Background
Cerebrospinal fluid (CSF) analysis remains an important tool in the assessment of infectious or autoimmune disorders of both the central and peripheral nervous systems. For CSF Total Protein (CSF-TP) obtained by lumbar puncture, a reference range of 0.15–0.45 g/L (or 15–45 mg/dL) is very frequently quoted in medical literature and has been widely implemented in hospital clinical laboratories. This specific range of values can be traced back to a monograph published by H. Houston Merritt in 1938.Reference Merritt and Fremont-Smith1
Despite its broad use across Canada and the world, concerns can be raised about this reference range: (1) values may be derived from small sample sizes studying young healthy volunteers, using outdated laboratory techniques; (2) only the upper limit typically is usually considered diagnostically relevant; and (3) the reference values are not incrementally adjusted in later decades of life. More recent reference studies done in a rigorous fashion, suggest that age-dependent and significantly higher upper limits (above 0.6 g/L after the age 50 years) are more representative.Reference McCudden, Brooks, Figurado and Bourque2, Reference Hegen, Auer, Zeileis and Deisenhammer3
We have recently reported results of a web-based survey to assess what CSF-TP upper reference values are used in clinical hospital laboratory hospitals worldwide.Reference Bourque, Breiner and Moher4 In the present paper, we offer an in-depth examination of data from Canadian hospital centers, with the goal of understanding current practice patterns in academic and community institutions and determining if there is a need to update reference limits in Canada.
Methods
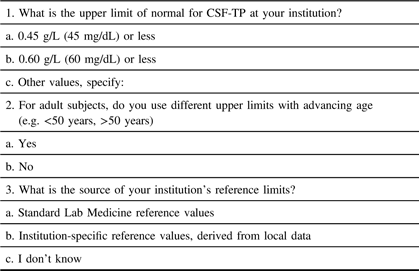
The Ottawa Hospital Research Institute Ethics Board approved the electronic distribution of a survey on institutional CSF-TP reference ranges (protocol #20170816–01H). This survey was distributed worldwide and was comprised of three simple questions (Table 1).Reference Bourque, Breiner and Moher4 For Canadian centers, permission was granted by the Canadian Neurological Sciences Federation (CNSF) for distribution to Full/Active members. In the event of duplicate responses for the same institution, the highest value was chosen if answers differed.
Table 1: Web-based questionnaire and response options
Additional responses to the survey question #1 were obtained by telephoning biochemistry technologists in individual clinical hospital laboratories across the country. We limited this question to sites where CSF protein is measured locally (as opposed to external/reference laboratory sample analysis). The contact numbers for these laboratories were obtained by consulting hospital directories of Canadian provincial and territorial health services websites. Every effort was made to include smaller community health centers and offer a wide geographical representation.
Web-based and telephone interview results were collated in a spreadsheet documenting geographical location, university-affiliated tertiary vs community hospital, CSF-TP upper limit in g/L, and whether an age-dependent adjustment was reported. Mean CSF-TP upper limit values were compared between tertiary and community hospitals using a T-test for independent samples.
Results
The web-based survey was distributed to 275 email addresses through the CNSF and yielded 96 responses, for a response rate of 34.9%. The survey completion rate was 100%. After the removal of duplicate hospital answers, there were 51 unique responses, with a predominance of tertiary academic hospitals with university affiliation (72.5%). All (100%) the responders indicated they did not use age-dependent CSF-TP reference ranges beyond the neonatal period. When asked about the origin of their institutional reference values, 78.8% chose “unknown” and 21.2% chose “institutional/local data.” An additional 91 telephone responses limited to CSF-TP upper limit were obtained from laboratory personnel. Only a single laboratory reported that a higher reference limit was applied to adults over the age of 60 years. The telephone contact list excluded university hospitals already covered in the CNSF web-based survey, resulting in a much higher proportion of community hospitals (84%).
Figure 1 provides a geolocation map for the total data set of 142 Canadian hospital laboratory unique responses, demonstrating country-wide representation. This included responses from 91 (64.1%) community hospitals and 51 (35.9%) academic/tertiary care institutions. The frequency distribution of CSF-TP upper limits is displayed in Figure 2. A value of 0.45 g/L or less was reported by 114 centers (80.4%). A value of 0.6 g/L or more was reported by 26 centers (18.2%). The mean CSF-TP upper limit was 0.468 g/L ± 0.060 for university-affiliated centers and 0.473 g/L ± 0.074 for community hospitals. Comparison of this data using a two-tailed T-test for independent samples did not show a statistically significant difference (p = 0.716).
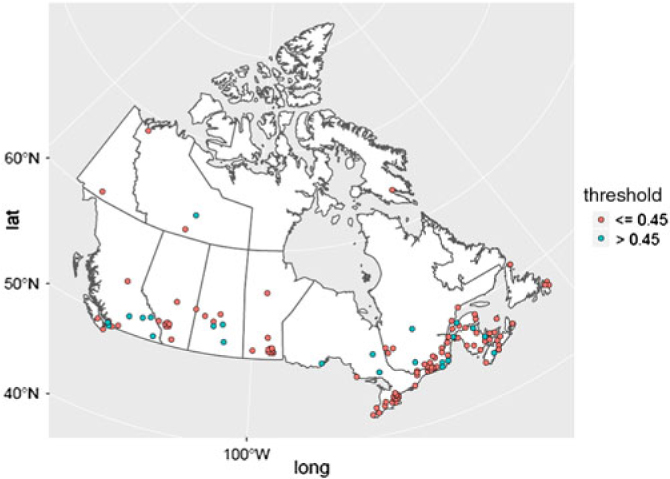
Figure 1: Canadian geolocation map for 142 hospital laboratories CSF-TP reference values. Hospital centers are represented in red if the CSF-TP upper limit is ≤ 0.45 g/L and in blue if the CSF-TP upper limit is >0.45 g/L. The geographical distribution is as follows: North West Territories (3), Yukon (1), Nunavut (1), British Columbia (13), Alberta (11), Saskatchewan (8), Manitoba (13), Ontario (28), Quebec (39), New Brunswick (9), Nova Scotia (10), Prince-Edward Island (2), and Newfoundland (4).
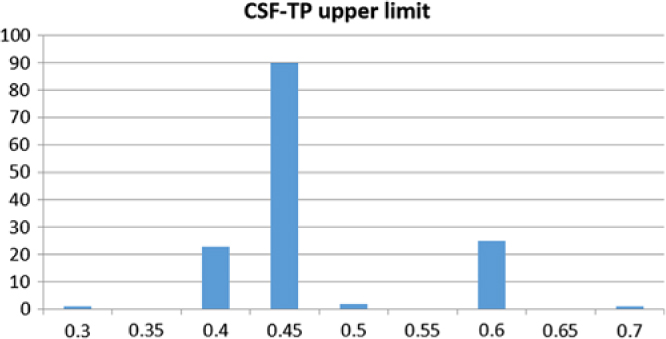
Figure 2: Frequency distribution of CSF-TP upper limit values (g/L, x-axis) in 142 Canadian hospital centers (y-axis).
Discussion
Our survey reveals that over 80% of 142 Canadian hospital laboratories report a CSF-TP upper reference range of 0.45 g/L or less, with no significant difference between academic or community hospitals. Similar findings were noted in a survey of 84 hospital laboratories in the UK where only 7% of centers used a value greater than 0.45 g/L and none reported age-adjusted reference ranges for adults.Reference Holbrook, Beetham and Cruickshank5 In the majority of cases (78.8%), physician and hospital laboratory personnel were not aware of how this reference limit was derived.
The commonly stated upper reference limit of 0.45 g/L can be traced back to literature from the 1930sReference Merritt and Fremont-Smith1 and has been subsequently widely reiterated in medical literature as well as in lab reference manuals. Lumbar punctures are invasive procedures, usually performed when there is a significant suspicion of a disorder of the central or peripheral nervous systems. Hospital laboratories would not be expected to routinely conduct institution-specific reference studies, but would typically rely on published literature.
Few studies have assessed neurologically normal volunteers, and such studies were comprised of small cohorts of young adults.Reference Ben Menachem, Persson, Schechter, Haegele, Huebert and Hardenberg6–Reference Merril and Harrington9 This limits the accuracy of the reference range and does not account for the rise in CSF-TP with increasing age. The best alternative strategy to define a reference sample may consist in analyzing CSF samples in a hospital laboratory database. The process of identifying suitable reference samples can start with CSF laboratory criteria, such as the exclusion of samples with abnormal white blood cell count, red blood cell count, or glucose level. It is imperative to conduct an additional clinical chart and broader laboratory review to exclude conditions known to be associated with CSF protein elevation, such as known spinal disorders, meningeal infection, tumors, multiple sclerosis, inflammatory or diabetic neuropathy, etc. The reference population thus selected is likely to consist of patients who eventually were found to have a neurologically benign final diagnosis such as non-aneurysmal headache, psychiatric disease, transient metabolic encephalopathy, or negative screening for malignancy. Obviously, this type of reference study might be confounded by an asymptomatic spinal spondylarthrosis, causing an elevation of CSF protein at the level of the lumbar cistern through a mechanism of sequestration.Reference Ahonen, Myllyla and Hokkanen10, Reference Skouen, Larsen, Gjerde, Hegrestad and Vollset11 Published reference studies have not required spinal imaging to systematically exclude this possibility, but it can be considered that it would similarly confound the assessment of CSF-TP in healthy volunteers. Two recent studies, published by groups in AustriaReference Hegen, Auer, Zeileis and Deisenhammer3 and CanadaReference McCudden, Brooks, Figurado and Bourque2 using modern laboratory equipment and very consistent selection criteria, reached remarkably similar results, stratified by age. Based on these studies, one can extrapolate a more rigorous CSF-TP upper reference limit of 0.5 g/L for age < 30 years, 0.55 g/L for age 30–49, 0.6 g/L for ages 50–59, and values reaching above 0.65 g/L beyond the age of 60 years.
A false positive determination of raised CSF-TP, based on an incorrect reference range, may lead to an erroneous diagnosis of albuminocytologic dissociation and the unwarranted suspicion of abnormal permeability of the blood–brain or blood–nerve barriers. It may trigger unnecessary neuroimaging, nerve conduction studies, repeat lumbar puncture, or additional investigations for inflammatory disorders of the central or peripheral nervous systems. A striking example is the risk of misdiagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), as was documented in a case series from a tertiary care referral center.Reference Allen and Lewis12 In this case series, an important source of error was undue reliance on “mild to moderate” CSF-TP elevation, possibly confounded by incorrect reference limits. The erroneous diagnosis of CIDP often leads to unnecessary treatment with immunomodulatory therapies including intravenous immunoglobulin.
Our study is subjected to the common limitations of web-based surveys. The response rate of 34.9% is in line with what can be expected for medical specialists in general, though one Canadian study documented a higher response rate for neurologists and neurosurgeons.Reference Cunningham, Quan and Hemmelgarn13 The web-survey distribution list obtained through the CNSF favored responses from academic hospitals. This bias had to be corrected by a complementary data collection strategy (direct phone contact), to allow for a wider representation of community hospital laboratories.
Apart from refining the CSF-TP reference range, it is also worthwhile to note that many academic research centers are moving toward analysis of other indices to determine the integrity of the blood–brain barrier, the increased intrathecal production, or the abnormal release or sequestration of specific brain proteins. Examples of these other indices include the CSF/serum albumin ratio (Q alb),Reference Deisenhammer, Bartos and Egg14 the detection of CSF oligoclonal bands,Reference Freedman, Thompson and Deisenhammer15 serum neuron-specific enolase,Reference Zandbergen, Hijdra and Koelman16 serum S-100β,Reference Branco, Oliveira and Sargento-Freitas17 and CSF tau,Reference Shaw, Arias and Blennow18 amyloid precursor protein,Reference Constantinides, Paraskevas and Emmanouilidou19 or synuclein.Reference Shi, Tang and Toledo20 However, for the time being, many of these are specialty tests which are not widely available and subject to the same challenge of defining reference populations. The readily accessible measure of CSF-TP remains by far the most widely used CSF protein measurement in Canada and in the world. Although it has well-acknowledged limitations in diagnostic sensitivity and specificity, its interpretation still warrants evidence-based reference limits. The survey we presented here reveals a significant gap in knowledge translation – despite strong evidence from recent publications, there has not yet been a shift in institutional standard operational procedures. The Canadian experience is likely reflective of a similar worldwide discrepancy, suggesting a need to widely apply data-driven, rather than historical, reference limits.
Acknowledgements
The authors extend thanks to Ms. Donna Irvin and the leadership of the CNSF for allowing a successful distribution of the survey, and to Ms. J. Klaas for her help with collating responses from clinical laboratories.
Disclosures
The authors have no conflicts of interest to declare.
Statements of authorship
PR: Survey distribution and data entry, and manuscript draft and revisions.
CRM: Critical review of manuscript, with emphasis on laboratory reference criteria.
JWC: Critical review of manuscript.
JB: Critical review of manuscript
HH: Critical review of manuscript, with emphasis on laboratory reference interpretation.
FD: Critical review of manuscript.
AB: Interpretation of data, literature review, and manuscript revision