Infant botulism results from the germination and outgrowth of ingested spores of botulinum neurotoxin-producing clostridia colonizing the large intestine of infants aged <1 year. Botulism should be suspected in infants aged <12 months showing constipation, lethargy, poor feeding, weak cry, bulbar palsies, and failure to thrive [Reference Lindström and Korkeala1, Reference Fenicia, Annibal and Aureli2]. Detection of C. botulinum in the stools or gut contents supports the diagnosis [Reference Nevas3]. The persistence of C. botulinum in samples of infant botulism patients has been reported to vary from 2 to 4 months [Reference Grant4, Reference Fenicia5]. Here we report a 3-month-old baby with type A infant botulism, who excreted botulinum neurotoxin and/or C. botulinum in his faeces for over 5 months after clinical recovery and discharge from hospital. Faecal excretion of C. botulinum can occur for much more prolonged periods of time than previously suspected, posing a possible health risk for caregivers and others in close contact with the infant.
In April 2010, a previously healthy infant was admitted to the Children's Hospital, University of Helsinki. He was breast-fed with formula supplementation. At age 3 months he became irritable and was feeding poorly for 2 days. On admission, he had flaccid palsy with severe bulbar symptoms. The baby was expressionless with ophthalmoplegia and inability to swallow or breathe.
On admission, routine laboratory tests, brain magnetic resonance (MR), and electroencephalography were conducted. Electroneuromyography (ENMG) was performed on days 6 and 17. Serum samples were collected on admission and on day 12. Stool samples were periodically collected during the hospital stay and after discharge from hospital until January 2011. A gastric content sample was collected on day 12.
At the infant's residence, the parents were interviewed and their stool samples were collected. The dust bags from a cylinder and handheld vacuum cleaner were taken. Blood and egg-yolk agar plates were left with the lid open for 1 h in the residence to capture airborne spores. Additional air sampling was done using a Particle Collector (Kymppitaito Oy, Finland) twice for 30 min. Dust and dirt particles were swabbed from windowsills, soles of shoes and from carpets. All swab samples were taken in duplicate. An opened bag of commercial plant soil, sawdust bedding and dry hay from the cage of a pet rabbit, and rabbit faeces from the cage were collected for examination. Informed consent was obtained from the patient's parents.
The serum, gastric content, and stool samples of the patient were tested for botulinum neurotoxin by the standard mouse bioassay. The mouse assays were performed under the license of appropriate authority (ESAVI-2010-03838-Ym-23).
For culture and polymerase chain reaction (PCR) analysis, all swab, faecal and gastric content samples were treated with or without ethanol and inoculated into tryptone-peptone-glucose-yeast (TPGY) extract broth, followed by anaerobic incubation (85% N2, 10% CO2, 5% H2; MK III, Don Whitley Scientific Ltd, UK) for 3 days at 30°C and 37°C. This was followed by subculture of 1 ml into fresh TPGY and overnight incubation at the respective conditions.
Detection of botulinum neurotoxin gene (bot) was revealed from the overnight cultures by multiplex PCR [Reference Lindström and Korkeala1, Reference De6]. Moreover, in order to detect bot directly from stool samples, DNA was isolated with faecal DNA miniPrep kit (Zymo Research Corporation, USA) and real-time PCR was used for detection [Reference Satterfield7]. To estimate the number of C. botulinum spores in the household dust, the most probable number method with PCR detection of bot was used [Reference Nevas8].
C. botulinum strains were isolated from bot-positive stool and household dust samples by growing them on egg-yolk agar plates. Colonies with lipase reaction were re-streaked onto egg-yolk agar plates and the presence of bot was confirmed [Reference Lindström and Korkeala1, Reference De6]. All isolates were compared with amplified fragment length polymorphism (AFLP) [Reference Keto-Timonen9] and their neurotoxin genes were sequenced to reveal the neurotoxin subtype.
Furthermore, 65 vacuum cleaner dust bags collected from volunteer households in the Helsinki area were collected and analysed for bot and the estimated C. botulinum count as described above.
On admission, the infant had no symptoms of respiratory infection, no vomiting, fever, constipation or diarrhoea. On the following day, respiratory weakness developed and subsequent apnoea necessitated mechanical ventilation. The routine laboratory test results were within normal limits. The laboratory findings with MR image did not support an acute septic infection, inflicted injury or metabolic crises. The electroencephalography revealed only decreased alertness, but no sign of epileptic discharges or slowing of background activity. The mild elevation of protein levels in CSF (329 mg/l, ref. 150–300 mg/l) suggested severe polyradiculitis. ENMG was performed on day 6 and revealed decreased motor responses and partial conduction block in the median nerve, but the distal latencies and nerve conduction velocities were within normal limits. No decrement was seen in repetitive nerve stimulations. The ENMG on day 6 was suggestive for polyradiculitis but did not show brief-duration, small-amplitude, polyphasic potentials (BSAPs) [Reference Arnon10]. The CSF protein levels had normalized (281 mg/l), and the spinal MR image was normal without nerve root enhancement.
The infant was given intravenous immunoglobulin (IVIG) therapy with no response. ENMG was repeated on day 17 and showed that the motor responses were decreased but at that time the repetitive stimulation at 3 Hz revealed a slight decrement and a 50–100% increment at 50 Hz, suggestive for presynaptic transmission disturbance supporting the possibility of infant botulism. BSAPs were observed, again suggestive of infant botulism [Reference Arnon10].
The diagnosis of infant botulism was confirmed by detecting botulinum neurotoxin type A in a serum sample taken at admission. At the time of the diagnosis, the clinical condition of the child had stabilized and no specific treatments were therefore initiated. The central symptoms with bulbar palsy were most severe at the beginning and restored slowly. The first signs of recovery were revival of the patient's smile, eye movements and distal movements of hands and feet. Within the total hospitalization of 52 days, ventilation support continued for 50 days.
The gastric content obtained at admission and the second serum sample, both taken on day 12, were negative for botulinum neurotoxin. However, a stool sample taken on day 6 was positive for botA with both multiplex and real-time PCR assays, suggesting the presence of C. botulinum type A. This was further confirmed by recovery of a type A1 isolate from the stool (Table 1).
Table 1. Analysis of Clostridium botulinum and botulinum neurotoxin in the patient and environmental samples
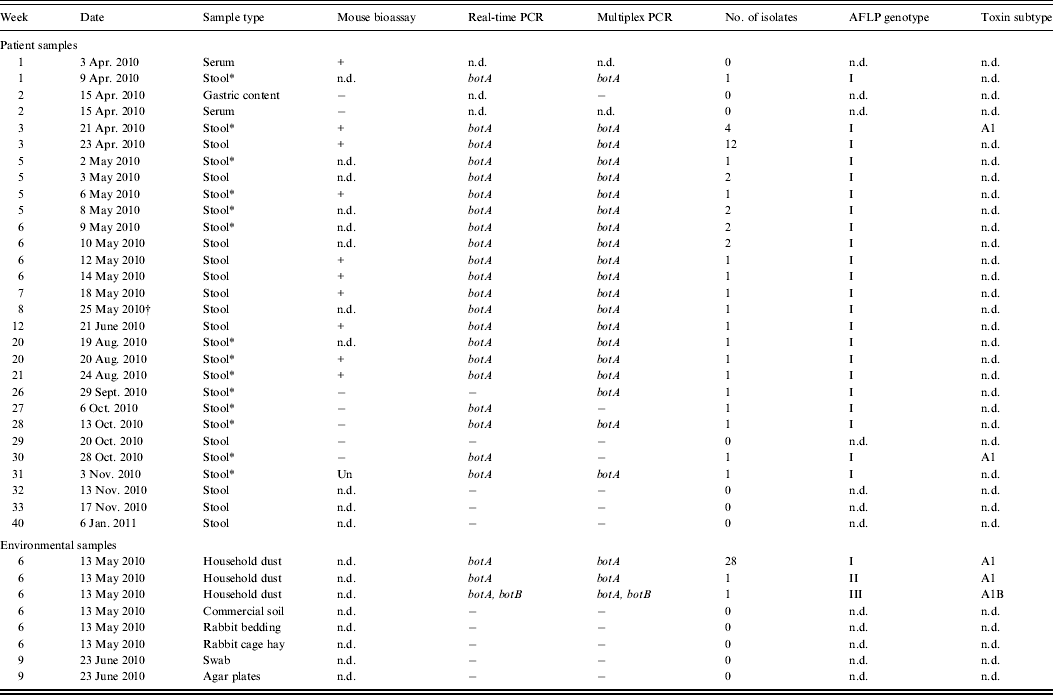
PCR, Polymerase chain reaction; AFLP, amplified fragment length polymorphism; n.d., no data; Un, unspecific symptom.
* Positive PCR and isolation only without ethanol treatment.
† Day of discharge from hospital.
The patient's stool was positive for C. botulinum type A during the hospital stay and on the day of discharge. Moreover, the stool samples were positive for botulinum neurotoxin for 4 months and positive for C. botulinum for at least 5 months after discharge from hospital (Table 1).
Field investigation revealed that the child had not ingested honey, neither were the parents employed in places that might increase exposure to C. botulinum spores. The family lived in the city of Helsinki in an area where there was no construction work in progress or windy conditions. Thus, the likelihood of contamination by environmental spores was not considered to be increased. The stool samples of the parents were negative for C. botulinum.
Two separate dust bags from the cylinder and handheld vacuum cleaners in the residence of the patient contained C. botulinum type A. The spore count in the two dust bags was estimated to be 210 and 454 spores/kg, respectively. None of the other environmental samples from the infant's residence or 65 randomly collected vacuum cleaner dust bags from volunteers contained C. botulinum.
Isolates carrying neurotoxin type A1 gene were recovered from stool and showed identical AFLP banding patterns with those recovered from the vacuum cleaner dust bags from the patient's home (Table 1). Moreover, a distinct strain isolated from the cylinder vacuum cleaner dust bag, was positive for botA and botB.
Here we report that in infant botulism, faecal excretion of botulinum toxin and C. botulinum may continue for an extremely long period of time, and most importantly, by patients that have fully recovered from clinical symptoms. Although cross-contamination cases have not been reported [Reference Koepke, Sobel and Arnon11], careful follow-up for toxin and C. botulinum excretion of all infant botulism patients is important to control the possible environmental and health risks these babies may pose to their families, kindergartens and other settings. Careful handling of infant botulism patients and limitation of close contact with other children and animals is thus urged [12].
We were able to isolate C. botulinum from all PCR-positive stool samples during and after the course of clinical infant botulism, which verifies the positive PCR findings. The fact that isolation was successful only from non-ethanol-treated samples but not from ethanol-treated samples on most occasions between April and November (Table 1) suggests the persistence of active C. botulinum vegetative cells but not spores in the infant's faeces for 7 months. Future investigations should address the properties of this particular C. botulinum type A strain which may have contributed to its efficient colonization in the infant's gut.
Apart from honey, ingestion of environmental C. botulinum spores frequently present in soil and dust has been proposed as the principal source of infant botulism [Reference Nevas3, Reference Grant4, Reference Arnon13]. Detection of identical C. botulinum genotypes in the patient's stool and household dust may suggest that dust was the initial source of the infection. The possibility of household dust being contaminated via the infected infant cannot be excluded, but is contradicted by the detection of multiple C. botulinum genotypes in the dust bags. Despite vigorous environmental sampling in the patient's residence, the source of C. botulinum found in the vacuum cleaner dust bags from the infant's home remains unclear. We could not find C. botulinum spores in 65 dust bags collected from various sources suggesting that C. botulinum spores are not a common finding in household dust in Finland.
ACKNOWLEDGEMENTS
This work was conducted at the Centre of Excellence in Microbial Food Safety Research and funded by the Academy of Finland (11860, 2141140) and the ABS Graduate School. We thank Hanna Korpunen, Heimo Tasanen and Maria Stark for technical assistance.
DECLARATION OF INTEREST
None.



