Deception is a normal component of human social interaction that follows a developmental trajectory. Reference Spence, Hunter, Farrow, Green, Leung and Hughes1 Disorders such as antisocial personality disorder and psychopathy, in particular, are characterised by high levels of deceptive behaviours and show a poor response to psychological treatment. Reference Hare, Cooke and Forth2 Improving our ability to detect deceptive behaviours in forensic samples may help assist in the process of risk assessment and management of high-risk antisocial individuals. Although there are few studies explicitly investigating the relationship between psychopathy and deception, there is some evidence to suggest that psychopathy is not associated with an increased ability to deceive, but may be associated with alterations in the non-verbal correlates of deception. Reference Klaver, Lee and Hard3 In recent years there has been a growth in interest in the use of functional magnetic imaging techniques (fMRI) to study the neural correlates of deception. In normal populations, fMRI neuroimaging studies of deception show activation in a variety of areas in the prefrontal cortex including the orbitofrontal/ventrolateral prefrontal cortex, Reference Spence, Hunter, Farrow, Green, Leung and Hughes1,Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4–Reference Kozel, Padgett and George10 and dorsolateral prefrontal cortex. Reference Lee, Liu, Tan, Chan, Mahankali and Feng5–Reference Abe, Suzuki, Mori, Itoh and Fujii13 In addition, a number of other brain areas have been implicated in the neural control of deception including the anterior cingulate cortex, Reference Ganis, Kosslyn, Stose, Thompson and Yurgelun-Todd11–Reference Langleben, Schroeder, Maldjian, Gur, McDonald and Ragland14 thalamus, Reference Nunez, Casey, Egner, Hare and Hirsch9,Reference Kozel, Johnson, Mu, Grenesko, Laken and George15 temporal lobes, Reference Kozel, Padgett and George12,Reference Langleben, Schroeder, Maldjian, Gur, McDonald and Ragland14,Reference Kozel, Johnson, Mu, Grenesko, Laken and George15 parietal lobes, Reference Lee, Liu, Tan, Chan, Mahankali and Feng5,Reference Lee, Ho-Ling, Chan, Yen-Bee, Fox and Gao6,Reference Langleben, Schroeder, Maldjian, Gur, McDonald and Ragland14,Reference Kozel, Johnson, Mu, Grenesko, Laken and George15 caudate Reference Lee, Liu, Tan, Chan, Mahankali and Feng5,Reference Nunez, Casey, Egner, Hare and Hirsch9 and insula. Reference Langleben, Schroeder, Maldjian, Gur, McDonald and Ragland14,Reference Kozel, Johnson, Mu, Grenesko, Laken and George15 Recently, structural MRI studies have demonstrated increases in frontal white matter, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti16,Reference Yang, Raine, Narr, Lencz, LaCasse and Colletti17 particularly in the orbitofrontal lobes, Reference Yang, Raine, Narr, Lencz, LaCasse and Colletti17 in populations with marked deceitful traits as measured by the Psychopathy Checklist Revised (PCL–R). Reference Hare18 To date there is only one published fMRI study of deception that has included a measure of psychopathic personality traits. In an fMRI study of autobiographical and non-autobiographical deception in a mixed gender sample, Nunez et al Reference Nunez, Casey, Egner, Hare and Hirsch9 found that higher coldheartedness scores on the Psychopathic Personality Inventory (PPI) Reference Lilienfeld and Andrews19 were associated with reduced blood oxygen level dependent (BOLD) responses in the posterior cingulate and precuneus cortices, during non-autobiographical deception. However, psychopathic personality traits have been shown to be less frequent in female than male samples Reference Salekin, Rogers and Sewell20,Reference Salekin, Rogers, Ustad and Sewell21 and the use of a mixed gender sample in the Nunez et al Reference Nunez, Casey, Egner, Hare and Hirsch9 study may have attenuated the nature and type of associations found between brain activity during deception and psychopathic personality traits.
The aim of the present study was to use a simple fMRI deception paradigm devised by Spence et al Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 to investigate the relationship between BOLD responses during deception and psychopathic personality traits measured using the PPI in a sample of male participants drawn from the normal population. Similar to Nunez et al, Reference Nunez, Casey, Egner, Hare and Hirsch9 a lie was defined by the three basic features described by Coleman & Kay. Reference Coleman and Kay22 That is, the intentional giving of a false response and awareness that the response is false rather than a mistake. We predicted that consistent with the findings of previous studies using the same paradigm, Reference Spence, Hunter, Farrow, Green, Leung and Hughes1,Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 deceitful responding (relative to truthful responding) would be associated with increased BOLD responses in the ventrolateral prefrontal cortex, and increased response times for false responses indicating an interference effect. We further predicted that scores on the PPI sub-scales would be significantly associated with BOLD responses in brain areas previously implicated in the neural control of deception.
Method
Participants
Twenty-four (21 right handed and 3 left handed) male participants aged 19–60 years (mean=30.04, s.d.=11.34) were recruited from University of Manchester ancillary staff and students. Specifically, participants were recruited using adverts placed in the University staff news letter and by approaching portering staff in each building. Students were recruited by targeting university sports teams (i.e. rugby teams) with the hypothesis that participants drawn from these populations may show higher levels of subclinical psychopathy spectrum personality traits. The majority (n=22) of the sample were White with the remaining participants of Asian ethnicity. The mean IQ of the sample measured using the National Adult Reading Test Reference Nelson23 was 113.87 (s.d.=7.60, range 96–128). The study was approved by the University of Manchester research ethics committee and participants gave written informed consent for participation in the study.
Measurement of psychopathic personality traits
Psychopathic personality traits were assessed using the Psychopathic Personality Inventory. Reference Lilienfeld and Andrews19 The PPI is a 187-item self-report questionnaire with a total score and 8 sub-scales designed to measure psychopathic personality traits in a dimensional manner.
These include:
-
(a) Machiavellian egocentricity which is characterised by ‘looking out for one's own interests before others’;
-
(b) social potency, or the ‘ability to be charming and influence others’;
-
(c) coldheartedness is the ‘propensity towards callousness, guiltlessness, and unsentimentality’;
-
(d) carefree non-planfulness, is the ‘non-planning component of impulsivity’;
-
(e) fearlessness, is the ‘absence of anxiety and harm concerning eagerness to take risks’;
-
(f) blame externalisation, is the ‘tendency to view others as source of problems’;
-
(g) impulsive non-conformity, is the ‘reckless lack of concern for social mores’;
-
(h) stress immunity, is the ‘absence of marked reactions to otherwise anxiety provoking events’ (pp. 500–2). Reference Lilienfeld and Andrews19
Each item consists of a statement to which participants must indicate how accurately it applies to them using a 4-point scale ranging from 1 ‘false’ to 4 ‘true’. The PPI has been shown to have good convergent and discriminant validity in both community and criminal samples. Reference Lilienfeld and Andrews19,Reference Nelson23–Reference Patrick, Edens, Poythress, Lilienfeld and Benning27 In particular, it shows good criterion related validity when compared with structured, collaboratively rated clinical assessments of psychopathy such as the PCL–R. Reference Hare18,Reference Poythress, Edens and Lilienfeld24 The PPI scores for the sample are shown in Table 1. The mean total PPI score for the sample was lower than that reported by Lilienfeld et al (S. Lilienfeld, personal communication, 2008), for a large sample of substance misusing male prisoners (see online supplement for details). However, individuals in the present sample did show total scores at or above the criminal mean and the fearlessness, social potency, coldheartedness and stress immunity scores for the present sample were remarkably similar to those reported for the criminal population. Additional figures demonstrating the sample distribution of scores on each sub-scale can be found in the online Fig. DS1.
Table 1 The Psychopathic Personality Inventory scores for the sample
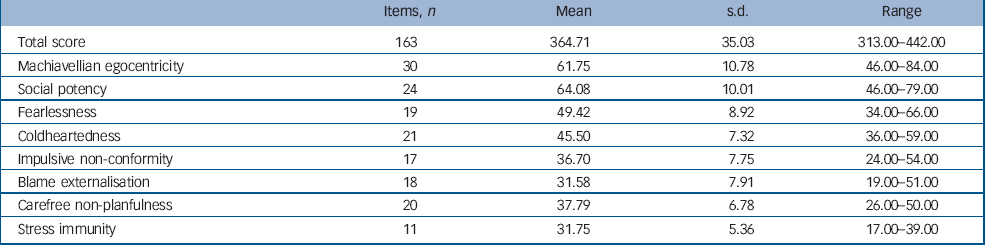
| Items, n | Mean | s.d. | Range | |
|---|---|---|---|---|
| Total score | 163 | 364.71 | 35.03 | 313.00–442.00 |
| Machiavellian egocentricity | 30 | 61.75 | 10.78 | 46.00–84.00 |
| Social potency | 24 | 64.08 | 10.01 | 46.00–79.00 |
| Fearlessness | 19 | 49.42 | 8.92 | 34.00–66.00 |
| Coldheartedness | 21 | 45.50 | 7.32 | 36.00–59.00 |
| Impulsive non-conformity | 17 | 36.70 | 7.75 | 24.00–54.00 |
| Blame externalisation | 18 | 31.58 | 7.91 | 19.00–51.00 |
| Carefree non-planfulness | 20 | 37.79 | 6.78 | 26.00–50.00 |
| Stress immunity | 11 | 31.75 | 5.36 | 17.00–39.00 |
Deception paradigm
The deception task used in the present study was based on the task reported by Spence et al. Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 Prior to scanning, participants were asked to fill in a questionnaire determining if they had performed 36 everyday acts during the current day (making the bed, taken a tablet, etc). Once in the scanner, participants were asked to lie or tell the truth about the performance of the 36 acts. In a standard ABAB block design, each participant was required to lie about the performance of each act once and tell the truth about the performance of each of the 36 acts once. Each of the 12 blocks contained 6 acts and each act was displayed visually on a screen for 5 s in the form ‘In the course of today have you… (made the bed)’. Participants were required to make a motor response on a button box in order to answer yes or no. They were instructed to lie or tell the truth depending on which prompt appeared on the screen. In order to increase task performance, participants were informed that an experimenter would be monitoring their responses in order to detect whether they were lying. Participants carried out a practice block prior to the main task. Response accuracy was calculated by comparing responses made to the truth or lie prompt during the task to the original response made in the 36-item questionnaire. Response times (seconds) were recorded for each trial and average response times during the truth and lie conditions were compared using a two-tailed paired-sample t-test. In addition, response times and response accuracy (relative to the original questionnaire items) were correlated with PPI sub-scales using Spearman's correlations.
MRI image acquisition
Images were acquired using a Philips (Eindhoven, Holland) 1.5 T Gyroscan ACS NT retrofitted with Powertrak 6000 gradients, operating at a software level 6.1.2 T2 *-weighted volumes were acquired using a singleshot echo-planar imaging pulse sequence. Each volume comprised 40 contiguous axial slices, (response time (TR)/echo time (TE) 5000/40 ms, 64×64 data matrix, 3.5 mm thickness with an inplane resolution of 3×3 mm). The stimuli were rear projected onto a screen using a liquid crystal display projection system. Task administration was coupled to image acquisition using personal computer software and hardware linked to a response button.
Analysis
Imaging data were analysed using Statistical Parametric Mapping (SPM5, Friston, The Welcome Department of Cognitive Neurology, London, UK). Images were corrected for motion and then realigned with the first scan serving as a reference. The scans were then normalised into a standard stereotactic space Reference Talairach and Tournoux28 using Montreal Neurological Institute templates. Images were finally smoothed with a 10 mm Gaussian filter to facilitate inter-individual averaging. After this spatial preprocessing, at an individual level, a general linear model with a delayed boxcar waveform was used to model BOLD signal changes during the task. The individual images were then combined in a random effects analysis that would allow inference to the general population using an independent samples t-test to investigate the main effect of the task. The main effect for the lie condition was the BOLD signal seen in the lie condition minus the BOLD signal seen in the truth condition. The main effect for the truth condition was the reverse subtraction.
The resulting statistical maps were thresholded at P<0.001 uncorrected with only cluster sizes of five or more contiguous voxels being reported. As the inferior (frontopolar/orbitofrontal/ventrolateral prefrontal), and superior frontal (dorsolateral/dorsomedial prefrontal) cortex are the regions in which activations are most consistently reported across different deception paradigms in the literature, we concentrated our primary analysis solely on these areas. In order to control for type I errors we applied small volume corrections Reference Worsley, Marrett, Neelin, Vandai, Friston and Evans29 for family-wise error at P⩽0.05 to these a priori regions of interest. Areas of activation at the P<0.001 uncorrected level are also reported when bilateral activations were seen.
In addition, we performed an exploratory analysis of signal in those areas less consistently identified by the past literature as active during deceptive responses; anterior cingulate, caudate, insula, thalamus, temporal lobes, temporal poles, posterior cingulate and precuneus. Again, in these regions we performed small volume corrections for family-wise error.
The association between BOLD responses during the lie condition and scores on the PPI sub-scales was investigated using simple regression analysis. During this analysis, for each a priori specified brain area of interest that exhibited a significant voxel-based correlation, the BOLD signal change observed during the lie condition was extracted from SPM and used in Spearman's correlational analysis (SPSS version 14 for Windows) in order to produce confirmatory r-values. As a further protection against type I errors we have only reported results where the probability value of the associated r-value exceeded P⩽0.001 for unilateral activations and P⩽0.01 for bilateral activations.
Finally, in order to control for possible within-sample variation in age, we examined the correlation between age and BOLD responses during the deception condition. Age was found to be positively correlated with BOLD response in the temporal poles and insula and was therefore entered as a nuisance covariate in any analyses where significant BOLD responses were exhibited in these areas.
Results
Behavioural data
Mean response accuracy was similar for the truth (91.06%, s.d.=11.66) and lie condition (91.55%, s.d.=12.21). The number of accurate responses (maximum of 36) for the lie condition showed a significant positive correlation with PPI stress immunity score (r=0.53, P=0.009).
Mean response times (seconds) were significantly slower during the lie condition compared with the truth condition (lie mean response time 2.66 s (s.d.=0.42), truth mean response time 2.56 s (s.d.=0.39); t=2.43, P=0.024).
There were no significant correlations between any of the other mean PPI sub-scales/factor scores and mean response accuracy or response time for the lie condition or truth condition.
Functional MRI data
Main effect of task
During the lie condition (relative to the truth condition) an increased BOLD response was seen in the left ventrolateral prefrontal cortex (Brodmann area (BA)47). An increased BOLD response at the P<0.001 uncorrected level was also seen in the right ventrolateral prefrontal cortex (Table 2 and online Fig. DS2).
Table 2 Main effect of the deception task
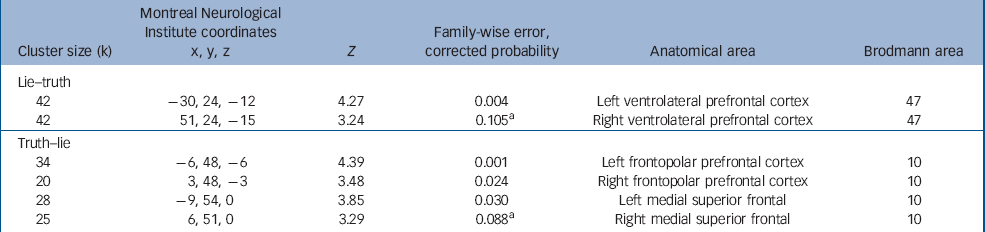
| Cluster size (k) | Montreal Neurological Institute coordinates x, y, z | Z | Family-wise error, corrected probability | Anatomical area | Brodmann area |
|---|---|---|---|---|---|
| Lie–truth | |||||
| 42 | –30, 24, −12 | 4.27 | 0.004 | Left ventrolateral prefrontal cortex | 47 |
| 42 | 51, 24, −15 | 3.24 | 0.105 a | Right ventrolateral prefrontal cortex | 47 |
| Truth–lie | |||||
| 34 | –6, 48, −6 | 4.39 | 0.001 | Left frontopolar prefrontal cortex | 10 |
| 20 | 3, 48, −3 | 3.48 | 0.024 | Right frontopolar prefrontal cortex | 10 |
| 28 | –9, 54, 0 | 3.85 | 0.030 | Left medial superior frontal | 10 |
| 25 | 6, 51, 0 | 3.29 | 0.088 a | Right medial superior frontal | 10 |
a. Significant at P<0.001, uncorrected
During the truth condition (relative to the lie condition) an increased BOLD response was seen bilaterally in the frontopolar area of the prefrontal cortex (BA10) extending into the medial superior frontal cortex (Table 2).
The results remained similar when the analysis was rerun excluding six participants who achieved less than 90% overall response accuracy for the task.
Relationship between the PPI sub-scales and BOLD response during the lie condition
The results of the correlational analysis are shown in Table 3. During the lie condition (relative to the truth), fearlessness scores were negatively correlated with BOLD responses in the right orbitofrontal cortex (Fig. 1). Coldheartedness scores were negatively correlated with BOLD responses bilaterally in the temporal poles (Fig. 2). Machiavellian egocentricity scores were negatively correlated with BOLD responses bilaterally in the caudate. Social potency scores were negatively correlated with BOLD responses bilaterally in the right posterior cingulate. Stress immunity scores were negatively correlated with BOLD response bilaterally in the insula. These associations remained significant after covarying for handedness and age (where appropriate).
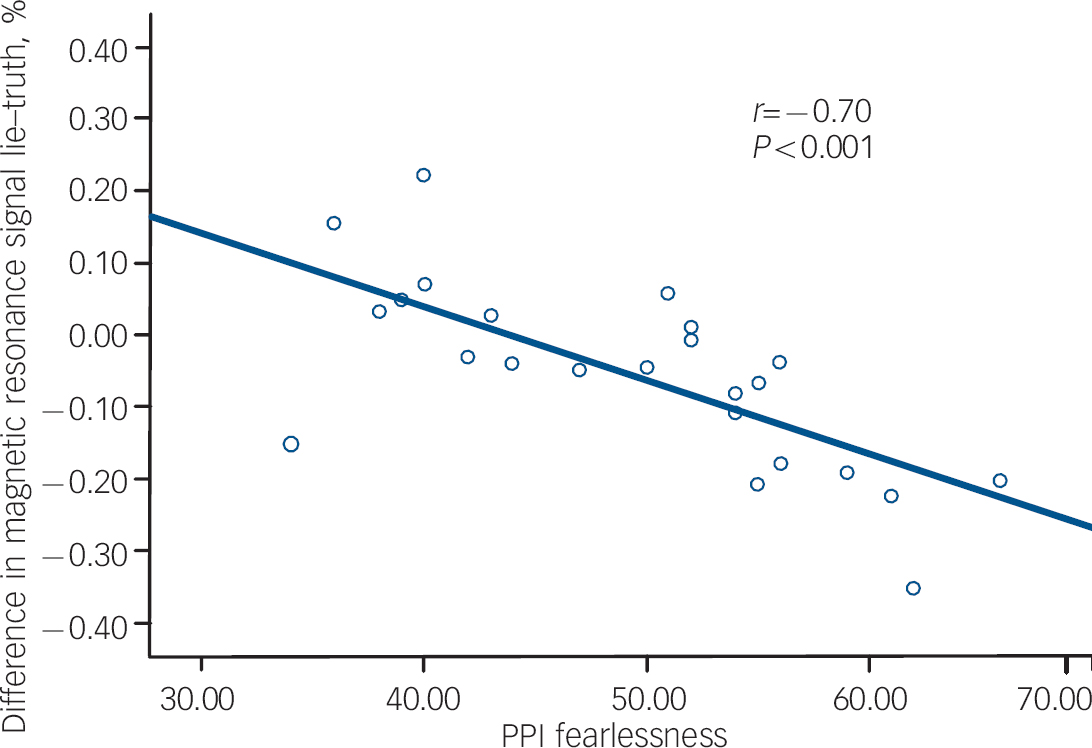
Fig. 1 The association between Psychopathic Personality Inventory (PPI) fearlessness and blood oxygen level dependent response in the right orbitofrontal cortex during deception.
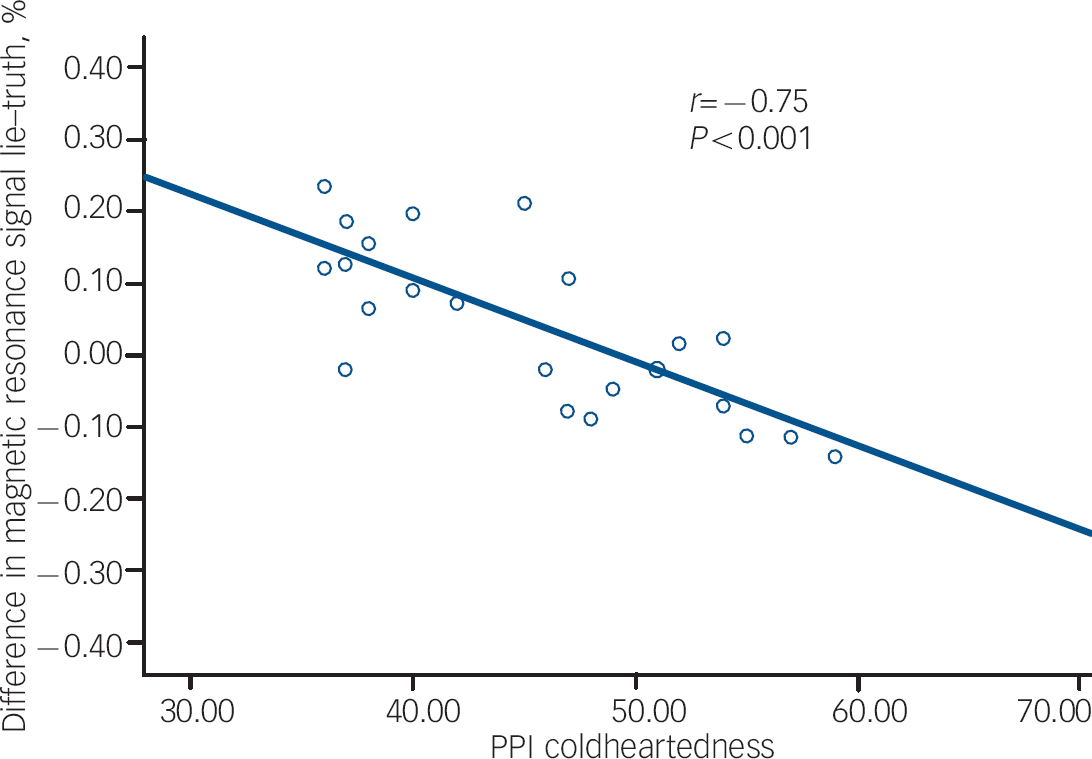
Fig. 2 The association between Psychopathic Personality Inventory (PPI) coldheartedness and blood oxygen level dependent response in the temporal poles during deception.
Table 3 The association between Psychopathic Personality Inventory (PPI) sub-scale scores and blood oxygen level dependent (BOLD) responses in brain areas of interest

| PSI scale | Cluster size (k) | Montreal Neurological Institute coordinates x, y, z | Z | Family-wise error, probability | Anatomical area | Brodmann area | Spearman's r |
|---|---|---|---|---|---|---|---|
| Fearlessness | 5 | 3, 21, −12 | 3.87 | 0.007 | Right orbitofrontal | 11 | –0.74, P<0.001 |
| 33 | 12, 42, −9 | 3.53 | 0.021 | Right orbitofrontal | 11 | –0.70, P<0.001 | |
| Coldheartedness | 61 | –45, 12, −24 | 4.21 | 0.005 | Left temporal pole a | 38 | –0.75, P<0.001 |
| 20 | 33, 18, −33 | 3.47 | 0.051 | Right temporal pole a | 38 | –0.61, P=0.001 | |
| Machiavellian egocentricity | 16 | 18, 0, 24 | 4.07 | 0.005 | Right caudate | n/a | –0.75, P<0.001 |
| 9 | –3, 6, −6 | 3.53 | 0.030 | Left caudate | n/a | –0.65, P=0.001 | |
| Social potency | 10 | 3, −42, 15 | 4.00 | 0.003 | Right posterior cingulate | 29 | –0.67, P<0.001 |
| Stress immunity | 13 | –39, −18, 21 | 3.70 | 0.035 | Left insula a | n/a | –0.54, P=0.006 |
| 31 | 33, −18, 6 | 3.89 | 0.019 | Right insula a | n/a | –0.75, P<0.001 |
a. Result remained significant after covarying for age
There were no significant associations between BOLD responses during the lie condition in any of the brain areas of interest and the PPI total score or impulsive non-conformity, carefree non-planfullness and blame externalisation sub-scale scores.
Discussion
The ability to non-invasively examine the neural correlates of deception in disorders such as antisocial personality disorder and psychopathy, where deception is prominent, could offer new insights into the neuropathology of these disorders. Despite the range of paradigms and scanning parameters used, BOLD fMRI studies in healthy volunteers suggest that the prefrontal cortex, anterior cingulate cortex, temporal and parietal lobes, and a number of subcortical areas are involved in the neural control of deception. Reference Spence, Hunter, Farrow, Green, Leung and Hughes1,Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4–Reference Kozel, Johnson, Mu, Grenesko, Laken and George15 Few studies have specifically looked at the role of psychopathic personality traits despite one report that callous unemotional traits may be associated with reduced activation in brain regions required for deceptive behaviours that have no personal significance. Reference Nunez, Casey, Egner, Hare and Hirsch9 This study examined the relationship between psychopathic personality trait scores and BOLD responses in brain areas of interest during a simple deception task devised by Spence et al Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 in male participants drawn from the normal population.
Behavioural data
Consistent with previous studies in this field Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4,Reference Nunez, Casey, Egner, Hare and Hirsch9 we found that the behavioural data indicated that mean response times were significantly longer for the lie compared with the truth condition but there was no effect of personality trait scores. It is possible that the relatively small sample size may account for the lack of an observed association between response time and personality traits. However, Nunez et al Reference Nunez, Casey, Egner, Hare and Hirsch9 also failed to find an association between response times during lie responses and scores on the PPI. We did find, however, that stress immunity, which reflects a lack of anxiety, was positively correlated with response accuracy in the lie condition. This would support the argument that interpersonal differences in trait anxiety can mediate behavioural and physiological responses during deception. Reference Wiley30,Reference Steinbrook31 This is an area that may warrant further investigation in imaging studies of deception.
Main effect of task imaging data
Consistent with our hypothesis, we found that the lie condition (relative to the truth condition) was associated with increased BOLD responses bilaterally in the ventrolateral prefrontal cortex. This is a replication of the finding reported by Spence et al Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 using the same task in a smaller sample, and as such represents the first between-laboratory replication of an fMRI deception finding (a research gap recently highlighted by Spence Reference Spence32 ). This finding also supports previous studies using different deception paradigms that have reported deception related BOLD responses in inferior frontal areas. Reference Lee, Ho-Ling, Chan, Yen-Bee, Fox and Gao6,Reference Luan Phan, Magalhaes, Ziemlewicz, Fitzgerald, Green and Smith8,Reference Nunez, Casey, Egner, Hare and Hirsch9,Reference Langleben, Schroeder, Maldjian, Gur, McDonald and Ragland14 As the ventrolateral prefrontal cortex has been shown to be active during a number of cognitive control paradigms Reference Ridderinkhof, van den Wildenberg, Segalowitz and Carter33–Reference Dove, Manly, Epstein and Owen36 this finding also adds further weight to the argument that deception engages executive prefrontal systems in order to achieve the production of a ‘lie’ at the same time as withholding the truth. Reference Spence, Hunter, Farrow, Green, Leung and Hughes1,Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 Similar to Spence et al Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4 we did not find any significant BOLD responses during deception in any of the other frontal areas of interest. Given that previous studies in the area Reference Spence, Farrow, Herford, Wilkinson, Zheng and Woodruff4–Reference Abe, Suzuki, Mori, Itoh and Fujii13 have shown deception-related activity in the dorsolateral prefrontal cortex and orbitofrontal cortex, it is possible that between-study deception paradigm and sample size differences may account for the current lack of findings in these areas.
We also found that the truth condition was associated with increased BOLD responses bilaterally in the frontopolar cortex. In a recent review of frontopolar function, Koechlin et al Reference Koechlin and Hyafil37 suggest that during decision-making tasks, lateral inferior frontal regions inhibit frontopolar regions in order to switch to and maintain a given response set. They also propose that frontopolar regions are able to store a previous response set in a back-up buffer in order to reinstate it following a reduction in top-down inhibition. In the present study, during the lie condition, the lateral inferior frontal cortex may have been exerting a strong inhibitory influence on the frontopolar regions in order to override the alternative truthful response set and switch to the deceitful set. In the truth condition, the frontopolar regions may have shown enhanced activation while accessing and returning to the use of the truthful response set. It is possible, therefore, that the underlying ‘task-switching’ nature of the deception paradigm used in the current study may be largely responsible for the activations seen during the truth condition. The majority of published studies in this area do not specifically examine BOLD responses during the truthful condition; however, of those that do, few reported any areas of activation during truthful responding. Reference Spence, Hunter, Farrow, Green, Leung and Hughes1,Reference Luan Phan, Magalhaes, Ziemlewicz, Fitzgerald, Green and Smith8–Reference Kozel, Padgett and George10 Despite these negative findings, Langleben et al Reference Langleben, Loughead, Bilker, Ruparel, Childress and Busch7 reported truth-related activations in the left medial frontal gyrus (BA46). The differences in findings may not only reflect inter-study differences in methodology, but also, in this case in particular, inter-laboratory differences in the ability to accurately image the frontopolar region without a large degree of airspace related signal drop out.
Relationship between personality factors and imaging findings
Psychopathy as a construct is generally considered to be characterised by high levels of callous unemotional traits and these traits are believed to be related to dysfunction in the limbic (amygdala) striatal prefrontal circuitry. Reference Blair38 The ability to measure key components of the psychopathy construct in less pathological samples allows us to postulate on the potential neuropathology of this disorder in clinical samples. The present study found evidence that BOLD responses during deception in a number of brain areas of interest were correlated with some, but not all, psychopathic personality trait scores. Specifically focusing on the prefrontal cortex, we found inverse correlations between fearlessness scores and BOLD responses in the right orbitofrontal lobe. This suggests that those with low levels of fear and harm avoidance may find it easier to lie and therefore do not activate the orbitofrontal cortex which is a key neural structure implicated in behavioural inhibition in a number of previous imaging studies. Reference Ridderinkhof, van den Wildenberg, Segalowitz and Carter33,Reference Horn, Dolan, Elliot, Deakin and Woodruff39 Our own work on the neuropsychology of behavioural inhibition in samples of offenders with antisocial personality disorder suggests that this clinical group are significantly impaired on tasks probing orbitofrontal cortex function compared with healthy controls. Reference Dolan and Park40 Given reports of increased white matter volume in some of the prefrontal subregions implicated in deception-related neurocircuitry in those who lie, cheat and manipulate others, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti16,Reference Yang, Raine, Narr, Lencz, LaCasse and Colletti17 this is a brain area that warrants more detailed investigation.
In our exploratory analysis, we found that coldheartedness was inversely correlated with temporal pole BOLD responses. As the temporal pole has been found to be active during theory of mind tasks, Reference Olson, Plotzker and Ezzyat41 our findings highlight the importance of looking at the role of callous unemotional traits and theory of mind/mentalising ability in tasks assessing the cognitive elements that may be involved in the deception and manipulation of others. Nunez et al Reference Nunez, Casey, Egner, Hare and Hirsch9 found that coldheartedness assessed using the PPI was negatively correlated with BOLD responses in the posterior cingulate and precuneus cortices. In the present study, BOLD signal in the posterior cingulate was inversely associated with PPI social potency scores. Differences between studies may reflect the gender differences in the nature of the samples studied. Base rates of psychopathic traits are lower in female populations, Reference Grann42 and female populations exhibit lower scores on some (but not all) specific symptoms such as callousness/lack of empathy on the PCL–R, Reference Grann42 PPI stress immunity, PPI social potency and on a factor similar to PPI coldheartedness. Reference Maesschalck, Vertommen and Hooghe43 In addition, there is some indication of a gender difference in the bio-behavioural correlates of psychopathy, with only male cohorts exhibiting a lack of physiological reactivity to aversive stimuli Reference Justus and Finn44 and stress. Reference O'Leary, Loney and Eckel45
In this study we also found that lower stress immunity scores (i.e. more anxiety/stress) were associated with greater BOLD responses in the bilateral insula. As the insula has been shown to be involved in error processing during Go/No Go tasks, Reference Hester, Fassbender and Garavan46 it is possible that stress immunity (which may relate to vigilance) may influence the function of the neural circuitry involved in the processing and monitoring of errors. Although at least some degree of anxiety is needed to engage error processing circuitry appropriately, it is possible that high levels may impair accuracy performance on behavioural tasks and moderate neural responses in imaging studies. Further studies are required to investigate the significance of both trait and state anxiety in deception-related brain activation patterns in those with antisocial and deceptive personality traits.
We found that higher Machiavellian egocentricity scores were associated with reduced BOLD responses in the bilateral caudate. Although there is a limited literature to compare the findings of the present study, decreased caudate activity appears to be associated with higher scores on the interpersonal (deceptive/superficial/grandiose) component of psychopathy, Reference Soderstrom, Hultin, Tullberg, Wikkelso, Ekholm and Forsman47 indicating that people with these and related personality traits, such as Machiavellian egocentricity, show reduced activation of caudate regions which are a key component of the subcortical straital network. A common finding across species and methodologies is the involvement of the striatum, the input structure of the basal ganglia, in a circuit responsible for mediating goal-directed behaviour. Reference Delgado48 In functional imaging studies, the caudate has been shown to be involved in the inhibition of both motor and mental responses Reference Mink49–Reference Durston, Davidson, Thomas, Worden, Tottenham and Martinez55 and appears to be specifically involved in the mediation of arousal. Reference Brown, Manuck, Flory and Hariri56
Overall, our findings fit with the previous literature suggesting that simple deception tasks activate prefrontal regions implicated in behavioural restraint and conflict monitoring and that lying results in greater activation than truthful responding. Our findings also tentatively suggest that specific personality traits may have a modulating effect on brain responses to deception tasks and that future studies examining brain activation during deception in offenders with and without psychopathic traits may be of value in understanding the neuropathology of psychopathy and antisocial personality disorder.
Limitations
Although the results of the present study are suggestive of an association between psychopathic traits and the neural processes involved in deception, there are a number of limitations that need to be taken into consideration. The deception paradigm used is highly constrained and similar in terms of cognitive demands to a Go/No Go test of behavioural inhibition. Recent imaging studies of deception are utilising more complex ecologically valid paradigms. For example, a recent study by Abe et al Reference Abe, Suzuki, Mori, Itoh and Fujii13 has demonstrated the involvement of the amygdala in a deception paradigm with a social component and it is possible that this neural response may also show some relationship with the callous/unemotional aspects of psychopathy. In addition, the use of the PPI in the present study may have limited the measurement of the core deceitful/manipulative components of psychopathy. The majority of the PPI items focus on impulsive/antisocial or fearless/dominant traits, Reference Benning, Patrick, Hicks, Blonigen and Krueger26 a more selective use of multiple measures of deception, such as those used by Yang et al, Reference Yang, Raine, Lencz, Bihrle, Lacasse and Colletti16,Reference Yang, Raine, Narr, Lencz, LaCasse and Colletti17 may have produced more specific neural correlates of a deceitful personality type.
Acknowledgements
This work was supported by an award of free MRI scanning time given by the Translational Imaging Unit, Faculty of Medical and Human Sciences, University of Manchester, UK.








eLetters
No eLetters have been published for this article.