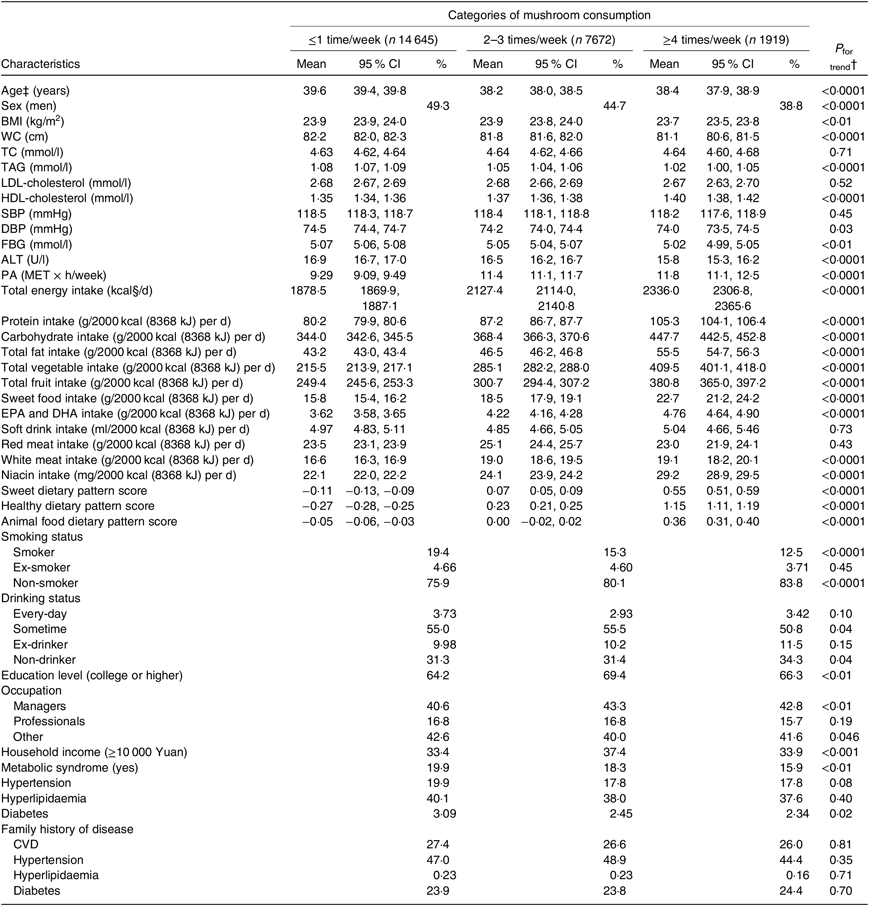
WC, waist circumference; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; ALT, alanine aminotransferase; PA, physical activity; MET, metabolic equivalent.
* Continuous variables are expressed as least square geometric means and 95 % CI and categorical variables are expressed as percentages.
† ANCOVA or logistic regression analysis adjusted for age where appropriate. The P for trend values were calculated by using the categories of mushroom consumption (≤1 time/week: 1; 2–3 times/week: 2 and ≥4 times/week: 3) as an ordinal variable.
‡ Values were not age-adjusted.
§ To convert kcal to kJ, multiply by 4·184.
We used logistic regression models to assess the association between mushroom intake and NAFLD or NAFLD with elevated ALT. The OR and corresponding 95 % CI were calculated using the ≤1 time/week group as the reference. We fitted five models. Model 1 was a crude model. Model 2 was adjusted for age (continuous: years), sex (categorical: men and women) and BMI (continuous: kg/m2). Model 3 was additionally adjusted for smoking status (categorical: current smoker, ex-smoker or non-smoker), drinking status (categorical: every-day drinker, sometime drinker, ex-drinker or non-drinker), educational level (categorical: < or ≥ college graduate), occupation (categorical: managers, professionals and other), household income (categorical: ≤ or >10 000 Yuan), PA (continuous: metabolic equivalent-h/week), family history of disease (including CVD, hypertension, hyperlipidaemia and diabetes (each yes or no)), individual history of disease (including hypertension, hyperlipidaemia and diabetes (each yes or no)) and total energy intake (continuous: kcal/d). Model 4 was further adjusted for FBG (continuous: mmol/l). Model 5 was adjusted for variables in model 4 plus niacin intake (quintiles) and three main dietary pattern scores (quintiles). Briefly, factor analysis with varimax rotation based on food items (mushrooms were not included in the calculation) was used to generate dietary patterns(Reference Xia, Xiang and Gu24). Three major dietary patterns were derived as follows: sweet pattern (higher factor loading of strawberry, kiwi fruit, persimmon, sweets, candied fruits, cakes, pineapple, cookies and ice cream), vegetable pattern (higher factor loading of celery, Chinese cabbage, cucumber, pumpkin, carrot, green vegetable, eggplant and raw vegetables) and animal food pattern (higher factor loading of animal organs, animal blood, preserved egg, sausage, instant noodle and seafood). Similar dietary patterns were derived in our previous study(Reference Xia, Xiang and Gu24). A higher dietary pattern score indicates stricter adherence to the dietary pattern. Moreover, P for trend values were calculated by using the categories of mushroom consumption (≤1 time/week: 1; 2–3 times/week: 2 and ≥4 times/week: 3) as an ordinal variable.
To assess effect modification, we calculated the P values for interactions between mushroom consumption and all covariates through the inclusion of cross-product terms in the fully adjusted model. In addition, to test the robustness of our results, multicollinearity among all covariates was assessed using variance inflation factor. Variance inflation factor exceeding 10 was a sign of multicollinearity. Moreover, we also conducted subgroup analyses by age, sex and BMI.
All statistical analyses were performed by using SAS version 9.4 for Windows (SAS, Inc.). All statistical tests were two-sided, and a P < 0·05 was considered to be statistically significant.
Results
Table 1 shows age-adjusted participant characteristics according to categories of mushroom consumption. Participants with higher mushroom intake tended to be younger and be more women and had lower BMI, waist circumference, TAG, diastolic BP, FBG, ALT and higher HDL. They were also more likely to be physically active, consumed more total energy and had higher sweet pattern score, vegetable pattern score and animal food pattern score. In addition, protein, carbohydrate, total fat, total vegetables, total fruits, sweet foods, EPA and DHA, white meat and niacin intake per 2000 kcal/d (8368 kJ/d) were higher in people with increased mushroom consumption. Furthermore, those with higher mushroom intake were more likely to be non-smokers and non-drinkers or employed as managers, and they were less likely to be occasional drinkers and had higher educational level and household income, and they had a lower prevalence of the metabolic syndrome and diabetes.
Table 2 shows age-adjusted participant characteristics by NAFLD status. Compared with participants without NAFLD, those with NAFLD tended to be older, men and to have higher BMI, waist circumference, total cholesterol, TAG, LDL, systolic BP, diastolic BP, FBG, ALT, PA, animal food pattern score and lower HDL. They also had higher protein, total fat, EPA and DHA, soft drinks, red meat, white meat and niacin intakes per 2000 kcal/d (8368 kJ/d), but had lower vegetable, fruit and sweet food intakes per 2000 kcal/d (8368 kJ/d). In addition, a higher proportion of these participants was current smokers or ex-smokers, sometime drinkers or ex-drinkers, employed as professionals or other and had the metabolic syndrome, hypertension, hyperlipidaemia, diabetes and family history of diabetes. A lower proportion of these participants was non-smokers, every-day drinkers, non-drinkers, with higher educational level, employed as managers and had family history of CVD or hyperlipidaemia.
Table 2. Age-adjusted characteristics of the participants by non-alcoholic fatty liver disease (NAFLD) status (n 24 236)*
(Least square geometric mean values and 95 % confidence intervals; percentages)

WC, waist circumference; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; ALT, alanine aminotransferase; PA, physical activity; MET, metabolic equivalent.
* Continuous variables are expressed as least square geometric means and 95 % CI and categorical variables are expressed as percentages.
† ANCOVA or logistic regression analysis adjusted for age where appropriate.
‡ Values were not age-adjusted.
§ To convert kcal to kJ, multiply by 4·184.
Table 3 presents the crude and adjusted associations between categories of mushroom consumption and newly diagnosed NAFLD or NAFLD with elevated ALT. The OR for newly diagnosed NAFLD decreased across increasing frequency of mushroom consumption. After adjustment for age, sex and BMI, the OR of newly diagnosed NAFLD across categories of mushroom consumption were 1·00 (reference) for ≤1 time/week, 0·90 (95 % CI 0·83, 0·99) for 2–3 times/week and 0·70 (95 % CI 0·59, 0·83) for ≥4 times/week (P for trend < 0·0001). Additional adjustments for sociodemographic and lifestyle factors as well as for FBG did not nearly change the inverse association. Further adjustments for dietary niacin intake and three major dietary patterns made the association slightly attenuated; the fully adjusted OR of newly diagnosed NAFLD were 1·00 (reference) for ≤1 time/week, 0·95 (95 % CI 0·86, 1·05) for 2–3 times/week and 0·76 (95 % CI 0·63, 0·92) for ≥4 times/week (P for trend = 0·01). Moreover, similar associations were also observed for NAFLD with elevated ALT. The fully adjusted OR of NAFLD with elevated ALT were 1·00 (reference) for ≤1 time/week, 0·92 (95 % CI 0·81, 1·04) for 2–3 times/week and 0·78 (95 % CI 0·62, 0·98) for ≥4 times/week (P for trend = 0·03).
Table 3. Association of mushroom consumption with non-alcoholic fatty liver disease (NAFLD) or NAFLD with elevated alanine aminotransferase (ALT) (n 24 236)
(Odds ratios and 95 % confidence intervals)
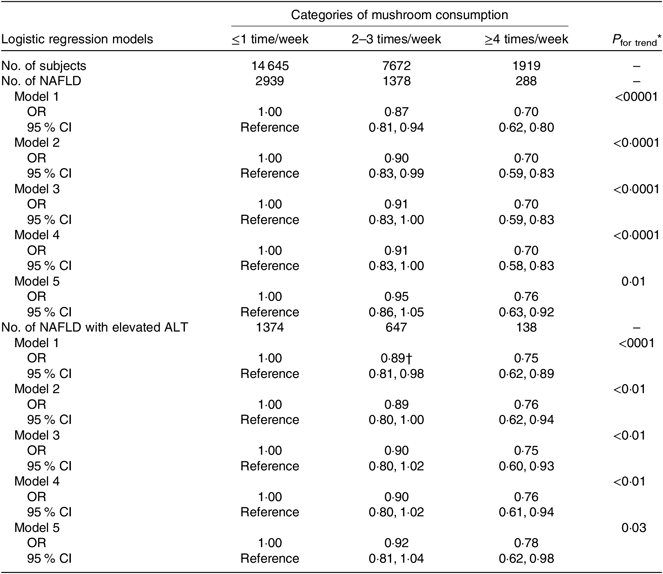
* Obtained by using multiple logistic regression analysis. The P for trend values were calculated by using the categories of mushroom consumption (≤1 time/week: 1; 2–3 times/week: 2 and ≥4 times/week: 3) as an ordinal variable.
† Model 1 was a crude model. Model 2 was adjusted for age (continuous: years), sex (men or women) and BMI (continuous: kg/m2). Model 3 was further adjusted for smoking status (categorical: current smoker, ex-smoker or non-smoker), drinking status (categorical: every-day drinker, sometime drinker, ex-drinker or non-drinker), educational level (categorical: < or ≥ college graduate), occupation (categorical: managers, professionals and other), household income (categorical: ≤ or >10 000 Yuan), physical activity (continuous: metabolic equivalent-h/week), family history of disease (including CVD, hypertension, hyperlipidaemia and diabetes (each yes or no)), individual history of disease (including hypertension, hyperlipidaemia and diabetes (each yes or no)) and total energy intake (continuous: kcal/d). Model 4 was additionally adjusted for fasting blood glucose (continuous: mmol/l). Model 5 was adjusted for variables in model 4 plus niacin intake (quintiles) and three main dietary pattern scores (quintiles). Mushroom intake was not included in the calculation of the dietary pattern score.
There was no evidence in the final model that the covariates modified the effect of the association between mushroom consumption and NAFLD (all P for interaction ≥ 0·10). For multicollinearity, all variance inflation factor values ranged from 1·01 to 2·44, indicating that no collinearity was accepted. In addition, the inverse association between mushroom intake and NAFLD persisted in subgroup analyses (data not shown).
Discussion
This is the first large population-based study to investigate the association between edible mushroom intake and NAFLD. The results suggested that high mushroom intake was associated with a low prevalence of NAFLD in the general Chinese population.
In the present study, we adjusted for a considerable number of confounding factors. First, we adjusted for age, sex and BMI. However, the results hardly changed after adjustment. Therefore, high mushroom intake was associated with a low prevalence of NAFLD, independent of age, sex and BMI. Second, sociodemographic variables, lifestyle factors, medical history and total energy intake(Reference Wehmeyer, Zyriax and Jagemann25) are closely associated with NAFLD. Therefore, we further adjusted for smoking status, drinking status, educational level, occupation, household income, PA, family history of disease, individual history of disease and total energy intake. However, the inverse association remained statistically significant when adjusted for these factors. Third, NAFLD is strongly associated with insulin resistance. Therefore, we further adjusted for FBG as a biomarker of insulin resistance. However, the inverse association between mushroom intake and NAFLD did not substantially change after adjustment for FBG. These results suggested that the impact of mushroom intake on NAFLD was independent of mediation by protection against insulin resistance. Finally, compared with individual nutrients or foods, dietary patterns represent a broader picture of nutrient and food consumption. It can be used as a covariate when examining a specific food, to determine whether the effect of the food is independent of the overall diet(Reference Hu26). Furthermore, a recent study has shown that high dietary niacin intake may have a favourable effect on the reduction of liver fat(Reference Linder, Willmann and Kantartzis27). Studies also have demonstrated that mushrooms contain high amounts of niacin(Reference Wang, Zhang and Wu6). Thus, in a final model, we further adjusted for three major dietary patterns and dietary niacin intake. However, the inverse association between mushroom consumption and NAFLD remained significant when adjusted for these dietary factors. These results indicated that the effect of mushroom on NAFLD is independent of niacin intake and overall diet.
A study conducted in mice demonstrated that 10 % Agaricus bisporus supplementation can reduce high-fat diet-induced NAFLD development after feeding 10 weeks(Reference Iniguez, Perez-Matute and Villanueva-Millan11). Similarly, another animal study also showed that dietary supplementation with 10 % air-dried powder of Mukitake mushrooms can alleviate NAFLD after an 8-week feeding period(Reference Nagao, Inoue and Inafuku12). These results from animal studies suggest that mushroom intake could have beneficial effects for mice with NAFLD. However, the association between mushroom consumption and NAFLD in humans remains unknown. The data from our human studies have been consistent with those of the animal studies. Therefore, our present study provides novel data that support a beneficial association between higher mushroom intake and a lower prevalence of NAFLD in the general population.
Many people in China consume a variety of edible mushrooms daily(Reference Chen, Cheng and Wu28). Mushrooms contain many nutritional components such as polyphenols, vitamins, minerals, fibres and polysaccharides(Reference Valverde, Hernandez-Perez and Paredes-Lopez29). Studies have suggested that edible mushrooms are potent antioxidants and anti-inflammatory agents(Reference Muszynska, Grzywacz-Kisielewska and Kala7,Reference Kozarski, Klaus and Jakovljevic8) that could play important roles in the development of NAFLD(Reference Masarone, Rosato and Dallio10,Reference Jung and Choi30) . Moreover, an animal study have indicated that supplementation with a variety of mushrooms (containing a 0·5 or 3 % mushroom mixture, which was equivalent to approximately 100 or 600 g of fresh mushroom intake per d in humans) can suppress visceral fat accumulation and improve gut microbiota(Reference Shimizu, Mori and Ouchi13). Furthermore, insulin resistance plays an important role in the pathogenesis of NAFLD. A randomised clinical trial showed that mushroom extract supplements (containing 500 mg Agaricus blazei Murill extract three times daily for 12 weeks) could improve insulin resistance(Reference Hsu, Liao and Lin31). Additionally, mushroom intake could suppress appetites, thereby reducing food intake(Reference Martel, Ojcius and Chang32). Therefore, the inverse association between mushroom intake and NAFLD development is biologically reasonable.
Notably, Table 2 shows NAFLD patients are more physically active than controls. Because men (geometric mean of PA was 11·8 (95 % CI 11·5, 12·1)) were more physically active than women (geometric mean of PA was 8·82 (95 % CI 8·62, 9·02)), we consider sex is an important confounding factor that explains the fact that NAFLD patients are more physically active than controls. Accordingly, we adjusted for age and sex. The results showed that age- and sex-adjusted geometric mean of PA was 10·2 (95 % CI 10·0, 10·4) in controls and 10·1 (95 % CI 9·68, 10·5) in NAFLD patients (P = 0·50). Therefore, the fact that NAFLD patients are more physically active than controls was confounded by sex.
To our knowledge, this is the first study that examined the association between mushroom consumption and NAFLD in humans. Strengths of our study include a large sample size and detailed measurement of habitual diet with a validated FFQ. Moreover, detailed information about lifestyle factors enabled us to conduct comprehensive analyses by taking into account many confounding factors.
The present study has several limitations. First, NAFLD was diagnosed using ultrasonography rather than liver biopsy (the ‘gold standard’ for diagnosing NAFLD). However, liver biopsy is time-consuming and expensive and not feasible in large observational studies. Second, diet was measured using an FFQ. Although validated, FFQ are subject to misclassification and random error. Therefore, the association was attenuated between mushroom intake and NAFLD. Third, high mushroom intake may be a marker of other healthy lifestyle factors. Baseline participant characteristics of the TCLSIH Cohort Study (Table 1) and Ohsaki Cohort 2006 Study(Reference Zhang, Tomata and Sugiyama33) show that participants with higher mushroom intake had many healthier dietary behaviours and lifestyle characteristics such as higher vegetable and fruit intake, less smoking and drinking and more physically active. However, adjustments for these factors did not substantially change the inverse association. Fourth, genetic and epigenetic factors play an important role in the pathogenesis of NAFLD(Reference Brunt, Wong and Nobili34,Reference Kim, Touros and Kim35) . Therefore, our results may not be generalisable to other populations. Finally, although we carefully adjusted for many potential confounding factors, there are likely to remain potentially important confounding differences that could affect the results(Reference Agoritsas, Merglen and Shah36).
Conclusions
In conclusion, the present study provides the first evidence of an association between edible mushrooms and NAFLD in humans. Further prospective cohort studies are needed to validate these results.
Acknowledgements
The authors gratefully acknowledge all the people that have made the present study.
The present study was supported by grants from the National Natural Science Foundation of China (no. 91746205).
S. Z. analysed data and wrote the paper. Y. G., M. L., J. F., Q. Z., L. L., G. M., Z. Y., H. W., X. B., S. S., X. W., M. Z., Q. J., K. S. and Y. W. conducted research. K. N. designed research and had primary responsibility for final content. All authors read and approved the final manuscript.
There are no conflicts of interest.







